Keywords: dopaminergic neurons, FGF signal, induced pluripotent stem cells, midbrain, neural differentiation, SHH signal, SMAD signal, WNT signal
Abstract
Midbrain dopaminergic neurons play an important role in the etiology of neurodevelopmental and neurodegenerative diseases. They also represent a potential source of transplanted cells for therapeutic applications. In vitro differentiation of functional midbrain dopaminergic neurons provides an accessible platform to study midbrain neuronal dysfunction and can be used to examine obstacles to dopaminergic neuronal development. Emerging evidence and impressive advances in human induced pluripotent stem cells, with tuned neural induction and differentiation protocols, makes the production of induced pluripotent stem cell-derived dopaminergic neurons feasible. Using SB431542 and dorsomorphin dual inhibitor in an induced pluripotent stem cell-derived neural induction protocol, we obtained multiple subtypes of neurons, including 20% tyrosine hydroxylase-positive dopaminergic neurons. To obtain more dopaminergic neurons, we next added sonic hedgehog (SHH) and fibroblast growth factor 8 (FGF8) on day 8 of induction. This increased the proportion of dopaminergic neurons, up to 75% tyrosine hydroxylase-positive neurons, with 15% tyrosine hydroxylase and forkhead box protein A2 (FOXA2) co-expressing neurons. We further optimized the induction protocol by applying the small molecule inhibitor, CHIR99021 (CHIR). This helped facilitate the generation of midbrain dopaminergic neurons, and we obtained 31–74% midbrain dopaminergic neurons based on tyrosine hydroxylase and FOXA2 staining. Thus, we have established three induction protocols for dopaminergic neurons. Based on tyrosine hydroxylase and FOXA2 immunostaining analysis, the CHIR, SHH, and FGF8 combined protocol produces a much higher proportion of midbrain dopaminergic neurons, which could be an ideal resource for tackling midbrain-related diseases.
Introduction
Dopaminergic neurons are an important neuronal type in the human brain and are associated with a variety of brain functions, including cognition, emotion, movement, and reward (Tian et al., 2022). Midbrain dopaminergic (mDA) neurons, occupying approximately 75% of all dopaminergic neurons, form three distinct neural clusters including the ventral tegmental area, red nucleus, and substantia nigra pars compacta. Substantial loss of these dopaminergic neurons due to brain injury or degeneration can cause functional abnormalities in specific brain regions, and eventually lead to the occurrence and progression of several neurological and psychiatric disorders, such as Parkinson’s disease (PD) (Tanudjojo et al., 2021), schizophrenia (Howes and Shatalina, 2022), and autism spectrum disorders (Bariselli et al., 2018). Stem cell-derived dopaminergic neurons are promising sources for cell transplantation therapies for neurodegenerative diseases (Zayed et al., 2022). A ground-breaking finding in reprogramming somatic cells into induced pluripotent stem cells (iPSC) under fully orchestrated patterning has made it possible to produce dopaminergic neurons from a reliable source in vitro (Takahashi et al., 2007). Transplantation of mDA neural precursor cells (NPC) derived from human iPSC (hiPSC) in a monkey model of PD not only replenishes mDA loss and functioning but improves symptoms such as dyskinesia (Kikuchi et al., 2017). However, the lack of potency and purity of in vitro-generated mDA neurons still limits their clinical application.
The development of mDA neurons has unique spatiotemporal characteristics. During embryonic development, the neural tube develops along the anterior-posterior (A-P) axis and gives rise to four major structures: the forebrain, midbrain, rhombencephalon, and neural tube terminations. The floor plate (FP), which is located in the ventral midline of the neural tube, is a source of mDA neurons. Cells located in the FP highly express the morphogen and dorsal-ventral (D-V) axis organizer molecule, sonic hedgehog (SHH) (Fuccillo et al., 2006; Ono et al., 2007). The morphogenetic gradient of SHH and bone morphogenetic protein (BMP) forms the D-V axis and specifies different types of neuronal populations along this axis (Rekler and Kalcheim, 2021). In addition, the isthmus organizer expresses Wnt family member 1 (WNT1) on the midbrain side and fibroblast growth factor 8 (FGF8) on the hindbrain side, which also contributes to mDA neuron development (Tian et al., 2022). NPC in the FP differentiate into mDA progenitors that express Forkhead box protein A2 (FOXA2) and LIM homeobox transcription factor 1, alpha (LMX1A) (Yeap et al., 2023). Nuclear receptor related 1 (Nurr1) expression in the midbrain enables mDA progenitors to differentiate into mature neurons (Jankovic et al., 2005). Previous studies have elucidated that the SHH, FGF, WNT, and BMP/suppressor of mother against decapentaplegic (SMAD) signaling pathways are critical in the proliferation and regulation of mDA progenitor cells (Wang et al., 1995; Ye et al., 1998; Castelo-Branco et al., 2003; Jovanovic et al., 2018). These pathways synergise with numerous transcription factors (TFs) to ensure that target genes are expressed at specific time windows and places, thereby controlling mDA development.
In recent years, tremendous progress has been made in generating mDA neurons by mimicking embryonic developmental signaling in stem cells, with some researchers having successfully conducted preclinical studies (Kikuchi et al., 2017; Doi et al., 2020; Xiong et al., 2021). In a landmark study, a dual SMAD pathway inhibition protocol was used to efficiently differentiate hiPSC into neurons, successfully yielding cells that express dopaminergic neural markers in vitro (Chambers et al., 2009). The effective formation of FP tissue is critical for the production of mDA neurons in the early neurodevelopmental stage. Accordingly, one study found that early exposure to high concentrations of SHH was effective in driving functional FP tissue formation in human embryonic stem cells (hESC)-derived neurodevelopment (Fasano et al., 2010). Another study induced dose-dependent activation of WNT signaling and controlled cell positional specification by adding a chemical inhibitor of glycogen synthase kinase 3 (GSK3), CHIR99021 (CHIR). They found that CHIR can effectively direct hESC towards a ventral mesencephalic fate and produce large numbers of FP progenitor cells (Kirkeby et al., 2012). In our study, we used SB431542 and dorsomorphin dual inhibitor combinations, SHH and FGF8 combinations, and CHIR, SHH, and FGF8 combinations to construct three different mDA differentiation schemes in vitro. Our results suggest that CHIR, SHH, and FGF8 combination can cause neural differentiation with a high proportion of mDA neurons.
Methods
hiPSC culture
The hiPSC resources in this study were obtained from the Guangzhou Institute of Biomedicine and Health. Cells were cultured in mTeSRTM medium (Stemcell Technologies, Vancouver, BC, Canada, Cat# 85850). mTeSR medium was supplemented with recombinant human basic FGF and recombinant human transforming growth factor β (TGF-β). For cell growth, 6-well plates were pre-coated with Matrigel (BD MatrigelTM, Waltham, MA, USA, hESC-qualified Matrix, Cat# 354277) for 30 minutes. Upon reaching 80% cell confluency, the attached cells were digested with ethylenediaminetetraacetic acid (5 × 10–4 M, Thermo Fisher Scientific, Grand Island, NY, USA, Cat# 25200056) and passaged into 12-well plates.
hiPSC differentiation in vitro
In the dual SMAD pathway inhibition protocol, NPC were obtained from hiPSC after 16 days of neural induction. NPC were grown in suspension culture using two SMAD signaling inhibitors, SB431542 and dorsomorphin. Upon reaching 95% confluency, cells were cultured in N2B27 medium (Thermo Fisher Scientific, Cat# 17502-048 and Cat# 17504044) with the addition of 5 µM SB431542 (Selleck, Houston, TX, USA, Cat# S1067) and 5 µM dorsomorphin (Selleck, Cat# S7840) for eight days. Neural rosettes were then scraped onto 6-well plates and fed with N2B27 medium without SB431542 and dorsomorphin for an additional 8 days. On day 8 of culture, rosettes were mechanically scraped into a T25 flask for suspension culture in N2B27 medium supplemented with 20 ng/mL FGF (Thermo Fisher Scientific, Cat# PHG0266) and 20 ng/mL epidermal growth factor (EGF) (Thermo Fisher Scientific, Cat# PHG0315) to eventually obtain NPC. NPC were then digested with Accutase and passaged three times (Thermo Fisher Scientific, Cat# A1110501). The cells were then dissociated into single cells and cultured at a density of 5 × 104 cells per well in 24-well plates pre-coated with mouse glia, forming a glial cell feeder layer. This was followed by culture in N2B27 medium with 1 µM cyclic adenosine monophosphate (cAMP) (Sigma-Aldrich, St. Louis, MO, USA, Cat# S7858) and 20 ng/μL brain-derived neurotrophic factor (BDNF) (PeproTech, Rocky Hill, NJ, USA, Cat# 450-02-10). The medium was changed every other day. Cells were collected and grown in coverslips, which were fixed and used for further immunofluorescence staining.
