Abstract
Stroke is a leading cause of disability and mortality worldwide, necessitating the development of advanced technologies to improve its diagnosis, treatment, and patient outcomes. In recent years, machine learning techniques have emerged as promising tools in stroke medicine, enabling efficient analysis of large-scale datasets and facilitating personalized and precision medicine approaches. This abstract provides a comprehensive overview of machine learning’s applications, challenges, and future directions in stroke medicine. Recently introduced machine learning algorithms have been extensively employed in all the fields of stroke medicine. Machine learning models have demonstrated remarkable accuracy in imaging analysis, diagnosing stroke subtypes, risk stratifications, guiding medical treatment, and predicting patient prognosis. Despite the tremendous potential of machine learning in stroke medicine, several challenges must be addressed. These include the need for standardized and interoperable data collection, robust model validation and generalization, and the ethical considerations surrounding privacy and bias. In addition, integrating machine learning models into clinical workflows and establishing regulatory frameworks are critical for ensuring their widespread adoption and impact in routine stroke care. Machine learning promises to revolutionize stroke medicine by enabling precise diagnosis, tailored treatment selection, and improved prognostication. Continued research and collaboration among clinicians, researchers, and technologists are essential for overcoming challenges and realizing the full potential of machine learning in stroke care, ultimately leading to enhanced patient outcomes and quality of life. This review aims to summarize all the current implications of machine learning in stroke diagnosis, treatment, and prognostic evaluation. At the same time, another purpose of this paper is to explore all the future perspectives these techniques can provide in combating this disabling disease.
Keywords: cerebrovascular disease, deep learning, machine learning, reinforcement learning, stroke, stroke therapy, supervised learning, unsupervised learning
Introduction
Stroke is one of the most common and devastating disorders, a leading cause of disability, and the second leading cause of death worldwide cause, with approximately 5.5 million deaths worldwide in 2015. Numerous advances have been made in understanding cerebrovascular diseases in recent years, but scientific research can still provide many improvements. Indeed, many steps remain to better characterize pathogenesis at the molecular level, identify risk profiles, prognostic stratification, and personalize treatment. Nevertheless, the significant advances in technology, which are increasingly beginning to have a place in the most modern approach to medicine, and the better knowledge of the pathophysiology of stroke open up new prospects for the development timing-based instrumental approach (Feigin et al., 2022).
Machine learning (ML) techniques have gained recognition in stroke medicine as valuable tools. They allow for the adequate examination of extensive datasets and support personalized and precise medical approaches. This summary thoroughly examines ML’s applications, obstacles, and future prospects in stroke medicine. ML algorithms that have been introduced recently are being widely utilized across various aspects of stroke medicine. These models have exhibited impressive precision in analyzing medical images, identifying different stroke subtypes, stratifying risks, aiding medical treatment decisions, and predicting patient outcomes.
The objective of this review is to consolidate the existing applications of machine learning in stroke diagnosis, treatment, and prognostic evaluation. Additionally, this paper seeks to investigate the potential avenues these techniques offer to address this debilitating condition.
Search Strategy
The searches were conducted during September 2022 in the databases CINAHL, Web of Science, and PubMed. The Mesh terms identified for the PubMed search were adapted to corresponding terms in CINAHL. Every individual search term was supplemented with relevant free-text terms. When appropriate, the free text terms have been truncated in order to include alternative word endings. The search result was limited to articles that were written in English as well as articles published during the last ten years. The full search strategy is included as an appendix. The database searches were complemented with a manual review of the reference lists of relevant articles, which resulted in a few additional articles included in the study.
Current Evidence on Stroke
Pathophysiology of stroke
A stroke or cerebrovascular accident is a sudden onset of a neurological deficit caused by a focal blood vessel obstruction.
The manifestation of a stroke is variable because of the brain’s and blood vessels’ complex anatomy. It is caused by a reduction in blood flow to the brain that lasts longer than a few seconds, leading to rapid energy failure in neurons and death of brain tissue if the flow continues to be stopped. If blood flow is restored, the symptoms may be temporary, called a transient ischemic attack. The reduction of blood flow to the brain depends on the site of occlusion, systemic blood pressure, and individual vascular anatomy. Infarction occurs when blood flow is below a certain threshold, while ischemia without infarction can occur if the flow is restored within a specific time frame. In this context, the surrounding reversibly dysfunctional tissue that will turn into infarction if the flow is not restored is defined as “The ischemic penumbra”. Infarction occurs via two pathways: necrotic and apoptotic. Ischemia causes cell death by depriving neurons of glucose and oxygen, leading to adenosine triphosphate failure and blockage of ion pumps, cellular depolarization, and cellular injury. It also causes the release of free radicals that induce further damage to cells.
Apoptotic cell death occurs with lesser degrees of ischemia, as seen in the penumbra. Fever and hyperglycemia worsen brain injury during ischemia, so it is recommended to suppress fever and prevent hyperglycemia. The value of mild hypothermia for improving stroke outcomes is still being researched (Kuriakose and Xiao, 2020).
