Abstract
This study showed both DNA and RNA NGS panels had high performance using EBUS-TBNA samples in patients with non-squamous NSCLC. Sampling more advanced nodal stations and samples with higher tumor cellularity are associated with higher success rates of DNA NGS. More EBUS-TBNA passes per station did not increase success rate of NGS.
Introduction/Background:
Samples from endobronchial ultrasound-guided fine needle aspiration (EBUS-TBNA) are frequently used for next generation sequencing (NGS) in patients with non-small cell lung cancer (NSCLC) to look for genetic driver mutations. The objective of the current study was to evaluate the performance of extended NGS panels using EBUS-TBNA samples in a real-world setting and identify factors associated with the success of NGS.
Materials and Methods:
This study included all patients who underwent EBUS and were diagnosed with non-squamous NSCLC with mediastinal metastasis from 2016 to 2019 at the University of Pennsylvania. We reviewed demographic information, imaging studies, procedure reports, pathology and NGS reports. Logistic regression was used to analyze factors associated with the success of NGS panels.
Results:
The success rates of NGS using EBUS-TBNA samples were 92.5%, and 91.5% for DNA and RNA NGS panels respectively. Samples from higher N stage (N2 and N3 lymph nodes) and with higher tumor cellularity (> 25%) resulted in higher success rate for DNA NGS. The effect of tumor cellularity remained borderline significant after enter ing multivar iable logistic regression. The short-axis diameter of the sampled lymph node on CT scan, FDG-avidity on PET CT and > 3 EBUS passes per lymph node during the procedure were not associated with NGS success.
Conclusion:
Both DNA and RNA extended-panel NGS had high performance using EBUS-TBNA samples. Sampling more advanced nodal stations and obtaining samples with higher tumor cellularity were associated with higher success rate of DNA NGS. Other imaging or procedural factors did not affect NGS performance.
Keywords: Success rate, Molecular analysis, Cytology samples, Bronchoscopy procedure, Imaging characteristics
Introduction
Lung cancer continues to be the leading cause of death in both men and women the United States, however, the mortality from lung cancer appears to be decreasing.1 Two factors likely contribute to this recent survival improvement - lung cancer screening programs2 as well as the newer treatment options, such as immunotherapy and targeted driver-mutation therapy.3 , 4 There are over 20 targeted therapies for patients with NSCLC and more under investigation. In order to assess candidacy for those therapies, it is essential to check for tumor genetic mutations. The College of American Pathologists has recommended evaluating 8 genes for alterations, and consider checking others for clinic trials.5 Given the large number of mutations to be tested, next generation sequencing (NGS) with a lung cancer specific oncogene panel has become the most efficient method. It allows parallel testing of hundreds of genetic mutations, requires smaller samples with lower concentrations of malignant cells, and can be finalized more rapidly than using multiple sequential single-gene assays.6 , 7 Furthermore, RNA NGS detects fusion genes, discriminates slicing isoforms, and quantifies fusion transcripts.8
Bronchoscopy with endobronchial ultrasound-guided transbronchial aspiration or fine needle aspiration (EBUS-TBNA or FNA) is the standard of care in diagnosing and staging of NSCLC.9 Commonly, FNA samples or cytology samples represent the sole diagnostic material for lung cancer.10 , 11 DNA NGS performance on FNA sample has been studied with heterogeneous results. For example, Hagemann et al. reported DNA NGS success rate of 40% on FNA samples from lymph node metastasis, compared with 100% from excisional biopsy.12 However, another study by Turner and colleagues reported higher success rate of 86.1% with EBUS-TBNA samples.13 There is even less data regarding the success of RNA NGS using FNA samples, given its more recent development and clinical application. RNA handling is more complicated as it is more susceptible to degradation.14 It is important to explore and identify factors that are associated with higher success rate for each of these emerging assays.
Our goal is to evaluate the pragmatic performance of EBUS-TBNA specimens on both extended DNA and RNA NGS panels in patients who presented for lung cancer mediastinal staging for non-squamous NSCLC. We also aimed to assess imaging, procedural and cytology-specific characteristics that could be associated with better performance status for DNA and RNA NGS.