In the SHH + FGF8 protocol, hiPSC were cultured according to the optimized dual SMAD pathway inhibition protocol to induce neural rosette formation during the first eight days of differentiation. The cells were then mechanically dissociated and cultured in N2B27 medium with SHH (100 ng/mL, R&D System, Minneapolis, MN, USA, Cat# 464-SH-025) and FGF8 (100 ng/mL, R&D System, Cat# 423-F8-025). Next, NPC were cultured according to the optimized dual SMAD pathway inhibition protocol with the addition of SHH and FGF8, digested with Accutase, and passaged three times. The cells were then dissociated into single cells by Accutase and grown in culture medium containing N2B27, 1 μM cAMP, 20 ng BDNF, 20 ng/mL glial cell line-derived neurotrophic factor (GDNF) (PeproTech, Cat# 16187S), and 0.2 mM ascorbic acid (AA) (Solarbio, Beijing, China, Cat# 50-81-7), on a glial cell feeder layer.
In the CHIR + SHH + FGF8 induction protocol. Induction relied on timely exposure to the dual signaling inhibitors, SB431542 and dorsomorphin, SHH, FGF8, 2 μM Purmorphamine (Selleck, Cat# S3042), and 0.7 μM CHIR99021 (Selleck, Cat# S1263). Upon reaching 80% confluency, hiPSC were cultured for 5 days in neural proliferation system I containing 10 µM SB431542 and 5 µM dorsomorphin. SHH, FGF8, and Purmorphamine were added on day 1 and removed on day 8. CHIR99021 was added on day 3 and removed on day 13. Neural Proliferation System I medium was gradually changed to Neural Proliferation System II containing N2B27 and 5 µM dorsomorphin from day 6 of differentiation. Cells were then digested with Accutase and seeded at a density of 1.5 × 105 cells per well in Neural Proliferation System III containing N2B27 supplemented with 1 μM cAMP, 20 ng BDNF, 20 ng/mL GDNF, and 0.2 mM AA from day 11. After 9 days, cells were digested again with Accutase and seeded at a density of 3–5 × 104 cells per well on Matrigel pre-coated glass coverslips in Neural Proliferation System III until the desired time point for neuronal maturation.
Virus transduction
NPC were digested into single cells and infected with DAT-Cherry (DAT, LV-SLC6A3(43030-1), Genechem, Shanghai, China, GOSL0165114, 5 × 108 TU/mL), in which Cherry fluorescence is expressed under the dopamine active transporter (DAT) promoter to label dopaminergic neurons. After 6 hours of infection, the cells were centrifuged and resuspended in fresh culture medium. The next day, the cells were cultured at a density of 6 × 104 cells per well on coverslips in 24-well plate with a glial cell feeder layer for neuronal development.
Immunofluorescence staining
Cells collected at different time points in three different induction protocols were fixed with 4% paraformaldehyde (PFA) for 8 minutes and rinsed with phosphate-buffered saline (PBS) for 5 minutes. After blocking in 3% bovine serum albumin (BSA) (Vetec™) in 0.3% PBST (0.3% Tween-20 [Solarbio, Cat# T8220] in PBS) at room temperature for 2 hours, the cells were incubated with primary antibodies diluted in 1% BSA at 4°C for 24 hours. Primary antibodies included: octamer-binding transcription factor 4 (OCT4), a marker used for the identification of iPSC (rabbit, 1:100, BioVision, Milpitas, CA, USA, Cat# 6765-100, RRID: AB_2936816), stage-specific embryonic antigen-4 (SSEA4), a marker used for identification of iPSC (mouse, 1:100, Invitrogen, Rockford, AL, USA, Cat# 41-4000, RRID: AB_2533506), SRY-box transcription factor 2 (SOX2), a marker used for identification of NPC (mouse, 1:500, R&D Systems, Minneapolis, MN, USA, Cat# MAB2018, RRID: AB_358009), Nestin, a marker used for identification of NPC (rabbit, 1:1000, Millipore, Darmstadt, Germany, Cat# ABD69, RRID: AB_2744681), microtubule-associated protein 2 (MAP2), a marker of dendrites (rabbit, 1:1000, Millipore, Burlington, MA, USA, Cat# AB5622, RRID: AB_91939), MAP2 (mouse, 1:1000, Millipore, Cat# AMAb91375, RRID: AB_2716657), beta III tubulin (TUJ1), a neuronal marker (mouse, 1:1000, Sigma-Aldrich, Cat# T8660, RRID: AB_477590), anti-human nuclei (HuNu), a marker of nuclei in human cells (mouse, 1:500, Millipore, Cat# MAB1281, RRID: AB_94090), anti-vesicular glutamate transporter 1 (VGLUT1), a glutamatergic neuronal marker (mouse, 1:250, Millipore, Cat# MAB5502, RRID: AB_262185), gamma-aminobutyric acid (GABA), a GABAergic neuronal marker (rabbit, 1:1000, Sigma-Aldrich, Cat# A2052, RRID: AB_477652), tyrosine hydroxylase (TH), a dopaminergic neuronal marker (rabbit, 1:1000, Millipore, Cat# AB152, RRID: AB_390204), TH (chicken, 1:500, Millipore, Cat# AB9702, RRID: AB_570923), FOXA2, a midbrain neuronal marker (rabbit, 1:500, Cell Signaling Technology, Danvers, MA, USA, Cat# 8186, RRID: AB_10891055), and paired like homeodomain 3 (PITX3), another midbrain neuronal marker (rabbit, 1:500, Millipore, Cat# AB5722, RRID: AB_91997). After primary antibody incubation, cells were washed three times with PBS. Next, they were incubated in 4’,6-diamidino-2-phenylindole (DAPI) (1:2000, Invitrogen, Cat# D1306, RRID: AB_2629482) and secondary antibodies diluted in PBS at room temperature for 2 hours, including Alexa-488 (anti-chicken, 1:500, Jackson Immuno Research, West Grove, PA, USA, Cat# 703-545-155, RRID: AB_2340375; anti-mouse, 1:1000, Invitrogen, Cat# A21202, RRID: AB_141607; anti-rabbit, 1:1000, Invitrogen, Cat# A21206, RRID: AB_2535792), Alexa-546 (anti-rabbit, 1:1000, Invitrogen, Cat# A10040, RRID: AB_2534016; anti-mouse, 1:1000, Invitrogen, Cat# A10036, RRID: AB_2534012), and Alexa-647 (anti-mouse, 1:1000, Invitrogen, Cat# A31571, RRID: AB_162542).
Brightfield images were obtained using an inverted fluorescence microscope (AxioobserverH1, Zeiss, Oberkochen, Germany). Immunofluorescence images of neurons were acquired using a Zeiss microscope (Imager Z2, Zeiss) and a laser scanning confocal microscope (Axiocam 506 mono, Zeiss). More than ten visual fields were randomly acquired from each coverslip. The number of MAP2-positive cells was counted in each visual field. Various neuronal differentiation ratios were calculated using number of MAP2-positive cells as the denominator and number of TH-, GABA-, and VGLUT1-positive cells as the numerator. In particular, the differentiation ratio of mDA neurons was calculated using number of TH-positive cells as the denominator and number of FOXA2- and PITX3-positive cells as the numerator.