Diagnosis in stroke: clinical approach
There is evidence that “Time is Brain” (von Kummer, 2019), and an accurate diagnosis is essential to enhancing stroke therapy. With evidence of early and long-lasting improvement in a patient treated with tissue-type plasminogen activator, 30% more likely to have minimal or no disability at three months on the assessment scales, according to the NINDS Study Group, an accurate diagnosis of a stroke can enable the appropriate intervention to be administered promptly, significantly improving the patient’s outcome and recovery (National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group, 1995).
A correct clinical approach is the first and most crucial step towards a correct diagnosis, evident from when the patient arrives at the emergency department. In fact, the manifestations of stroke can vary greatly depending on the topographic pattern of the lesion.
The close correlation between clinical neurological signs and symptoms and acute ischemic or hemorrhagic stroke has gradually led to the development of a practical neurological scale that can help identify stroke patients in the emergency room and measure stroke severity (Kothari et al., 1999; Harbison et al., 2003; Kwah and Diong, 2014).
In the Cincinnati Prehospital Stroke Scale study, the scoring of each scale item showed a high sensitivity of 66% and specificity of 87% for identifying a stroke patient when any of the three-stroke scale items were observed, with high reproducibility observed among prehospital providers for the total score and each scale item, and excellent intraclass correlation (Kothari et al., 1999).
The Face Arm Speech Test also proved to be a good tool for stroke diagnosis, accurately identifying 144 out of 183 (79%) stroke patients presented to the emergency department (Harbison et al., 2003).
The National Institutes of Health Stroke Scale has moderate to high reliability in assessing stroke severity when administered by both medical and non-medical personnel (intra-rater κ = 0.66 to 0.77; inter-rater κ = 0.69). It has also been shown to be very reliable when clinicians rate videos of patients (intra-rater ICC = 0.93; inter-rater ICC = 0.95) (Kwah and Diong, 2014).
The progressive approach to stroke patients is the most effective in identifying stroke by neuroimaging techniques. Timing is also essential for the correct characterization of ischemic/hemorrhagic lesions by CT and MRI.
A comparison of the sensitivity of MRI and CT in a prospective study of 356 patients, of whom 217 had a final clinical diagnosis of acute stroke, showed a sensitivity of 83% (181 of 217; 78–88%) and 26% (56 of 217; 20–32%) for MRI and CT, respectively, for the diagnosis of any acute stroke (Chalela et al., 2007).
In another study, the sensitivity of both techniques appears to increase after 48 hours of stroke onset, with 85% of positive diagnoses for CT (75/89), 93.5% (115/123) for MRI, and 98.8% (79/80) for DWI (Smajlović and Sinanović, 2004).
Outcome prediction in stroke
The proper therapy is not enough to guarantee long-term survival after a stroke. The risk of death after a stroke is estimated to be 28% at 28 days, 41% at one year, and 60% at five years, and the risk of death between four weeks and one year after a first stroke is five times higher in patients with non-fatal stroke than in the general population, and the risk of death after one year is twice as high (Boysen et al., 2009).
This trend is also consistent with the results of a cohort study in which, of the 2447 patients followed for ten years after a primary minor ischemic stroke or transient ischemic attack, 1489 (60%) died, and 1336 (54%) had at least one vascular event. The 10-year risk of death was 42.7% (95% CI 40.8–44.7). Age over 65 years, diabetes, claudication, previous peripheral vascular surgery, and pathological Q waves on the baseline electrocardiogram were all associated with an increased risk of death. The 10-year risk of a vascular event was 44.1% (42.0–46.1), and the predictive factors for vascular events were similar to those for the risk of death. The annual risk of a vascular event increased over time after decreasing in the first three years (van Wijk et al., 2005).
In this setting, mortality prediction may assist clinicians in prognosticating outcomes, developing supportive care plans, choosing the right therapy, coordinating rehabilitation services, facilitating patient and family counseling, fair comparisons of hospital outcomes, and performance assessment related to stroke mortality.
Several previous studies have attempted to predict stroke outcomes, such as the I-Score, taking into account multivariable predictors of 30-day and 1-year mortality, including older age, male sex, severe stroke, non-lacunar stroke subtype, glucose ≥ 7.5 mM (135 mg/dL), history of atrial fibrillation, coronary artery disease, congestive heart failure, cancer, dementia, kidney disease on dialysis, and dependency before stroke.
This study retrospectively analyzed data on 12,262 patients with acute ischemic stroke from several hospitals in Ontario between 2003 and 2008. Patients were identified from the Canadian Stroke Network registry and the Ontario Stroke Audit, with 8223 in the derivation cohort, 4039 in the internal validation cohort, and 3720 in the external validation cohort. The 30-day mortality rates for the derivation and internal validation cohorts were 12.2% and 12.6%, respectively, and the 1-year mortality rates were 22.5% and 22.9% (Saposnik et al., 2011).
Machine Learning: New Light on Stroke
The above-mentioned studies have highlighted the efforts being made to develop reliable predictive models that can be used to guide clinical decision-making. For example, accurately predicting the likelihood of a particular outcome can help clinicians choose the most appropriate therapy and tailor their treatment plans to individual patients. However, these approaches are limited by classical biostatistics, which focuses on reducing bias due to study design, whereas analyzing data from large populations would provide a real-world perspective.
