The blood-brain barrier (BBB) (discovered and defined by Max Lewandowsky and Lina Stern, and not, as it is universally, and yet erroneously believed, by Paul Ehrlich (Verkhratsky and Pivoriunas, 2023)) that separates the nervous system from the circulation is evolutionarily conserved from arthropods to man. The primeval BBB of the invertebrates and some early vertebrates was made solely by glial cells and secured (in invertebrates) by septate junctions; in most vertebrates, including mammals, the barrier is associated with endothelial cells and secured with tight junctions. This, however, is a simplified view, as brain-fluid barriers in general and the BBB in particular, are complex structures, which dynamically control traffic between nervous tissue and the circulation. The vascular part of the BBB complex (Figure 1) includes hemocompatible glycocalyx, which outlines and protects the single-cell layer of brain endothelial cells (BECs) clamped together with tight and adherence junctions that restrict paracellular transport. Tight junctions form a highly efficient barrier for ion fluxes that translates into a very high (up to 5000 Ω/cm2) electrical resistance (generally referred to as transendothelial electrical resistance or TEER) of the brain endothelial barrier, which is > 100 times larger as compared to peripheral capillaries where TEER varies between 2 and 20 Ω/cm2. Brain endotheliocytes are in direct contact with pericytes, several subtypes of which are associated with capillaries and pre- and post-capillary vessels. Pericytes and endothelial cells are covered with the vascular basement membrane. In arterioles and venules, the basement membrane is surrounded by smooth muscle cells regulating vasodilatation and vasoconstriction. The parenchymal part of the BBB is made by endfeet of protoplasmic (in grey matter) or fibrous (in white matter) astrocytes; every protoplasmic astrocyte extends at least one (and usually several) perivascular processes (Hosli et al., 2022). Astrocytic endfeet rest on the parenchymal basement membrane; together they form the glia limitans perivascularis (which also includes processes of juxtavascular microglia). The composition of vascular and parenchymal basal membranes is different, reflecting distinct extracellular matrix components secreted by endotheliocytes and astrocytes. At the level of arterioles and venules, vascular and parenchymal membranes are separated by perivascular space filled with cerebrospinal fluid; this perivascular space, together with glia limitans, provides the anatomical substrate for the brain-wide glymphatic system. At the level of capillaries, both basement membranes join while perivascular space disappears.
Figure 1.
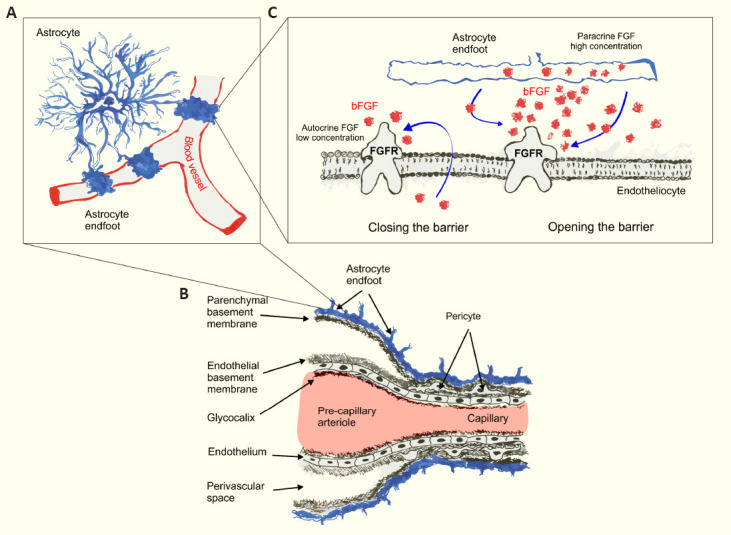
Structural organization of the vasculature in the brain.
(A) Schematic representation of astrocyte endfeet coverage of brain capillaries. (B) Cellular components of the blood-brain barrier. (C) Proposed dual effect of bFGF on the regulation of permeability of the blood-brain barrier endothelial cells. bFGF: Basic fibroblast growth factor; FGFR: fibroblast growth factor receptor.
The BBB is an integral part of the brain’s active milieu, which brings together all cell types to create a functionally active nervous tissue (Semyanov and Verkhratsky, 2021). The complex of cells related to the BBB (i.e., endothelial cells, pericytes, smooth muscle cells, astrocytes, microglia, and neurons) is formally defined as a neurogliovascular unit. The astrocyte, which (in rodents) occupies an individual territorial domain, is considered as an integrator that brings all the aforementioned cells together to form a functional and relatively independent unit (Schaeffer and Iadecola, 2021). Of note, the territorial domain organization of astrocytes, which is quite prominent in rodents, may not extend to all species; for example, in ferret and human cortex, astrocytes seem to display a high degree of overlapping and criss-crossing each other’s territories.