Electrophysiological recordings
Artificial cerebrospinal fluid, patch clamp amplifier (MultiClamp 700B, Molecular Devices, San Jose, CA, USA), digital-to-mode converter (Digidata 1440A, Molecular Devices), and clamps were used for electrophysiological recordings. Cells with clear and optimized cytological morphology were selected as target cells. A borosilicate glass microelectrode was pulled with an electrode tractor; the internal fluid resistance of the electrode was 3–6 Ω. The microelectrode was filled with filling electrode fluid and then mounted onto a microelectrode clamp, ensuring a tight closure. A positive voltage was applied to the microelectrode. When the microelectrode contacted the cell surface, fluid outflow from the electrode caused a vacuole to form on the cell surface, which resulted in a slight increase in electrode resistance. Positive pressure was then removed and negative pressure gently applied to establish a high impedance seal. By maintaining a seal resistance of –70 mV, a stable high impedance seal to 1 GΩ could be achieved more quickly and effectively, especially when the seal resistance exceeded 400 MΩ. The vacuum within the microelectrode was increased to absorb the cell membrane, allowing for recording of cell parameters. The voltage was held at –70 mV and the sodium current was triggered by increasing the voltage by 5 mV every 300 ms from –20 mV to 50 mV. The peak and threshold values of the sodium current were recorded. In the current clamp mode, the current was then increased by 10 pA every 1000 ms from –30 pA to 80 pA to record the action potential frequency.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad Software, San Diego, CA, USA, www.graphpad.com), and data are presented as mean ± SEM. Data differences between groups were analyzed by two-tailed unpaired Student’s t-test, P < 0.05 was considered statistically significant.
Results
The dual SMAD pathway inhibition protocol produces multiple subtypes of neurons including dopaminergic neurons
Based on a previous study (Chambers et al., 2009), we used 5 µM dorsomorphin and 5 µM SB431542 in an early induction culture system from day 0 to day 8 to promote neural differentiation in hiPSC. This showed that hiPSC morphology started to become organized columnar epithelial cells after the addition of both inhibitors. On day 9, we found that the cell clusters gradually showed NPC characteristics. On day 16, densely adherent cell tissue was mechanically separated into homogeneous cell clusters by a scraper approach. These cell clusters were then cultured in a suspension system that included EGF and FGF. NPC were passaged for three generations and then cultured with an astrocyte feeder for further differentiation into neurons, which were cultured with BDNF and c-AMP. The results showed that this modified protocol could successfully induce hiPSC into neurons after 24 days (Figure 1). This protocol was named the dual SMAD pathway inhibition protocol.
Figure 1.
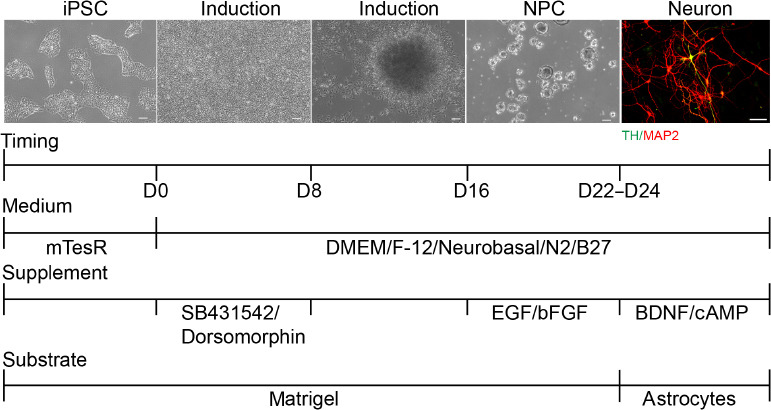
The modified dual SMAD pathway inhibition protocol.
A modified monolayer differentiation protocol was used, in which the rosettes were mechanically scraped from the plate bottom for suspension culture. Timepoint, medium, and supplemented growth factors for human induced pluripotent stem cell (hiPSC) to neuron are indicated. Representative microscope images of the stages of the neural differentiation protocol are also shown. Scale bars: 50 μm. At least five independent replicates were performed for each experiment. BDNF: Brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; cAMP: cyclic adenosine monophosphate; DMEM/F-12: Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12; EGF: epidermal growth factor; iPSC: induced pluripotent stem cell; MAP2: microtubule-associated protein 2; NPC: neural precursor cell; TH: tyrosine hydroxylase.
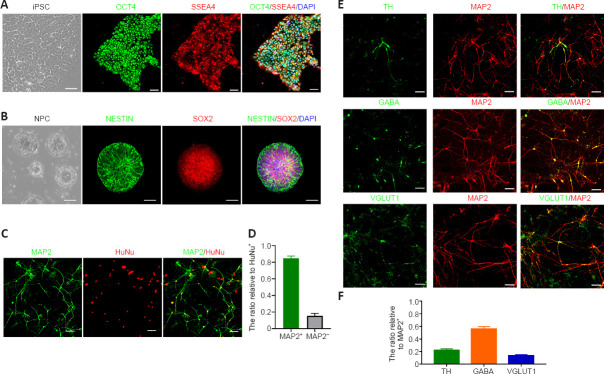
As shown, hiPSC expressed OCT4 and SSEA4 before differentiation, reflecting the stem cell characteristics and multi-differentiation potential of the hiPSC in our study (Figure 2A). The morphology of hiPSC changed during culture from day 0 to day 8 with dual inhibitor. On day 20, both SOX2 and Nestin were highly expressed, indicating that cells at this stage showed NPC characteristics (Figure 2B). Overall, 84% of cells successfully differentiated into neurons, based on MAP2 immunofluorescence staining (Figure 2C and D). On day 33, we observed the expression of multiple subtypes of neuronal markers such as dopaminergic neurons (Figure 2D). The results showed that different subtypes of neurons including 13% glutaminergic neurons, 21% dopaminergic neurons, and 56% GABAergic neurons (Figure 2E and F). However, few cells expressed mDA biomarkers.
Figure 2.
The modified dual SMAD pathway inhibition protocol yielded a low proportion of dopaminergic neurons.
(A) Representative images of human induced pluripotent stem cells (hiPSC) with immunostaining of pluripotency markers, octamer-binding transcription factor 4 (OCT4) and stage-specific embryonic antigen-4 (SSEA4). Scale bars: 50 μm. (B) Representative images of neural precursor cells (NPC) induced from hiPSC with immunostaining of SRY-box transcription factor 2 (SOX2) and Nestin. Scale bars: 50 μm. (C) Representative images of hiPSC-derived neurons with immunostaining of microtubule-associated protein 2 (MAP2) (green) and human nuclei (HuNu) antibody (red). Scale bars: 50 μm. (D) Analysis of neuronal differentiation efficiency (number of cells expressing HuNu: n = 443 cells in dual SMAD pathway inhibition protocol). (E) Representative images of hiPSC-derived neurons with immunostaining of markers for different neuronal subtypes: tyrosine hydroxylase (TH), gamma-aminobutyric acid (GABA), vesicular glutamate transporter 1 (VGLUT1) (all in green), and MAP2 (red) grown on mouse astrocytes. Scale bars: 50 μm. (F) Differentiation ratio of dopaminergic neurons, GABAergic neurons, and glutamatergic neurons were assessed based on the proportion of TH, GABA, and VGLUT1 positive cells among MAP2 positive cells, respectively. At least three independent replicates were performed of each experiment. SMAD: Suppressor of mother against decapentaplegic.
SHH and FGF8 contribute to a higher induction efficiency of dopaminergic neurons derived from hiPSC
An increasing number of studies have shown that the application of growth factors or certain small molecules (such as smoothened agonists, SHH, TGF-β3, FGF8, retinoic acid, LIF, stromal cell-derived factor 1a) are beneficial to the induction of dopaminergic neurons (Storch et al., 2001; Guyon et al., 2006; Katsuki et al., 2009; Schwartz et al., 2012; Blaess and Ang, 2015). Considering that SHH and FGF8 can control the fate of dopaminergic neurons along the D-V and A-P axes, cooperating to specify dopaminergic neurons, we added SHH and FGF8 into the culture medium from day 8 to day 22. For better survival of dopaminergic neurons, supplemental addition of 20 ng/mL GDNF and 0.2 mM AA was essential for neuronal differentiation. After the addition of SHH and FGF8, hiPSC were successfully differentiated into neurons with a higher ratio of dopaminergic neurons after 24 days (Figure 3). This optimized protocol was named the SHH + FGF8 differentiation protocol.
Figure 3.