With the rise of big data, the increasing use of electronic health records, and the parallel development of machine learning algorithms, healthcare professionals can now tackle population health problems that were once thought impossible. This shift towards using clinical data at a population level has fundamentally changed the way we make inferences about a population, allowing us to identify and address health issues with greater precision and accuracy.
One of the key benefits of machine learning is its ability to identify patterns and correlations in complex data sets, which can provide valuable insights into disease diagnosis, prognosis, and treatment. In addition, by automating the data analysis process, machine learning reduces human bias and improves the accuracy of predictions.
Machine learning algorithms are well suited to handling “big data”. Big data refers to large, complex, and diverse variables that cannot be processed or analyzed using traditional data processing tools or techniques. Machine learning is able to assimilate large amounts of data, both structured and unstructured, generated from a variety of sources. Big data is characterized by its high volume, velocity, and variety, and requires specialized technologies and methodologies to manage, process, and analyze it. Big data analysis can provide valuable information for decision-making, research, and innovation by revealing insights and patterns that would be difficult to discern from smaller, more structured data sets (Zhou et al., 2017).
Machine learning: how it works
Supervised learning and unsupervised learning represent the main type of machine learning (Figure 1).
Figure 1.
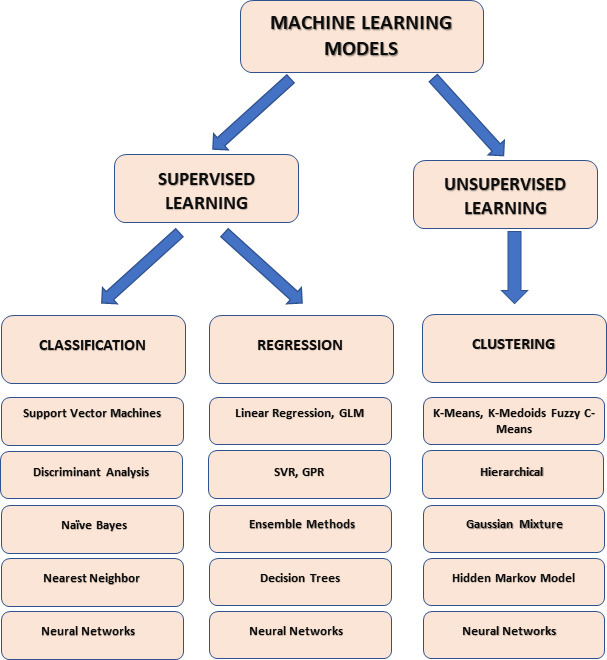
Principal types of machine learning models.
Created with Microsoft PowerPoint. GLM: Generalized linear models; GPR: Gaussian process regression; SVR: support vector regression.
Supervised learning is a machine learning technique in which a model is trained on labeled data to predict new, unseen data output. It is used in a wide range of applications, including image classification, speech recognition, natural language processing, and predictive modeling in healthcare, finance, and marketing. In supervised learning, the algorithm is given a set of inputs and the corresponding correct outputs, also known as labels. Next, it learns to map the inputs to the outputs through a process called training. Once trained, the model can then make predictions about new data based on what it has learned during training (Figure 2).
Figure 2.
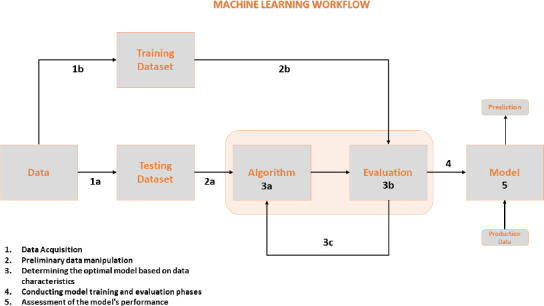
Machine learning workflow.
Popular supervised learning algorithms include linear regression, logistic regression, support vector machines, neural networks, and decision tree algorithms (Ayodele, 2010).
Neural networks have several advantages that make them useful for solving complex problems. One of these advantages is scalability, as they can be trained on large datasets using distributed computing resources. The scalability makes neural networks suitable for a wide range of applications, allowing them to tackle complex problems. Another advantage is adaptability, which means neural networks can adapt to new data and situations and generalize well to unseen data. This ability is advantageous in applications where the input data may change over time. Finally, neural networks are easily parallelized, allowing them to take advantage of modern hardware architectures such as graphical processing units and tensor processing units. With their scalability, adaptability, and parallel processing capabilities, neural networks are a powerful tool for solving complex problems in a wide range of fields.
Backpropagation is the best-known example of an algorithm used to train a neural network. Backpropagation calculates the gradient vector of the error surface, which indicates the direction of the steepest descent from the current point. Moving along this vector will decrease the error, and a series of such moves can eventually lead to finding a minimum. However, determining the appropriate step size can be challenging. Significant steps can lead to faster convergence but can also overshoot the solution or veer off course if the error surface is complex. On the other hand, small steps are more reliable but require more iterations. The step size is, therefore, proportional to the gradient and a learning rate constant, which is usually determined by experimentation and may also vary over time.