Astrocytes do not contribute to the embryonic BBB formation and function; the vascular permeability in the mouse embryo starts to decrease from embryonic day 13 and BBB is functional as early as on embryonic day 16, whereas astrogenesis does not start until embryonic day 18. Astrocytes join the BBB complex in earnest in the postnatal brain with astrocyte endfeet coverage of blood vessels in the mouse brain being completed at postnatal day 5 (P5) in the cortex and at P13 in pial vessels. Astrocyte perivascular endfeet maturation is characterized by MLC1 and GlialCAM proteins forming a complex during P10–P15 and this correlates with BBB maturation as shown by increased levels of claudin-5 and P-glycoprotein in BECs (Gilbert et al., 2019).
Basement membranes prevent direct parenchymal cell-vascular cell contacts; hence astrocyte-pericyte and astrocyte-endotheliocyte interactions occur through a paracrine route. Astrocyte-conditioned medium as well as non-contact co-culturing of BECs with astrocytes up-regulates expression of tight junction proteins directly responsible for maintaining barrier properties in BECs, increases TEER and reduces paracellular permeability in vitro (Siddharthan et al., 2007). However, in vivo studies are far more ambiguous as the contribution of astrocytes in the regulation of BBB function under physiological conditions remains obscure, waiting for appropriate animal models to answer this fundamental question. Removal of perivascular astrocytic endfeet by laser ablation in mice did not compromise the integrity of the BBB, while re-coverage of vessels by another endfoot coming from neighboring intact astrocyte began as soon as fifty minutes after ablation (Kubotera et al., 2019). Arguably, the duration of loss of astrocyte-endothelial interaction was too short to induce BBB damage. In contrast, inducible astrocyte-specific ablation in genetically engineered mice leading to a sustained removal of astrocytes revealed their critical role in the maintenance of the BBB in the adult brain. Loss of astrocytes led to extravasation of plasma fibrinogen, reduction of expression of main tight junction protein zonula occludens-1; the BBB leakage sustained for several weeks after ablation (Heithoff et al., 2021).
Astrocytes are the main secretory cells in the central nervous system (CNS); however, most paracrine factors affecting the BBB in vitro are not expressed or expressed at a low level under physiological conditions. These include Sonic hedgehog, glial-derived neurotrophic factor, angiopoietin, basic fibroblast growth factor (bFGF), and vascular endothelial growth factor A (VEGF-A) (Heithoff et al., 2021; Verkhratsky and Pivoriunas, 2023). In pathology, expression of BBB permeability-inducing factors like VEGF, matrix metalloproteinases, and nitric oxide is increased, whereas in certain contexts astrocytes secrete BBB integrity-promoting factors, such as glial-derived neurotrophic factor or retinoic acid. Reactive astrocyte-derived Sonic hedgehog, for example, restores BBB integrity after CNS injury by upregulating the expression of tight junction proteins although its expression in astrocytes in the healthy brain is low with neurons being the main source (Heithoff et al., 2021).
The Wnt/β-catenin signaling pathway is one of the most powerful regulators of angio- and barrier-genesis. This signaling cascade plays a key role during development as well as in maintaining the BBB integrity in the adult healthy brain and is intimately involved in the pathophysiology of various disorders linked to BBB destruction (Wang et al., 2022). Components of the Wnt pathway in the developing CNS are mainly secreted by neural precursor cells. In the postnatal brain, neuronal secretion of Wnt significantly decreases although continuous Wnt/β-catenin signaling aimed at BECs is required for proper BBB functioning. Neither pericytes nor smooth muscle cells express Wnt proteins; astrocytes, however, act as the main source of Wnt factors in the adult brain. Inhibiting astrocytic Wnt release by the cell-specific deletion of the Wnt secretion mediator evenness interrupted gene from ~90% of astrocytes results in a decreased expression of Wnt/β-catenin target genes in BECs, instigated brain edema, increased extravasation of fluorescent tracers and up-regulation of caveolin-1. The Wnt signaling pathway target genes were downregulated in astrocytes as well; at the same time, astrocytic endfeet coverage of blood vessels was altered and swollen endfeet were observed (Guerit et al., 2021).