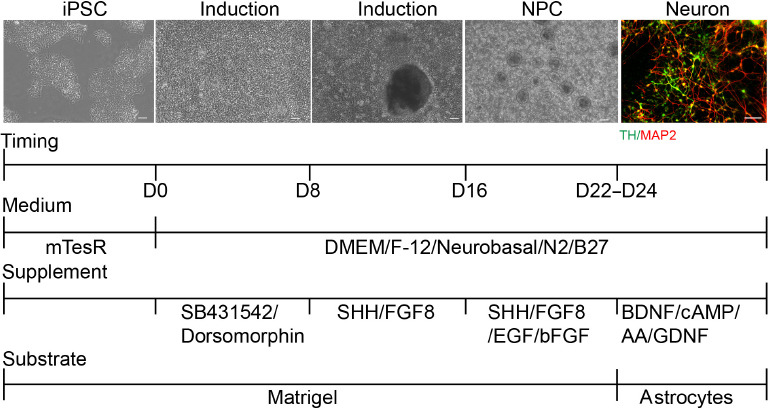
The SHH + FGF8 differentiation protocol.
The addition of sonic hedgehog (SHH) and fibroblast growth factor 8 (FGF8) in the early stage of a modified dual SMAD pathway inhibition protocol, along with small molecules during induction of the neural differentiation period. This protocol yielded a high proportion of dopaminergic neurons. Scale bars: 50 μm. At least three independent replicates were performed of each experiment. BDNF: Brain-derived neurotrophic factor; bFGF: basic fibroblast growth factor; cAMP: cyclic adenosine monophosphate; DMEM/F-12: Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12; EGF: epidermal growth factor; FGF8: fibroblast growth factor 8; iPSC: induced pluripotent stem cell; MAP2: microtubule-associated protein 2; NPC: neural precursor cell; SHH: Sonic Hedgehog; TH: tyrosine hydroxylase.
On day 20 of the SHH + FGF8 differentiation protocol, induced cell clusters in suspension culture showed neurosphere-like characteristics, and the neurospheres were Nestin- and SOX2-positive (data not shown), indicating that these neurosphere cells had NPC characteristics. Further, NPC at this stage expressed FOXA2 and SOX2, indicating that these cells also have FP progenitor properties (Figure 4A). Immunofluorescence staining of cells on day 33, showed that MAP2-positive neurons accounted for 83% of the total induced cells and TH-positive cells for 83%, which is similar to the SMAD protocol (Figure 4B and C). Moreover, application of SHH and FGF8, significantly increased the proportion of dopaminergic neurons to approximately 75% of the total differentiated neurons, compared with only 21% in the previous protocol (Figure 4D and E). To verify whether differentiated TH-positive neurons have the potential to develop to the maturation stage, we transfected single cells after NPC digestion with a DAT-Cherry lentivirus on day 22, and then examined expression of TH by immunofluorescence staining. Co-expressed DAT-Cherry and TH neurons were observed on day 33 in neural induction cultures (Figure 4F), suggesting that induced dopaminergic neurons partially show early maturation properties. In addition, we also confirmed that a small number of these dopaminergic neurons were positive for the midbrain marker, FOXA2 (Figure 4G). This indicates that the SHH + FGF8 protocol not only greatly improved the differentiation efficiency of dopaminergic neurons, but also benefited the induction of mDA neurons. It is known that mDA neurons play an important role in cognitive, emotional, and neurodegenerative disorders. Thus, there is a need to further optimize the induction protocol to increase the differentiation efficiency of mDA neurons.
Figure 4.
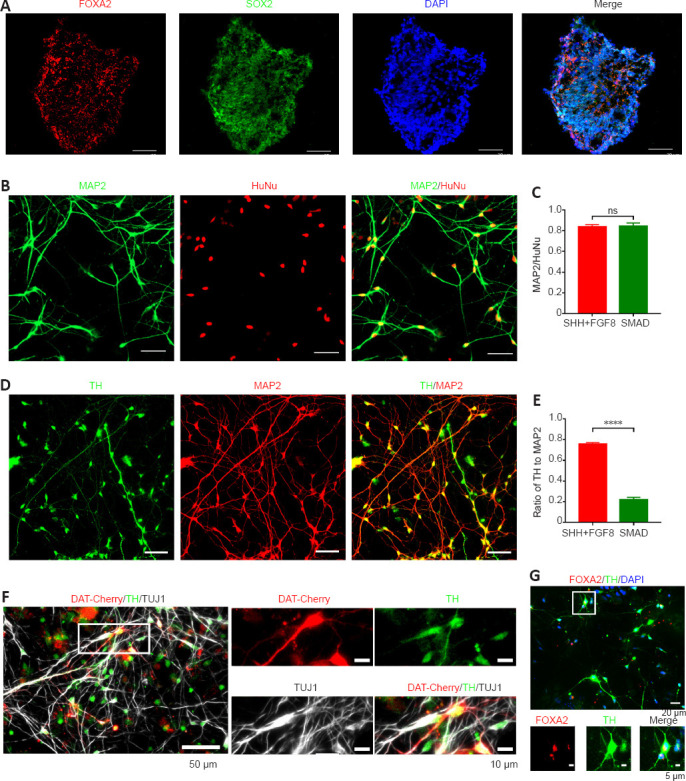
The SHH + FGF8 differentiation protocol yielded a high proportion of dopaminergic neurons, with a small fraction of neurons that expressed FOXA2, a marker of midbrain dopaminergic neurons.
(A) Representative images of neural precursor cells (NPC) induced from human induced pluripotent stem cells (hiPSC) with immunostaining of Forkhead box protein A2 (FOXA2) and SRY-box transcription factor 2 (SOX2). Scale bars: 50 μm. (B) Representative images of hiPSC-derived neurons in the SHH + FGF8 protocol with immunostaining of microtubule-associated protein 2 (MAP2) (green) and human nuclei (HuNu) antibody (red). Scale bars: 50 μm. (C) Comparison of the differentiation efficiency of neurons in the SHH + FGF8 protocol and modified dual SMAD pathway inhibition protocol (number of cells expressing HuNu: n = 607 cells in SHH+FGF8 protocol, n = 443 cells in dual SMAD pathway inhibition protocol). (D) Representative images of hiPSC-derived neurons in the SHH + FGF8 protocol with immunostaining of tyrosine hydroxylase (TH) (green) and MAP2 (red). Scale bars: 50 μm. (E) The proportion of dopaminergic neurons in the SHH + FGF8 protocol and modified dual SMAD pathway inhibition protocol was determined by calculating the percentage of TH-positive cells in MAP2-positive cells. (number of cells expressing MAP2: n = 581 cells in SHH + FGF8 protocol, n = 339 cells in dual SMAD pathway inhibition protocol). (F) The white box area in the image is enlarged and displayed on the right. NPC were infected with a DAT-Cherry lentivirus and then cultured on a glial cell feeder layer to label dopaminergic neurons in the later differentiation stage. Representative images of hiPSC-derived neurons in the SHH + FGF8 protocol with immunostaining of TH (green) and beta III tubulin (TUJ1) (white) on day 33. Scale bars: 50 μm (left), 10 μm (right). (G) The white box area in the image is enlarged and displayed at the bottom. Immunofluorescence staining of FOXA2 (red) and TH (green). Scale bars: 20 μm (upper panels), 5 μm (lower panels). At least three independent replicates were performed for each experiment. Data are presented as mean ± SEM, ****P < 0.0001. Statistical significance was evaluated by two-tailed unpaired Student’s t-test (C, E). FGF8: Fibroblast growth factor 8; ns: not significant; SHH: Sonic Hedgehog; TH: tyrosine hydroxylase.
Application of CHIR promotes the differentiation of mDA neurons
Previous studies have shown that CHIR99021 (Kriks et al., 2011), a potent GSK3β inhibitor, can strongly activate WNT signaling and promote the differentiation of floor plate progenitors, which is particularly important for the development of mDA neurons. The addition of Purmorphamine in combination with SHH (a small molecule agonist) activates SHH signaling (Fasano et al., 2010). Thus, we further improved the induction protocol by directly adding 0.7 μM CHIR99021 and 2 μM Purmorphamine at the early stage of the SHH + FGF8 differentiation protocol. However, no NPC-like or neuron-like cells were observed throughout the entire induction course. We next modified the protocol by adding SHH, FGF8, and Purmorphamine into the neuronal induction system from day 1 to day 7 of hiPSC induction, with 0.7 μM CHIR99021 added from day 3 to day 13. This allowed us obtain a high proportion of mDA neurons (Figure 5). This protocol was named the CHIR + SHH + FGF8 induction protocol.
Figure 5.