According to the definition of González et al. (2005), a support vector machine (SVM) is a classification algorithm that constructs an N-dimensional hyperplane to separate data into two categories optimally. SVM models have similarities to neural networks, and an SVM model using a sigmoid kernel function is equivalent to a two-layer perceptron neural network. SVM models and classical multilayer perceptron neural networks are closely related, and SVMs are an alternative training method for polynomial, radial basis functions and multilayer perceptron classifiers. Instead of solving a non-convex, unconstrained minimization problem, as in standard neural network training, the network weights are determined by solving a quadratic programming problem with linear constraints.
Random forest and decision tree algorithms are both used in machine learning for classification and regression tasks. A decision tree is a tree-like model in which each internal node represents a test on an attribute, each branch represents a test result, and each leaf node represents a class label. The tree is constructed by recursively partitioning the data into subsets based on the best attribute to partition on, with the goal of minimizing impurity or maximizing information gain. Decision trees have the advantage of being easy to interpret but are prone to overfitting and can be unstable. Random forest is an ensemble method that uses multiple decision trees to improve prediction accuracy and reduce overfitting (Ngiam and Khor, 2019).
Machine Learning in Stroke Medicine: Current Research and Development
Machine learning has numerous applications in stroke medicine, contributing to improved diagnosis, treatment, and patient outcomes (Figure 3).
Figure 3.
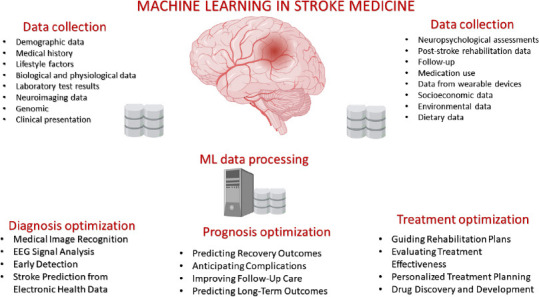
Principal application of machine learning in stroke medicine.
EEG: Electroencephalography; ML: machine learning.
The application of machine learning methods to stroke medicine began several years ago. In a comparative review published in Methods of Information in Medicine, Linder et al. (2006) first attempted to validate the use of machine learning to predict stroke outcomes by comparing logistic regression analysis and machine learning algorithms proposed with artificial neural networks. This comparative review was based on the German Stroke Database. The authors included a data set of 1754 prospectively recruited patients with acute ischemic stroke. They developed two prognostic models based on logistic regression and artificial neural networks with the aim of predicting a return to functional independence and survival at 100 days. The study showed no difference between the algorithms, except for the model that predicted fully versus incompletely restituted or deceased patients. Although the results were encouraging, the authors concluded that the use of logistic regression remains the gold standard for prognostic modeling. Nevertheless, more than fifteen years ago, artificial neural networks were touted as a “quick and easy” alternative to multivariate analysis.
Since then, all areas of medicine, particularly genetics, oncology, and cardiology, have seen an exponential increase in the use of machine learning techniques (Awan et al., 2018; Zampieri et al., 2019; Sultan et al., 2020).
Over the years, the reliability of predictions has increased significantly due to the refinement of techniques for analyzing data sets (Table 1).
Table 1.
Principal fields of application of Machine Learning Models in Stroke Medicine
| Study title | Objective | ML-model | Conclusion and clinical application | Reference |
|---|---|---|---|---|
| Comparison of different machine learning approaches to model stroke subtype classification and risk prediction | Diagnosis of stroke subtypes and mortality | RF | Prediction of the stroke type and associated outcomes that a patient may face | Garcia-Temza et al., 2019 |
| Ischemic stroke identification based on EEG and EOG using ID convolutional neural network and batch normalization | Diagnosis of ischemic stroke through EEG | 1D CNN vs. various models (NB, Classification Tree, ANN, RF, kNN, LR) | The findings suggest that EEG has significant potential for differentiating individuals with stroke from the general population, highlighting its feasibility as a diagnostic tool. | Giri et al., 2016 |
| An automated detection method for the MCA dot sign of acute stroke in unenhanced CT | Identification of the MCA dot sign in non-contrast CT scans | SVM | Potential detection of the MCA dot sign of acute stroke on unenhanced CT images | Takahashi et al., 2014 |
| Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks | Automatically segment stroke lesions in DWI | CNN | AI-enabled automated segmentation of acute ischemic stroke lesions on DWI achieves high accuracy, aiding swift diagnosis and treatment decisions. | Chen et al., 2017; Bentley et al., 2014 |
| 3D convolutional neural networks applied to CT angiography in the detection of acute ischemic stroke. | Ischemic stroke detection | 3D CNN | 3D convolutional neural networks can effectively detect acute ischemic stroke lesions from CTA-SI, with contralateral hemisphere data aiding false positive reduction. | Öman et al., 2019 |
| Prediction of tissue outcome and assessment of treatment effect in acute ischemic stroke using deep learning. | Prediction of final infarct volume | CNN | Deep convolutional neural network accurately predicts final lesion volume in acute ischemic stroke, enhancing personalized treatment planning. | Nielsen et al., 2018 |
| Automatic machine-learning-based outcome prediction in patients with primary intracerebral hemorrhage | Prediction of the functional outcome (Measured by mRS) at the 1st and 6th mon | RF | Machine learning technique using a random forest model accurately predicts functional outcomes in primary intracerebral hemorrhage patients at 1st and 6th mon, aiding clinical decisions and patient care. | Wang et al., 2019 |
| Machine learning-based model for prediction of outcomes in acute stroke. | Prediction of mRS score (0–2 vs. 3–6) at 90 d | DNN | Machine learning algorithms, especially the deep neural network, significantly improve the prediction of long-term outcomes in ischemic stroke patients compared to traditional scoring methods. | Heo et al., 2019 |
| Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study | Automated detection of head CT scan abnormalities | DNN | Deep learning algorithms accurately identify abnormalities in head CT scans. Possibility to automate the triage process for urgent cases. | Chilamkurthy et al., 2018 |
| Machine learning to predict mortality after rehabilitation among patients with severe stroke | Predicting 3-yr mortality in stroke patients | RF, ADA-B, GB | Machine learning algorithms outperformed logistic regression for predicting 3-yr mortality in stroke patients. | Scrutinio et al., 2020 |
ADA-B: AdaBoost; AI: artificial intelligence; ANN: artificial neural network; CNN: convolutional neural network; CT: computed tomography; CTA: computed tomography angiography; DNN: deep neural network; DWI: diffusion-weighted imaging; EEG: electroencephalography; GB: gradient boosting; kNN: k-nearest neighbors; LR: logistic regression; MCA: middle cerebral artery; ML: machine learning; mRS: modified Rankin scale; NB: Naive Bayes; RF: random forest; SI: source images; SVM: support vector machine.