Astrocyte-specific neogenin (Neo-1) knock-out mice were used to demonstrate that Neo-1 depletion results in various defects in the BBB: an increase in dextran leakage, disrupted basal membrane, reduced pericyte coverage, and decreased blood flow. Neo-1 is a netrin receptor that has a role in cell migration and axonal guidance, while netrins can act as angiogenesis-inducing or suppressing factors. Moreover, during the study, netrin-1 (Ntn-1) expression was shown to be significantly reduced in astrocytes. The addition of Ntn-1 to Neo-1 knock-out mice condition medium attenuated BECs proliferation and revealed the Neo-1/Ntn-1 pathway to be involved in BBB maintenance in a healthy brain (Yao et al., 2020).
Another factor that may regulate BBB function in a healthy brain is bFGF, which is expressed in astrocytes. Genetic manipulations demonstrated that FGF signaling keeps astrocyte reactivity at bay as cell-specific deletion of FGF receptors triggered astrogliosis. This effect could be seen in both healthy and injured brains. However, the BBB remained intact as no leakage of tracer molecules to the brain tissue was observed (Kang et al., 2014). Suppression of bFGF signaling in BECs using FGF receptor 1 inhibitor PD173074 reduced TEER. Exogenous bFGF in concentrations exceeding 4 ng/mL impaired barrier integrity through the ERK1/2 signaling pathway in a concentration-dependent manner as shown by decreased TEER values in human BECs monolayers. This demonstrates a dual role of bFGF in the regulation of barrier properties – autocrine FGF signaling is required to maintain high TEER values, whereas at high concentrations bFGF impairs the integrity of the BBB. Increased expression of bFGF in BECs, astrocytes and pericytes was associated with various CNS injuries (Kriauciunaite et al., 2023). However, the exact mechanism of barrier integrity disruption by bFGF remains unknown. bFGF could indirectly contribute to the regulation of the BBB by increasing the expression of permeability-inducing factors. In particular, bFGF promotes the expression of VEGF and angiopoietin-2 (Ang-2) (Fujii and Kuwano, 2010). A study in rats, characterized by increased expression of Ang-2, demonstrated a negative effect of Ang-2 on BBB permeability: decreased expression of tight junction protein claudin-5 and adherens junction molecule VE-cadherin, increased expression of endothelial transcytosis-related molecule caveolin-1 in brain endotheliocytes led to increased intracellular and transcellular permeability of the BBB (Gurnik et al., 2016). On the other hand, several studies have shown the protective effect of bFGF on the BBB after intracerebral hemorrhage and traumatic brain injury (Wang et al., 2016). However, most of the studies of the effects of bFGF on the BBB were conducted under pathological conditions, and there are very little data on its impact on the healthy brain. It is also important to consider the administration method. BECs are characterized by polarised apical and basolateral expression of transporters, metabolite-degrading enzymes, and receptors (Schaeffer and Iadecola, 2021). Thus, it is possible that the same factor could have distinct effects depending on whether it was administrated from the luminal or abluminal side of BECs. Future studies using cell-type specific knockouts of bFGF secretion in astrocytes and (or) FGF receptor depletion in brain endotheliocytes together with simultaneous monitoring of the BBB permeability in vivo will be instrumental for understanding the role of astrocytic bFGF in the regulation of the BBB.
Surprisingly, little is known about the effects of astrocyte-secreted factors on the integrity of the BBB under physiological conditions. Recent evidence indicates that local changes in the secretion of paracrine factors by astrocytes can be important for the dynamic regulation of the BBB. We expect that future studies employing conditional knockouts of astrocytic paracrine factor secretion and (or) expression of cognate receptors in BECs and pericytes will help to clarify these issues.
APociūtė, APivoriūnas and AV received funding from European Regional Development Fund (project No 13.1.1-LMT-K-718-05-0005) under grant agreement with the Research Council of Lithuania (LMTLT). Funded as European Union’s measure in response to COVID-19 pandemic.
Additional file: Open peer review report 1 (80.1KB, pdf) .
Footnotes
Open peer reviewer: Takafumi Inoue, Waseda University, Japan.