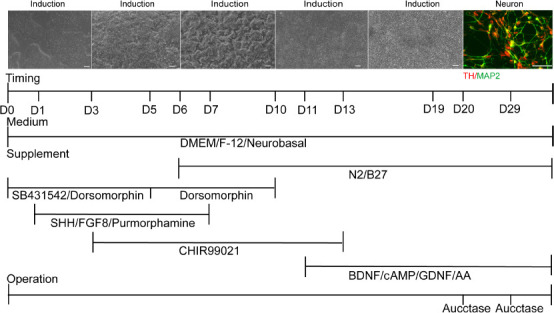
The CHIR + SHH + FGF8 induction protocol.
The addition of CHIR99021 (a small molecule inhibitor), sonic hedgehog (SHH), fibroblast growth factor 8 (FGF8), synergistic SB431542 (a SMAD signaling pathway inhibitor), and a dorsomorphin dual inhibitor at different time points during induction and differentiation. Scale bars: 50 μm. At least three independent replicates were performed. AA: Ascorbic acid; BDNF: brain-derived neurotrophic factor; cAMP: cyclic adenosine monophosphate; D: day; DMEM/F-12: Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12; FGF8: fibroblast growth factor 8; GDNF: glial cell line-derived neurotrophic factor; SHH: Sonic Hedgehog.
The CHIR + SHH + FGF8 induction protocol showed that approximately 80% of the induced cells expressed FOXA2, a key marker of mDA neuronal development, in the early stage of differentiation on day 6 (Figure 6A). We found no statistical difference in neural differentiation efficiency between the CHIR + SHH + FGF8 induction protocol and SHH + FGF8 differentiation protocol (Figure 6B and C). However, the percentage of differentiated dopaminergic neurons was significantly higher (74%) with the addition of CHIR99021, compared with the dual SMAD pathway inhibition protocol (Figure 6D and E). We also found that more dopaminergic neurons were co-labeled with midbrain markers such as FOXA2 and PITX3 on day 41 under the CHIR + SHH + FGF8 induction condition, compared with the SHH+FGF8 differentiation protocol (Figure 6F–I). We found that about 31% of TH-positive neurons induced in this protocol were co-labeled with FOXA2, whereas only 15% were co-labeled in the SHH + FGF8 differentiation protocol (Figure 6F and G). About 37% of TH-positive neurons in this protocol were co-labeled with PITX3, whereas only 7% were co-labeled in the SHH + FGF8 differentiation protocol (Figure 6H and I). Of particular note, 80% of the cells expressed FOXA2-formed FP progenitors by day 6 of cell culture in the CHIR + SHH + FGF8 induction protocol. On day 41, approximately 31% of TH-positive neurons were co-labeled with FOXA2 and approximately 37% of TH-positive neurons were co-labeled with PITX3 (Figure 6G and I). FOXA2 is critical for determining the ventral midbrain identity of neurons, but not all mDA neurons express FOXA2 after maturation of mDA neurons (Kirkeby et al., 2017; Yeap et al., 2023). In addition, PITX3 is critical for the precise induction of mDA neuronal maturation and is more highly expressed in mature mDA neurons (Kouwenhoven et al., 2017). Therefore, neither FOXA2 nor PITX3 alone is sufficient to define all mDA neurons. Experimental data in this study showed that the percentage of TH-positive neurons was approximately 74%, therefore we speculated that the percentage of mDA neurons induced by the CHIR + SHH + FGF8 induction protocol varies between 31–74%.
Figure 6.
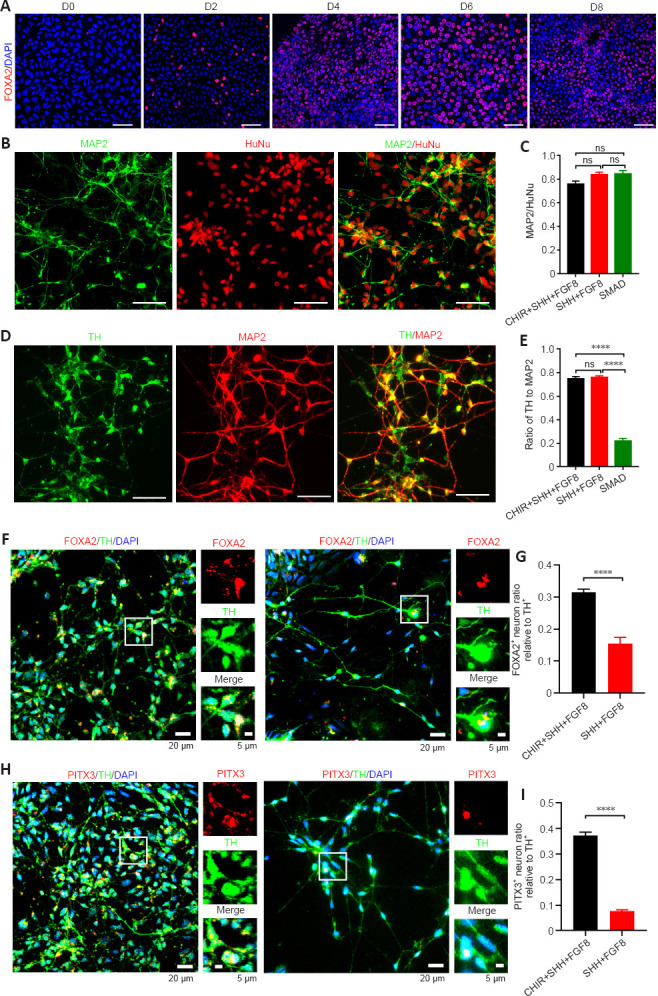
The CHIR + SHH + FGF8 induction protocol more effectively induced hiPSC differentiation into mDA neurons.
(A) Representative images of temporal expression (from day 0 to day 8) of Forkhead box protein A2 (FOXA2) (red) in the CHIR + SHH + FGF8 induction protocol. Scale bars: 50 μm. (B, C) Differentiation efficiency of neurons in the three neural induction protocols (number of cells expressing human nuclei [HuNu]: n = 1599 cells in CHIR + SHH + FGF8 protocol, 607 cells in SHH + FGF8 protocol, and 443 cells in dual SMAD pathway inhibition protocol). Scale bars: 50 μm. (D, E) The proportion of dopaminergic neurons in three neural induction protocols (number of cells expressing microtubule-associated protein 2 [MAP2]: n = 497 cells in CHIR + SHH + FGF8 protocol, 581 cells in SHH + FGF8 protocol, and 339 cells in dual SMAD pathway inhibition protocol). Scale bars: 50 μm. (F) Representative images of human induced pluripotent stem cells (hiPSC)-derived neurons in the CHIR + SHH + FGF8 induction protocol (left) and SHH + FGF8 protocol (right) with immunostaining of FOXA2 (red), tyrosine hydroxylase (TH) (green), and 4’,6-diamidino-2-phenylindole (DAPI) (blue). Scale bars: 20 μm (left), 5 μm (right). (G) Co-expression analysis of FOXA2 and TH in the SHH + FGF8 and CHIR + SHH + FGF8 induction protocols (number of cells expressing TH: n = 414 cells in CHIR + SHH + FGF8 protocol, n = 296 cells in SHH + FGF8 protocol). (H) Representative images of hiPSC-derived neurons in the CHIR + SHH + FGF8 induction protocol (left) and SHH + FGF8 protocol (right) with immunostaining of paired like homeodomain 3 (PITX3) (red), TH (green), and DAPI (blue). Scale bars: 20 μm (left), 5 μm (right). (I) Co-expression analysis of PITX3 and TH in the SHH+FGF8 and CHIR + SHH + FGF8 induction protocols (number of cells expressing TH: n = 612 cells in CHIR + SHH + FGF8 protocol, n = 564 cells in CHIR + SHH + FGF8 protocol). Data are presented as mean ± SEM, ****P < 0.0001. Statistical significance was evaluated by two-tailed unpaired Student’s t-test (C, E, G, I). CHIR: CHIR99021 (small molecule inhibitor); FGF8: fibroblast growth factor 8; iPSC: induced pluripotent stem cells; ns: not significant; SHH: Sonic Hedgehog.