In the context of big data, deep neural networks have the potential to improve the diagnosis, prevention, and treatment of stroke. By processing vast amounts of data, deep neural networks can identify patterns and relationships that are difficult for humans to detect, including subtle risk factors and early warning signs of stroke.
Deep neural networks can also help develop personalized treatment plans by analyzing data from individual patients, such as medical history, lifestyle factors, and genetic information. This technology can improve outcomes and reduce the risk of complications by helping healthcare providers tailor treatments and interventions to each patient’s specific needs.
In addition, deep neural networks can be used to develop predictive models that identify patients at high risk of stroke, enabling early intervention and prevention. By analyzing large datasets, these models can identify patterns and risk factors that may not be apparent using traditional methods, leading to more accurate risk assessments and targeted prevention strategies.
Machine learning in stroke diagnosis
The urgency of stroke care underscores the need for accurate and timely tools to aid its diagnosis. With the emergence of various artificial intelligence-based diagnostic imaging algorithms, the field of brain imaging has advanced significantly in recent years (Mainali et al., 2021).
Since the development of computer-aided diagnosis (CAD) in 1980, CAD has evolved considerably, and the development of powerful artificial intelligence algorithms has accelerated its evolution towards what is defined as the main difference between modern CAD and classical CAD. Modern CAD uses representation learning based algorithms such as deep learning, which can learn to automatically discover and extract meaningful patterns and features from raw data (Mokli et al., 2019).
An example is the study by Chilamkurthy et al. (2018), who retrospectively collected a dataset of 313,318 head CT scans with clinical reports from 20 centers in India between 2011 and 2017. A randomly selected subset of the dataset was used for validation (Qure25k dataset), and the rest was used for algorithm development. In addition, an additional validation dataset was collected from different centers (CQ500 dataset).
Areas under the receiver operating characteristic curves (AUCs) were primarily used to assess the algorithms for automated detection of intracranial hemorrhage and its types (i.e., intraparenchymal, intraventricular, subdural, extradural, and subarachnoid), calvarial fractures, midline shift, and mass effect. The algorithms achieved high AUCs for detecting different types of intracranial hemorrhage on both the Qure25k and CQ500 datasets. In addition, the AUCs for skull fractures, midline shift, and mass effect were also high. Overall, the study demonstrated the potential of using deep learning algorithms to detect and diagnose intracranial abnormalities on CT scans accurately (Chilamkurthy et al., 2018).
With the potential of fully detecting stroke lesions characteristic of deep learning algorithms, some companies have developed Food and Drug Administration-cleared software to detect and alert clinicians of large vessel occlusion (LVO), such as Viz LVO® (No authors listed, 2018). This software has demonstrated high detection accuracy in a recent single-center study in real-world clinical practice. In this study, 1167 CTAs were analyzed, of which 404 were stroke protocols. Of the total, 75 patients had a LVO, and the system detected 61 of them, giving a sensitivity of 0.81, a negative predictive value of 0.99, and an accuracy of 0.94. In the subgroup of stroke protocol patients, 72 out of 404 patients had a LVO, and the system identified 59 of them, achieving a sensitivity of 0.82, a negative predictive value of 0.96, and an accuracy of 0.89 (Yahav-Dovrat, 2021).
Machine learning in stroke prognosis and outcome prediction
While diagnosing acute stroke and determining the onset time are critical steps in comprehensive stroke management, predicting the prognosis after stroke is of great importance, particularly for treatment selection, such as identifying which patients will benefit from a particular type of treatment. It is also essential to determine long-term outcomes, such as motor, cognitive and functional abilities, and to plan rehabilitation by setting appropriate goals.