P-Reviewer: Inoue T; C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Fujii T, Kuwano H. Regulation of the expression balance of angiopoietin-1 and angiopoietin-2 by Shh and FGF-2. In Vitro Cell Dev Biol Anim. 2010;46:487–491. doi: 10.1007/s11626-009-9270-x. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert A, Vidal XE, Estevez R, Cohen-Salmon M, Boulay AC. Postnatal development of the astrocyte perivascular MLC1/GlialCAM complex defines a temporal window for the gliovascular unit maturation. Brain Struct Funct. 2019;224:1267–1278. doi: 10.1007/s00429-019-01832-w. [DOI] [PubMed] [Google Scholar]
- 3.Guerit S, Fidan E, Macas J, Czupalla CJ, Figueiredo R, Vijikumar A, Yalcin BH, Thom S, Winter P, Gerhardt H, Devraj K, Liebner S. Astrocyte-derived Wnt growth factors are required for endothelial blood-brain barrier maintenance. Prog Neurobiol. 2021;199:101937. doi: 10.1016/j.pneurobio.2020.101937. [DOI] [PubMed] [Google Scholar]
- 4.Gurnik S, Devraj K, Macas J, Yamaji M, Starke J, Scholz A, Sommer K, Di Tacchio M, Vutukuri R, Beck H, Mittelbronn M, Foerch C, Pfeilschifter W, Liebner S, Peters KG, Plate KH, Reiss Y. Angiopoietin-2-induced blood-brain barrier compromise and increased stroke size are rescued by VE-PTP-dependent restoration of Tie2 signaling. Acta Neuropathol. 2016;131:753–773. doi: 10.1007/s00401-016-1551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heithoff BP, George KK, Phares AN, Zuidhoek IA, Munoz-Ballester C, Robel S. Astrocytes are necessary for blood-brain barrier maintenance in the adult mouse brain. Glia. 2021;69:436–472. doi: 10.1002/glia.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosli L, Zuend M, Bredell G, Zanker HS, Porto de Oliveira CE, Saab AS, Weber B. Direct vascular contact is a hallmark of cerebral astrocytes. Cell Rep. 2022;39:110599. doi: 10.1016/j.celrep.2022.110599. [DOI] [PubMed] [Google Scholar]
- 7.Kang W, Balordi F, Su N, Chen L, Fishell G, Hebert JM. Astrocyte activation is suppressed in both normal and injured brain by FGF signaling. Proc Natl Acad Sci U S A. 2014;111:E2987–2995. doi: 10.1073/pnas.1320401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kriauciunaite K, Pociute A, Kausyle A, Verkhratsky A, Pivoriunas A. Basic fibroblast growth factor opens and closes the endothelial blood-brain barrier in a concentration-dependent manner. Neurochem Res. 2023;48:1211–1221. doi: 10.1007/s11064-022-03678-x. [DOI] [PubMed] [Google Scholar]
- 9.Kubotera H, Ikeshima-Kataoka H, Hatashita Y, Allegra Mascaro AL, Pavone FS, Inoue T. Astrocytic endfeet re-cover blood vessels after removal by laser ablation. Sci Rep. 2019;9:1263. doi: 10.1038/s41598-018-37419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaeffer S, Iadecola C. Revisiting the neurovascular unit. Nat Neurosci. 2021;24:1198–1209. doi: 10.1038/s41593-021-00904-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semyanov A, Verkhratsky A. Astrocytic processes: from tripartite synapses to the active milieu. Trends Neurosci. 2021;44:781–792. doi: 10.1016/j.tins.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 2007;1147:39–50. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkhratsky A, Pivoriunas A. Astroglia support regulate and reinforce brain barriers. Neurobiol Dis. 2023;179:106054. doi: 10.1016/j.nbd.2023.106054. [DOI] [PubMed] [Google Scholar]
- 14.Wang Q, Huang X, Su Y, Yin G, Wang S, Yu B, Li H, Qi J, Chen H, Zeng W, Zhang K, Verkhratsky A, Niu J, Yi C. Activation of Wnt/beta-catenin pathway mitigates blood-brain barrier dysfunction in Alzheimer's disease. Brain. 2022;145:4474–4488. doi: 10.1093/brain/awac236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ZG, Cheng Y, Yu XC, Ye LB, Xia QH, Johnson NR, Wei X, Chen DQ, Cao G, Fu XB, Li XK, Zhang HY, Xiao J. bFGF protects against blood-brain barrier damage through junction protein regulation via PI3K-Akt-Rac1 pathway following traumatic brain injury. Mol Neurobiol. 2016;53:7298–7311. doi: 10.1007/s12035-015-9583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yao LL, Hu JX, Li Q, Lee D, Ren X, Zhang JS, Sun D, Zhang HS, Wang YG, Mei L, Xiong WC. Astrocytic neogenin/netrin-1 pathway promotes blood vessel homeostasis and function in mouse cortex. J Clin Invest. 2020;130:6490–6509. doi: 10.1172/JCI132372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