We then performed electrophysiological tests (Figure 7A) on the neurons induced by our neural induction protocol. The results showed that the neurons exhibited sodium currents when the voltage was held at –70 mV (Figure 7B and C), and a hyperpolarization-induced “sag” potential characteristic when action potentials were evoked (Figure 7D); this is consistent with characteristics of dopaminergic neuronal subtypes (Yang et al., 2001; Niclis et al., 2017; Zou et al., 2018). Therefore, our evidence suggests that the application of SHH, FGF8, and Purmorphamine combined with CHIR99021 during neural induction improved the efficiency of mDA neuronal differentiation. This offers the possibility of generating a large number of mDA neurons for translational medicine research.
Figure 7.
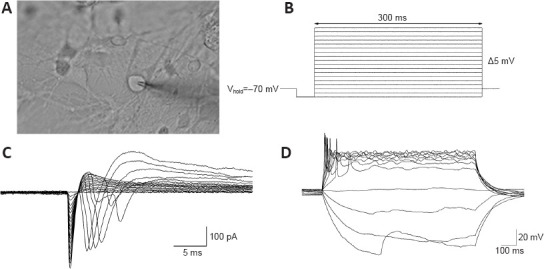
Electrophysiological properties of dopaminergic neurons derived from the CHIR + SHH + FGF8 induction protocol.
(A) Schematic representation of neurons during whole cell recordings. (B) Parameters of sodium currents recorded from dopaminergic neurons. (C) Schematic diagram of sodium current distribution in dopaminergic neurons. (D) Representative images of human induced pluripotent stem cell-derived neurons show a hyperpolarization-induced sag potential, which is characteristic of dopaminergic neurons. At least three independent replicates were performed of each experiment. CHIR: CHIR99021 (small molecule inhibitor); FGF8: fibroblast growth factor 8; SHH: Sonic Hedgehog.
Discussion
Our study sought to develop a stable and efficient protocol to differentiate hiPSC into mDA neurons. This method can be used to replenish aging or damaged brain tissues, and represents a cellular platform to study neuronal developmental processes and mechanisms of neurological diseases. Our protocol was optimized based on previous studies (Chambers et al., 2009; Fasano et al., 2010; Kim et al., 2010; Kirkeby et al., 2012). Initially, we optimized a protocol for inducing neural differentiation by dual inhibition of the SMAD pathway. While this protocol converted most hiPSC into neuronal cells, it did not specifically increase the proportion of differentiated dopaminergic neurons. To address this, we modified the protocol by adding SHH and FGF8 on day 8 of cell culture and continuing the suspension culture of NPC. This significantly improved the differentiation ratio of dopaminergic neurons without decreasing the efficiency of hiPSC differentiation into neurons. To generate more dopaminergic neurons that harbored midbrain properties, it was necessary to produce enough FOXA2-expressing FP precursors during the early stage of hiPSC differentiation. Therefore, we further optimized the protocol by including SHH and FGF8 on day 1 of cell culture and adding Purmorphamine to enhance activation of the SHH pathway. CHIR99021 was also used to provide strong activation of the WNT pathway. Although the adherent culture lost some of its neural differentiation efficiency, this protocol caused the induction of a relatively high proportion of hiPSC-derived mDA neurons.
Recently reported protocols for inducing stem cells to dopaminergic neurons have yielded variable proportions of dopaminergic neurons. In a similar study, three key factors (SHH, FGF8, and CHIR99021) were added at the early stage of hiPSC induction, but only SB431542 was used to inhibit the SMAD pathway (Song et al., 2020). Although this produced more than 90% TH-positive cells on day 28, only 20% FOXA2-positive cells among the total cell population were induced. This supports the use of dual SMAD pathway inhibitors in our protocol for improving the overall differentiation rate of dopaminergic neurons. Notably, instead of using FGF8 to interfere with the diencephalic fate of the cells, one study enhanced WNT signaling by increasing the concentration of CHIR99021 at an early stage to bolster the mesencephalic fate of the cells (Kim et al., 2021). This modified protocol facilitated robust induction of mDA neurons expressing engrailed-1 (EN1) on day 11 of differentiation. Moreover, early exposure to retinoic acid and activation of the SHH pathway (rather than activation of the FGF8 and WNT pathways) promoted rapid specification of LMX1A/FOXA2/OTX2 ventral midbrain progenitors, which are potent to differentiate into mDA neurons (Alekseenko et al., 2022). One study overexpressed two TFs, achaete-scute homolog 1 (ASCL1) and LMX1A, for hiPSC reprogramming. While 59.1% of dopaminergic neurons were obtained on day 28 of cell culture, the number of neurons with a midbrain identity was low (Nishimura et al., 2022). In parallel, another study found that expression of ASCL1 was sufficient to induce dopaminergic neurons from hESC, which surged with the activation of WNT1 signaling, but these TH-positive cells lacked midbrain markers (Ng et al., 2021). Although dopaminergic neurons can be directly and rapidly generated by inducing stem cells to express relevant TFs without going through an intermediate NPC stage, the current combinations of TFs are not potent in achieving highly pure mDA neurons.
Differentiation of embryonic stem cells (ESC) into trophoblast or ectoderm requires regulation of the BMP/SMAD signaling pathway. Blocking the BMP pathway with Noggin has been shown in several studies to promote ESC differentiation into nervous system cells (Smith and Harland, 1992; Karvas et al., 2022). The TGF-β/Activin/Nodal signaling pathway is associated with mesoderm/endoderm formation and specification in early development (Galiakberova and Dashinimaev, 2020; Volpicelli et al., 2020). When this pathway is inhibited, it induces differentiation of ESC along the standard ectoderm lineage (Blaess and Ang, 2015). Seemingly paradoxically, another study claimed that this pathway was needed to work with the FGF signaling pathway to maintain ESC totipotency (Vallier et al., 2005). Under fine-tuned culture conditions, simultaneous blockade of these two pathways with Noggin and SB431542 (to induce ectodermal transformation of hESC) led to rapid and uniform neural transformation of iPSC without germline formation. The use of dual SMAD signaling inhibition greatly improved the efficiency of neural induction, resulting in over 80% of hESC being induced to differentiate into neural progenitors, which is undoubtedly a breakthrough in comparison to the single inhibition protocol (Chambers et al., 2009). Moreover, this dual SMAD signaling inhibition protocol could partially overcome the innate propensity to differentiate between diverse strains of ESC or iPSC, which can strongly induce neural transformation (Osafune et al., 2008; Kim et al., 2010). Our optimized protocol allowed up to 84% conversion from hiPSC to NPC. The differentiation efficiency was slightly improved compared with the pre-optimized scheme when the stem cell source was not taken into account. In contrast to a recent study (Kim et al., 2021a), our percentage of induced differentiated NPC of the total cells was consistently stable. Our protocol uses dorsomorphin, rather than Noggin, to block the BMP pathway, primarily because of the smaller and more stable molecular weight of dorsomorphin; therefore it can more easily penetrate the cell mass. In addition, dorsomorphin blocks activation of the SMAD1/5/8 and non-SMAD signaling pathways through the MAPK p38, ERK1/2, and Akt pathways. This allowed it to interfere with the BMP-induced signaling cascade (Boergermann et al., 2010). Not to be overlooked, we also included experimental manipulation of the suspension culture and passaged three times during the cell culture process. Suspension cultures are a type of three-dimensional culture that are more suitable for simulating intercellular and extracellular matrix interactions than two-dimensional monolayer cultures. Suspension cultures are not only used to maintain cell vitality but also to promote the differentiation of human stem cells (Hookway et al., 2016). Therefore, the inhibitor dorsomorphin and the suspension culture approach used in our protocol may be more conducive to improving differentiation efficiency.