In acute management, assessing a patient’s likelihood of developing symptomatic intracranial hemorrhage is critical. In this area, clinician-based prognostic tools such as SEDAN (Sugar, Early Infarct signs, Dense cerebral artery sign, Age, and National Institutes of Health Stroke Scale) and Hemorrhage After Thrombolysis (HAT) have recently been compared with an automated SVM machine learning algorithm. In this retrospective study by Bentley et al. (2014), the AUC of the automated SVM using both National Institutes of Health Stroke Scale and raw imaging data (0.744) was found to be better than the AUCs of the SEDAN and HAT scores, regardless of whether the original or adapted versions of these scores were used (0.626–0.720; P < 0.01 for all; Bentley et al., 2014).
Machine learning has proven to be a powerful tool in stroke outcome prediction, outperforming the logistic regression model in predicting three-year mortality, as shown by Scrutinio et al. (2020) In the comparison, the logistic regression model had an AUC of 0.745 and, when properly calibrated, achieved an accuracy of 75.7%, a positive predictive value of 33.9%, and a negative predictive value of 91.0% at the optimal risk threshold. With the use of the synthetic minority oversampling technique and random forests, the ML algorithm outperformed the logistic regression model with an AUC of 0.928 and an accuracy of 86.3%, a positive predictive value of 84.6%, and a negative predictive value of 87.5% (Scrutinio et al., 2020).
The deep neural network was also superior to the Angioplasty and Stenting for Renal Artery Lesions (ASTRAL) score in predicting functional outcomes in a study of 2604 patients with acute ischemic stroke, with the area under the curve for the deep neural network model significantly higher than that of the ASTRAL score (0.888 vs. 0.839; P < 0.001).
Using an automated machine learning random forest algorithm to predict functional outcomes after intracerebral hemorrhage has proven to be a valuable tool. This prediction model can provide clinicians with meaningful information to guide appropriate medical care for patients and their caregivers regarding their functional outcomes. The random forest model was trained on data from 333 patients with primary intracerebral hemorrhage. The accuracy of predicting functional outcome at month one was 83.1%, with a sensitivity of 77.4%, specificity of 86.9%, and an AUC of 0.899. At 6 months, the overall accuracy was 83.9%, with a sensitivity of 72.5%, a specificity of 90.6%, and an AUC of 0.917 (Wang et al., 2019).
Linear SVM regression has also been used to predict the outcome of the rehabilitation process in the early stages of stroke. The predicted and measured outcomes (T1 Barthel index, T1 motor functional independence measure (FIM), T1 cognitive FIM, and T1 total FIM) showed good correlation in the test samples, ranging from 0.75 to 0.81. However, the mean absolute deviation percentage was high for the T1 Barthel index (83.96%), while the other predicted responses (T1 Motor FIM, T1 Cognitive FIM, T1 Total FIM) had a lower mean absolute deviation percentage of 30%. The root mean square error ranged from 4.28 for T1 cognitive FIM to 22.6 for T1 Barthel index (Sale et al., 2018).
In 2019, Heo et al. examined if machine learning methods may be used to foresee long-term outcomes in ischemic stroke patients. Patients with acute ischemic stroke were enrolled in a prospective cohort for this retrospective analysis. They created three machine learning models (deep neural network, random forest, and logistic regression) and tested how well they predicted good outcomes (modified Rankin score < 3) compared with ASTRAL, one of the most widely used stroke outcome prediction scores. Of the 2604 patients eligible for the study, 78% had a favorable outcome. In addition, the deep neural network model performed significantly better than the ASTRAL score, with a significantly larger area under the ROC curve (0.888 vs. 0.839; P < 0.001). The results of this study once again demonstrated the reliability and accuracy of machine learning techniques compared to canonical techniques.
One of the most important studies to evaluate outcome prediction from clinical and instrumental data was recently published in Circulation (Raghunath et al., 2021). Raghunath et al. (2021) attempted to create a prediction model for new-onset atrial fibrillation by analyzing 1.6 million resting 12-lead digital electrocardiogram (ECG) traces from 430,000 patients collected over a 35-year period from 1984 to 2019. The study aimed to use deep neural network analysis to individualize recurrent ECG patterns in patients without a history of atrial fibrillation (AF), which may help identify patients at risk of AF-related stroke. Applying machine learning models to the subset of patients who developed a subsequent ischemic stroke, the authors showed that 62% of patients with an AF-related stroke within three years of an ECG were predicted to be at high risk for new-onset AF. Therefore, it seems reasonable to assume that if these patients had been considered at high risk of developing AF, they might have benefited from anticoagulant treatment and thus reduced the prognostic impact of ischemic stroke. Analyzing repetitive patterns of graphical data, such as a surface ECG, to develop prognostic models is proving to be a breakthrough in risk prediction for therapeutic purposes, a goal that traditional statistical methods cannot achieve.
Conclusion
ML has the potential to significantly improve the diagnosis, care, and outcomes of ischemic stroke patients.
The diagnosis and detection of ischemic stroke is an area where machine learning can be beneficial. Large amounts of medical data, such as brain imaging scans, a patient’s medical history, and clinical symptoms, can be analyzed by machine learning algorithms to find patterns and estimate the likelihood of a stroke. As a result, ischemic stroke can be diagnosed earlier and more accurately, improving patient outcomes.
In addition, machine learning can predict the likelihood of stroke and identify people more likely to have the disease. Machine learning algorithms can identify risk factors and predict the likelihood of stroke by studying patient data. This information can guide treatment decisions and help prevent strokes.