Some studies suggest that activation of the BMP/SMAD pathway can promote iPSC differentiation into mDA neurons. This is also an important cue for regulating the production of mDA neurons in mammals (Jovanovic et al., 2018). Another study found that BMP/SMAD and TGF-β/SMAD signaling serves as an upstream regulator to regulate WNT signaling during iPSC differentiation into mDA neurons (Cai et al., 2013). However, we blocked this pathway in the early stages of cell culture to achieve efficient differentiation of hiPSC into neurons. This may explain why our induction protocol of inhibiting SMAD signaling allowed only a small number of hiPSCs to differentiate into dopaminergic neurons. The successful induction and differentiation of FP cells determines the generation of mDA neurons, requiring a combination of SHH from the FP and FGF8 from isthmus tissue for regulation (Liu et al., 2021). Thus, after strong inhibition of the SMAD pathway, we added SHH and FGF8 on day 8 of cell culture; this significantly increased the number and proportion of dopaminergic neurons obtained on day 22 of cell culture. However, this protocol was still not sufficient to specify midbrain identity of the neurons. We further optimized the protocol using a combination of SHH and FGF8 in the early stages of cell culture to enhance SHH signaling, and activated the WNT pathway, an alteration that further increased the proportion of mDA neurons. Growing evidence shows that the addition of high concentrations of SHH in early cell culture is beneficial for inducing expression of the FP marker, FOXA2, hinting that the early stage of stem cell differentiation is the optimal time window for exerting these effects (Fasano et al., 2010). In addition, the proliferation of mDA progenitors requires regulation of the WNT pathway. Differentiation of FP cells into mDA requires SHH and FGF8 to regulate the balance between the WNT-LMX1A and SHH-FOXA2 mDA pathways (Blaess and Ang, 2015). One study demonstrated that WNT signaling plays different roles at different stages of early midbrain development, and that the optimal WNT signaling strength to determine cellular midbrain identity varies (Kim et al., 2021b). Researchers have induced expression of the midbrain marker, EN1, on the first day of cell differentiation without the addition of FGF8, through the so-called biphasic activation of WNT signaling. Because of this complex mechanism of midbrain embryonic development and the involvement of SHH, FGF, WNT, and other signaling pathways, further studies are needed to develop more precise and efficient protocols based on the spatiotemporal characteristics of midbrain development to improve the purity of in vitro generated mDA neurons.
Some limitations of this study should be addressed. We noted that some studies have used cell sorting (Doi et al., 2020) or the lysis of undifferentiated cells by certain drugs (Song et al., 2020) to achieve a higher purity of induced mDA neurons for better application in the treatment of neurodegenerative diseases. These studies can provide clues for further optimization of our protocol. The differentiation of hiPSC into mDA neurons is time-consuming and cost demanding, thus acceleration of this process is necessary. The signal transduction process of mDA neurons during embryonic development is complex, and the underlying mechanisms involved warrant further exploration. Integration of these studies will help control and precisely induce hiPSC differentiation into mDA neurons in vitro. Most importantly, the differentiated mDA neurons in our protocol need to be further validated by cell transplantation in animal models to investigate cell viability, neuronal functioning, neural circuit integration, and therapeutic efficacy for neurodegenerative diseases, this being the next step of our study.
In summary, we here report an hiPSC-specific protocol for stable and efficient differentiation into mDA neurons of high purity. This will be beneficial for investigating neurological disease mechanisms, drug screening studies, and cell transplantation applications of hiPSC-derived mDA neurons.
Funding Statement
Funding: This study was supported by the National Natural Science Foundation of China, No. 81771222 (to LS); Guangzhou Key Research Program on Brain Science, Nos. 202007030011, 202206060001 (to LS); the Program of Introducing Talents of Discipline to Universities of China, No. B14036 (to KFS).
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest. KFS is the Editor-in-Chief of Neural Regeneration Research. KFS did not participate in the peer review of decision-making of the paper.
Data availability statement: All relevant data are within the paper.
C-Editor: Zhao M; S-Editor: Li CH; L-Editors: Li CH, Song LP; T-Editor: Jia Y
References
- 1.Alekseenko Z, Dias JM, Adler AF, Kozhevnikova M, van Lunteren JA, Nolbrant S, Jeggari A, Vasylovska S, Yoshitake T, Kehr J, Carlén M, Alexeyenko A, Parmar M, Ericson J. Robust derivation of transplantable dopamine neurons from human pluripotent stem cells by timed retinoic acid delivery. Nat Commun. 2022;13:3046. doi: 10.1038/s41467-022-30777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bariselli S, Hörnberg H, Prévost-Solié C, Musardo S, Hatstatt-Burklé L, Scheiffele P, Bellone C. Role of VTA dopamine neurons and neuroligin 3 in sociability traits related to nonfamiliar conspecific interaction. Nat Commun. 2018;9:3173. doi: 10.1038/s41467-018-05382-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaess S, Ang SL. Genetic control of midbrain dopaminergic neuron development. Wiley Interdiscip Rev Dev Biol. 2015;4:113–134. doi: 10.1002/wdev.169. [DOI] [PubMed] [Google Scholar]
- 4.Boergermann JH, Kopf J, Yu PB, Knaus P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad p38 and Akt signalling in C2C12 cells. Int J Biochem Cell Biol. 2010;42:1802–1807. doi: 10.1016/j.biocel.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai J, Schleidt S, Pelta-Heller J, Hutchings D, Cannarsa G, Iacovitti L. BMP and TGF-βpathway mediators are critical upstream regulators of Wnt signaling during midbrain dopamine differentiation in human pluripotent stem cells. Dev Biol. 2013;376:62–73. doi: 10.1016/j.ydbio.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castelo-Branco G, Wagner J, Rodriguez FJ, Kele J, Sousa K, Rawal N, Pasolli HA, Fuchs E, Kitajewski J, Arenas E. Differential regulation of midbrain dopaminergic neuron development by Wnt-1, Wnt-3a and Wnt-5a. Proc Natl Acad Sci U S A. 2003;100:12747–12752. doi: 10.1073/pnas.1534900100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doi D, Magotani H, Kikuchi T, Ikeda M, Hiramatsu S, Yoshida K, Amano N, Nomura M, Umekage M, Morizane A, Takahashi J. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson's disease. Nat Commun. 2020;11:3369. doi: 10.1038/s41467-020-17165-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fasano CA, Chambers SM, Lee G, Tomishima MJ, Studer L. Efficient derivation of functional floor plate tissue from human embryonic stem cells. Cell Stem Cell. 2010;6:336–347. doi: 10.1016/j.stem.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 11.Galiakberova AA, Dashinimaev EB. Neural stem cells and methods for their generation from induced pluripotent stem cells in vitro. Front Cell Dev Biol. 2020;8:815. doi: 10.3389/fcell.2020.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyon A, Skrzydelsi D, Rovère C, Rostène W, Parsadaniantz SM, Nahon JL. Stromal cell-derived factor-1alpha modulation of the excitability of rat substantia nigra dopaminergic neurones: presynaptic mechanisms. J Neurochem. 2006;96:1540–1550. doi: 10.1111/j.1471-4159.2006.03659.x. [DOI] [PubMed] [Google Scholar]
- 13.Hookway TA, Butts JC, Lee E, Tang H, McDevitt TC. Aggregate formation and suspension culture of human pluripotent stem cells and differentiated progeny. Methods. 2016;101:11–20. doi: 10.1016/j.ymeth.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Howes OD, Shatalina E. Integrating the neurodevelopmental and dopamine hypotheses of schizophrenia and the role of cortical excitation-inhibition balance. Biol Psychiatry. 2022;92:501–513. doi: 10.1016/j.biopsych.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Prog Neurobiol. 2005;77:128–138. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Jovanovic VM, Salti A, Tilleman H, Zega K, Jukic MM, Zou H, Friedel RH, Prakash N, Blaess S, Edenhofer F, Brodski C. BMP/SMAD pathway promotes neurogenesis of midbrain dopaminergic neurons in vivo and in human induced pluripotent and neural stem cells. J Neurosci. 2018;38:1662–1676. doi: 10.1523/JNEUROSCI.1540-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karvas RM, David L, Theunissen TW. Accessing the human trophoblast stem cell state from pluripotent and somatic cells. Cell Mol Life Sci. 2022;79:604. doi: 10.1007/s00018-022-04549-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsuki H, Kurimoto E, Takemori S, Kurauchi Y, Hisatsune A, Isohama Y, Izumi Y, Kume T, Shudo K, Akaike A. Retinoic acid receptor stimulation protects midbrain dopaminergic neurons from inflammatory degeneration via BDNF-mediated signaling. J Neurochem. 2009;110:707–718. doi: 10.1111/j.1471-4159.2009.06171.x. [DOI] [PubMed] [Google Scholar]