Treatment planning and selection are other areas where machine learning could be helpful. For example, by evaluating patient data, machine learning algorithms can assist physicians in selecting the best therapies for specific patients based on their particular features and medical history. This approach could lead to more personalized and successful treatment programs that improve patient outcomes.
In conclusion, machine learning has the potential to significantly improve the diagnosis, treatment, and outcomes of patients with ischemic stroke. As technology advances, we can expect machine learning to play an increasingly important role in the management of this condition.
Footnotes
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
Conflicts of interest: The authors declare no conflicts of interest.
Data availability statement: Not applicable.
References
- 1.Awan SE, Sohel F, Sanfilippo FM, Bennamoun M, Dwivedi G. Machine learning in heart failure: ready for prime time. Curr Opin Cardiol. 2018;33:190–195. doi: 10.1097/HCO.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 2.Ayodele T. Types of machine learning algorithms. InTech. doi: 10.5772/9385 2010 [Google Scholar]
- 3.Bentley P, Ganesalingam J, Carlton Jones AL, Mahady K, Epton S, Rinne P, Sharma P, Halse O, Mehta A, Rueckert D. Prediction of stroke thrombolysis outcome using CT brain machine learning. Neuroimage Clin. 2014;4:635–640. doi: 10.1016/j.nicl.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boysen G, Marott JL, Grønbaek M, Hassanpour H, Truelsen T. Long-term survival after stroke: 30 years of follow-up in a cohort the Copenhagen City Heart Study. Neuroepidemiology. 2009;33:254–260. doi: 10.1159/000229780. [DOI] [PubMed] [Google Scholar]
- 5.Chalela JA, Kidwell CS, Nentwich LM, Luby M, Butman JA, Demchuk AM, Hill MD, Patronas N, Latour L, Warach S. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369:293–298. doi: 10.1016/S0140-6736(07)60151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L, Bentley P, Rueckert D. Fully automatic acute ischemic lesion segmentation in DWI using convolutional neural networks. Neuroimage Clin. 2017;15:633–643. doi: 10.1016/j.nicl.2017.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chilamkurthy S, Ghosh R, Tanamala S, Biviji M, Campeau NG, Venugopal VK, Mahajan V, Rao P, Warier P. Deep learning algorithms for detection of critical findings in head CT scans: a retrospective study. Lancet. 2018;392:2388–2396. doi: 10.1016/S0140-6736(18)31645-3. [DOI] [PubMed] [Google Scholar]
- 8.Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, Fisher M, Pandian J, Lindsay P. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke. 2022;17:18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 9.García-Temza L, Risco-Martín JL, Ayala JL, Roselló GR, Camarasaltas JM. Comparison of different machine learning approaches to model stroke subtype classification and risk prediction. 2019 Spring Simulation Conference (SpringSim) 2019 DOI: 10.23919/SpringSim.2019.8732846. [Google Scholar]
- 10.Giri EP, Fanany MI, Arymurthy AM, Wijaya SK. Ischemic stroke identification based on EEG and EOG using ID convolutional neural network and batch normalization. 2016 International Conference on Advanced Computer Science and Information Systems (ICACSIS) 2016 doi: 10.1109/ICACSIS.2016.7872780. [Google Scholar]
- 11.González L, Angulo C, Velasco F, Català A. Unified dual for Bi-class SVM approaches. Pattern Recognit. 2005;38:1772–1774. [Google Scholar]
- 12.Harbison J, Hossain O, Jenkinson D, Davis J, Louw SJ, Ford GA. Diagnostic accuracy of stroke referrals from primary care, emergency, room physicians and ambulance staff using the face arm speech test. Stroke. 2003;34:71–76. doi: 10.1161/01.str.0000044170.46643.5e. [DOI] [PubMed] [Google Scholar]
- 13.Heo J, Yoon JG, Park H, Kim YD, Nam HS, Heo JH. Machine learning–based model for prediction of outcomes in acute stroke. Stroke. 2019;50:1263–1265. doi: 10.1161/STROKEAHA.118.024293. [DOI] [PubMed] [Google Scholar]
- 14.Kothari RU, Pancioli A, Liu T, Brott T, Broderick J. Cincinnati Prehospital Stroke Scale: reproducibility and validity. Ann Emerg Med. 1999;33:373–378. doi: 10.1016/s0196-0644(99)70299-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuriakose D, Xiao Z. Pathophysiology and treatment of stroke: present status and future perspectives. Int J Mol Sci. 2020;21:7609. doi: 10.3390/ijms21207609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS) J Physiother. 2014;60:61. doi: 10.1016/j.jphys.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Linder R, König IR, Weimar C, Diener HC, Pöppl SJ, Ziegler A. Two models for outcome prediction - a comparison of logistic regression and neural networks. Methods Inf Med. 2006;45:536–540. [PubMed] [Google Scholar]