- 19.Kikuchi T, Morizane A, Doi D, Magotani H, Onoe H, Hayashi T, Mizuma H, Takara S, Takahashi R, Inoue H, Morita S, Yamamoto M, Okita K, Nakagawa M, Parmar M, Takahashi J. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson's disease model. Nature. 2017;548:592–596. doi: 10.1038/nature23664. [DOI] [PubMed] [Google Scholar]
- 20.Kim DS, Lee JS, Leem JW, Huh YJ, Kim JY, Kim HS, Park IH, Daley GQ, Hwang DY, Kim DW. Robust enhancement of neural differentiation from human ES and iPS cells regardless of their innate difference in differentiation propensity. Stem Cell Rev Rep. 2010;6:270–281. doi: 10.1007/s12015-010-9138-1. [DOI] [PubMed] [Google Scholar]
- 21.Kim HM, Noh HB, Lee SH, Lee KG, Chang B, Cheong E, Lee CJ, Hwang DY. Fine-tuning of dual-SMAD inhibition to differentiate human pluripotent stem cells into neural crest stem cells. Cell Prolif. 2021a;54:e13103. doi: 10.1111/cpr.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim TW, Piao J, Koo SY, Kriks S, Chung SY, Betel D, Socci ND, Choi SJ, Zabierowski S, Dubose BN, Hill EJ, Mosharov EV, Irion S, Tomishima MJ, Tabar V, Studer L. Biphasic activation of WNT signaling facilitates the derivation of midbrain dopamine neurons from hESCs for translational use. Cell Stem Cell. 2021b;28:343–355.e345. doi: 10.1016/j.stem.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkeby A, Grealish S, Wolf DA, Nelander J, Wood J, Lundblad M, Lindvall O, Parmar M. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 2012;1:703–714. doi: 10.1016/j.celrep.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Kirkeby A, Nolbrant S, Tiklova K, Heuer A, Kee N, Cardoso T, Ottosson DR, Lelos MJ, Rifes P, Dunnett SB, Grealish S, Perlmann T, Parmar M. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson's disease. Cell Stem Cell. 2017;20:135–148. doi: 10.1016/j.stem.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouwenhoven WM, von Oerthel L, Smidt MP. Pitx3 and En1 determine the size and molecular programming of the dopaminergic neuronal pool. PLoS One. 2017;12:e0182421. doi: 10.1371/journal.pone.0182421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, Yang L, Beal MF, Surmeier DJ, Kordower JH, Tabar V, Studer L. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature. 2011;480:547–551. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Deng J, Liu Y, Li W, Nie X. FGF, mechanism of action role in Parkinson's disease and therapeutics. Front Pharmacol. 2021;12:675725. doi: 10.3389/fphar.2021.675725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng YH, Chanda S, Janas JA, Yang N, Kokubu Y, Südhof TC, Wernig M. Efficient generation of dopaminergic induced neuronal cells with midbrain characteristics. Stem Cell Reports. 2021;16:1763–1776. doi: 10.1016/j.stemcr.2021.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niclis JC, Gantner CW, Alsanie WF, McDougall SJ, Bye CR, Elefanty AG, Stanley EG, Haynes JM, Pouton CW, Thompson LH, Parish CL. Efficiently specified ventral midbrain dopamine neurons from human pluripotent stem cells under Xeno-free conditions restore motor deficits in parkinsonian rodents. Stem Cells Transl Med. 2017;6:937–948. doi: 10.5966/sctm.2016-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura K, Nitta T, Doi K, Takata K. Rapid conversion of human induced pluripotent stem cells into dopaminergic neurons by inducible expression of two transcription factors. Stem Cells Dev. 2022;31:269–277. doi: 10.1089/scd.2021.0363. [DOI] [PubMed] [Google Scholar]
- 31.Ono Y, Nakatani T, Sakamoto Y, Mizuhara E, Minaki Y, Kumai M, Hamaguchi A, Nishimura M, Inoue Y, Hayashi H, Takahashi J, Imai T. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007;134:3213–3225. doi: 10.1242/dev.02879. [DOI] [PubMed] [Google Scholar]
- 32.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA. Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol. 2008;26:313–315. doi: 10.1038/nbt1383. [DOI] [PubMed] [Google Scholar]
- 33.Rekler D, Kalcheim C. From neural crest to definitive roof plate: the dynamic behavior of the dorsal neural tube. Int J Mol Sci. 2021;22:3911. doi: 10.3390/ijms22083911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz CM, Tavakoli T, Jamias C, Park SS, Maudsley S, Martin B, Phillips TM, Yao PJ, Itoh K, Ma W, Rao MS, Arenas E, Mattson MP. Stromal factors SDF1α sFRP1 and VEGFD induce dopaminergic neuron differentiation of human pluripotent stem cells. J Neurosci Res. 2012;90:1367–1381. doi: 10.1002/jnr.23064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- 36.Song B, Cha Y, Ko S, Jeon J, Lee N, Seo H, Park KJ, Lee IH, Lopes C, Feitosa M, Luna MJ, Jung JH, Kim J, Hwang D, Cohen BM, Teicher MH, Leblanc P, Carter BS, Kordower JH, Bolshakov VY, et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson's disease models. J Clin Invest. 2020;130:904–920. doi: 10.1172/JCI130767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storch A, Paul G, Csete M, Boehm BO, Carvey PM, Kupsch A, Schwarz J. Long-term proliferation and dopaminergic differentiation of human mesencephalic neural precursor cells. Exp Neurol. 2001;170:317–325. doi: 10.1006/exnr.2001.7706. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 39.Tanudjojo B, Shaikh SS, Fenyi A, Bousset L, Agarwal D, Marsh J, Zois C, Heman-Ackah S, Fischer R, Sims D, Melki R, Tofaris GK. Phenotypic manifestation of α-synuclein strains derived from Parkinson's disease and multiple system atrophy in human dopaminergic neurons. Nat Commun. 2021;12:3817. doi: 10.1038/s41467-021-23682-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian L, Al-Nusaif M, Chen X, Li S, Le W. Roles of transcription factors in the development and reprogramming of the dopaminergic neurons. Int J Mol Sci. 2022;23:845. doi: 10.3390/ijms23020845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- 42.Volpicelli F, Perrone-Capano C, Bellenchi GC, Colucci-D'Amato L, di Porzio U. Molecular regulation in dopaminergic neuron development cues to unveil molecular pathogenesis and pharmacological targets of neurodegeneration. Int J Mol Sci. 2020;21:3995. doi: 10.3390/ijms21113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang MZ, Jin P, Bumcrot DA, Marigo V, McMahon AP, Wang EA, Woolf T, Pang K. Induction of dopaminergic neuron phenotype in the midbrain by Sonic hedgehog protein. Nat Med. 1995;1:1184–1188. doi: 10.1038/nm1195-1184. [DOI] [PubMed] [Google Scholar]
- 44.Xiong M, Tao Y, Gao Q, Feng B, Yan W, Zhou Y, Kotsonis TA, Yuan T, You Z, Wu Z, Xi J, Haberman A, Graham J, Block J, Zhou W, Chen Y, Zhang SC. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell. 2021;28:112–126.e116. doi: 10.1016/j.stem.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B. GDNF acutely modulates excitability and A-type K(+) channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 46.Ye W, Shimamura K, Rubenstein JL, Hynes MA, Rosenthal A. FGF and Shh signals control dopaminergic and serotonergic cell fate in the anterior neural plate. Cell. 1998;93:755–766. doi: 10.1016/s0092-8674(00)81437-3. [DOI] [PubMed] [Google Scholar]
- 47.Yeap YJ, Teddy TJW, Lee MJ, Goh M, Lim KL. From 2D to 3D: development of monolayer dopaminergic neuronal and midbrain organoid cultures for Parkinson's disease modeling and regenerative therapy. Int J Mol Sci. 2023;24:2523. doi: 10.3390/ijms24032523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zayed MA, Sultan S, Alsaab HO, Yousof SM, Alrefaei GI, Alsubhi NH, Alkarim S, Al Ghamdi KS, Bagabir SA, Jana A, Alghamdi BS, Atta HM, Ashraf GM. Stem-cell-based therapy: the celestial weapon against neurological disorders. Cells. 2022;11:3476. doi: 10.3390/cells11213476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zou L, Xue Y, Jones M, Heinbockel T, Ying M, Zhan X. The effects of quinine on neurophysiological properties of dopaminergic neurons. Neurotox Res. 2018;34:62–73. doi: 10.1007/s12640-017-9855-1. [DOI] [PubMed] [Google Scholar]