- 18.Mainali S, Darsie ME, Smetana KS. Machine learning in action: stroke diagnosis and outcome prediction. Front Neurol. 2021;12:734345. doi: 10.3389/fneur.2021.734345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mokli Y, Pfaff J, dos Santos DP, Herweh C, Nagel S. Computer-aided imaging analysis in acute ischemic stroke –background and clinical applications. Neurol Res Pract. 2019;1:23. doi: 10.1186/s42466-019-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 21.Ngiam KY, Khor IW. Big data and machine learning algorithms for health-care delivery. Lancet Oncol. 2019;20:e262–e273. doi: 10.1016/S1470-2045(19)30149-4. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen A, Hansen MB, Tietze A, Mouridsen K. Prediction of tissue outcome and assessment of treatment effect in acute ischemic stroke using deep learning. Stroke. 2018;49:1394–1401. doi: 10.1161/STROKEAHA.117.019740. [DOI] [PubMed] [Google Scholar]
- 23.Öman O, Mäkelä T, Salli E, Savolainen S, Kangasniemi M. 3D convolutional neural networks applied to CT angiography in the detection of acute ischemic stroke. Eur Radiol Exp. 2019;3:8. doi: 10.1186/s41747-019-0085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.No authors listed. FDA approves stroke-detecting AI software. Nat Biotechnol. 2018;36:290. doi: 10.1038/nbt0418-290. [DOI] [PubMed] [Google Scholar]
- 25.Raghunath S, Pfeifer JM, Ulloa-Cerna AE, Nemani A, Carbonati T, Jing L, vanMaanen DP, Hartzel DN, Ruhl JA, Lagerman BF, Rocha DB, Stoudt NJ, Schneider G, Johnson KW, Zimmerman N, Leader JB, Kirchner HL, Griessenauer CJ, Hafez A, Good CW, et al. Deep neural networks can predict new-onset atrial fibrillation from the 12-lead ECG and help identify those at risk of atrial fibrillation-related stroke. Circulation. 2021;143:1287–1298. doi: 10.1161/CIRCULATIONAHA.120.047829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sale P, Ferriero G, Ciabattoni L, Cortese AM, Ferracuti F, Romeo L, Piccione F, Masiero S. Predicting motor and cognitive improvement through machine learning algorithm in human subject that underwent a rehabilitation treatment in the early stage of stroke. J Stroke Cerebrovasc Dis. 2018;27:2962–2972. doi: 10.1016/j.jstrokecerebrovasdis.2018.06.021. [DOI] [PubMed] [Google Scholar]
- 27.Saposnik G, Raptis S, Kapral MK, Liu Y, Tu JV, Mamdani M, Austin PC. Investigators of the Registry of the Canadian Stroke Network and the Stroke Outcome Research Canada Working Group. The iScore predicts poor functional outcomes early after hospitalization for an acute ischemic stroke. Stroke. 2011;42:3421–3428. doi: 10.1161/STROKEAHA.111.623116. [DOI] [PubMed] [Google Scholar]
- 28.Scrutinio D, Ricciardi C, Donisi L, Losavio E, Battista P, Guida P, Cesarelli M, Pagano G, D'Addio G. Machine learning to predict mortality after rehabilitation among patients with severe stroke. Sci Rep. 2020;10:20127. doi: 10.1038/s41598-020-77243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smajlović D, SinanovićO Sensitivity of the neuroimaging techniques in ischemic stroke. Med Arh. 2004;58:282–284. [PubMed] [Google Scholar]
- 30.Sultan AS, Elgharib MA, Tavares T, Jessri M, Basile JR. The use of artificial intelligence, machine, learning and deep learning in oncologic histopathology. J Oral Pathol Med. 2020;49:849–856. doi: 10.1111/jop.13042. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi N, Lee Y, Tsai DY, Matsuyama E, Kinoshita T, Ishii K. An automated detection method for the MCA dot sign of acute stroke in unenhanced CT. Radiol Phys Technol. 2014;7:79–88. doi: 10.1007/s12194-013-0234-1. [DOI] [PubMed] [Google Scholar]
- 32.van Wijk I, Kappelle LJ, van Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, Gorter JW, Algra A. LiLAC study group. Long-term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet. 2005;365:2098–2104. doi: 10.1016/S0140-6736(05)66734-7. [DOI] [PubMed] [Google Scholar]
- 33.von Kummer R. Time is brain. Stroke. 2019;50:552–553. doi: 10.1161/STROKEAHA.118.024214. [DOI] [PubMed] [Google Scholar]
- 34.Wang HL, Hsu WY, Lee MH, Weng HH, Chang SW, Yang JT, Tsai YH. Automatic machine-learning-based outcome prediction in patients with primary intracerebral hemorrhage. Front Neurol. 2019;10:910. doi: 10.3389/fneur.2019.00910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yahav-Dovrat A, Saban M, Merhav G, Lankri I, Abergel E, Eran A, Tanne D, Nogueira RG, Sivan-Hoffmann R. Evaluation of artificial intelligence-powered identification of large-vessel occlusions in a comprehensive stroke center. AJNR Am J Neuroradiol. 2021;42:247–254. doi: 10.3174/ajnr.A6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zampieri G, Vijayakumar S, Yaneske E, Angione C. Machine and deep learning meet genome-scale metabolic modeling. PLoS Comput Biol. 2019;15:e1007084. doi: 10.1371/journal.pcbi.1007084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou L, Pan S, Wang J, Vasilakos AV. Machine learning on big data: opportunities and challenges. Neurocomputing. 2017;237:350–361. [Google Scholar]