Materials and Methods
Study Cohort
A retrospective review of all patients undergoing diagnostic bronchoscopy and staging EBUS from September, 2016 to May, 2019 was used to build the cohort. This study was approved by University of Pennsylvania (Philadelphia, PA) institutional review board (IRB protocol # 848770). All patients with the diagnosis of non-squamous NSCLC and pathologically confirmed metastasis to mediastinal lymph nodes were included. A total of 292 patients and 300 EBUS procedures were identified and included in the final analysis. We reviewed cytology, surgical pathology and NGS reports as well as demographics, imaging studies and procedure reports.
EBUS-TBNA
EBUS-TBNA was performed in the operating room using a convex probe ultrasound bronchoscope (Olympus BF-UC180F; Olympus America Inc, Central valley, PA). All patients were placed under general anesthesia using a continuous infusion of propofol and remifentanil. The bronchoscope was inserted orally and a systematic nodal evaluation was done according to clinical guidelines.15 Any lymph node that had a short axis greater than 5mm under ultrasound was sampled. Number of passes and agitations were obtained per operator’s discretion, with the standard being 3 passes and 30 agitations per pass with rapid forward motion during agitation. If the primary tumor was also accessible using the convex probe ultrasound bronchoscope, it was sampled after lymph node staging. We used 22-gauge needles (NA-201SX-4022, Olympus America Inc, Central valley, PA) to obtain FNA samples in most cases. Occasionally, 19-gauge needles (Vizishot FLEX 19 G, Olympus America Inc, Central valley, PA) or miniforceps (CoreDx forceps, Boston Scientific) were used for sampling when alternative diagnosis, such as lymphoma, was suspected. Rapid on-site evaluation (ROSE) was not routinely performed, and was used at bronchoscopist’s discretion.
Flexible Bronchoscopy (FB)
Endobronchial biopsy (EBBx) was performed if an endobronchial lesion was present. Peripheral lesions in lung parenchyma were biopsied using needles, cytobrushes and/or forceps. Cytology samples (ie FNA, brushing) were placed in normal saline or CytoLyt; surgical pathology samples (ie EBBx, transbronchial biopsy or TBBx) in formalin for further histological and molecular analysis.
NGS and Immunohistochemistry Stains (IHC)
After the diagnosis of NSCLC was made, reflex IHC and molecular analyses were performed using a health system wide, pre-determined pathway for lung cancer relevant driver mutations. The algorithm evolved during the 3-year study period as the number of lung cancer-associated genes of interest expanded. The most recent version is shown in Figure 1. The solid tumor panel(STP) was a NGS assay used for DNA sequencing of 152 genes (Haloplex assay, Agilent, Santa Clara, CA); while the fusion transcription panel(FTP) was a RNA sequencing assay of 55 gene rearrangements (ArcherDx, Boulder, CO). RNA NGS was launched in Aug, 2017. Both tests were performed by the Center for Personalized Diagnostics (CPD) lab at the Hospital of the University of Pennsylvania. Testing was triaged according to available tissue. Assays were previously internally validated for cytologic and surgical pathology specimens.16 Patients who underwent paired testing demonstrated 100% concordant mutations between cytologic samples and pathology samples during validation process. EBUS-TBNA samples (cytology specimens) are routinely collected in normal saline or Thin-Prep Cytolyt solution. The samples were concentrated by usual cytologic methods. In anticipation of the molecular testing needs in NSCLC, our lab reserves material for this purpose in a “cytology molecular vial” while processing the initial specimen. Cytology molecular vials were created by placing an additional drop(s) of concentrated cellular material into a second PreservCyt vial. Cytology molecular vials were used as the preferred sample for NGS. The minimum requirement to run each NGS assay was 10% tumor cellularity. The percentage of tumor cellularity was calculated by estimating the number of tumor cells versus non-tumor cells present on a representative slide. If the cytologic sample was inadequate for molecular testing or the surgical pathology specimen was much more cellular, the surgical pathology specimen was utilized for NGS testing instead.
Figure 1.
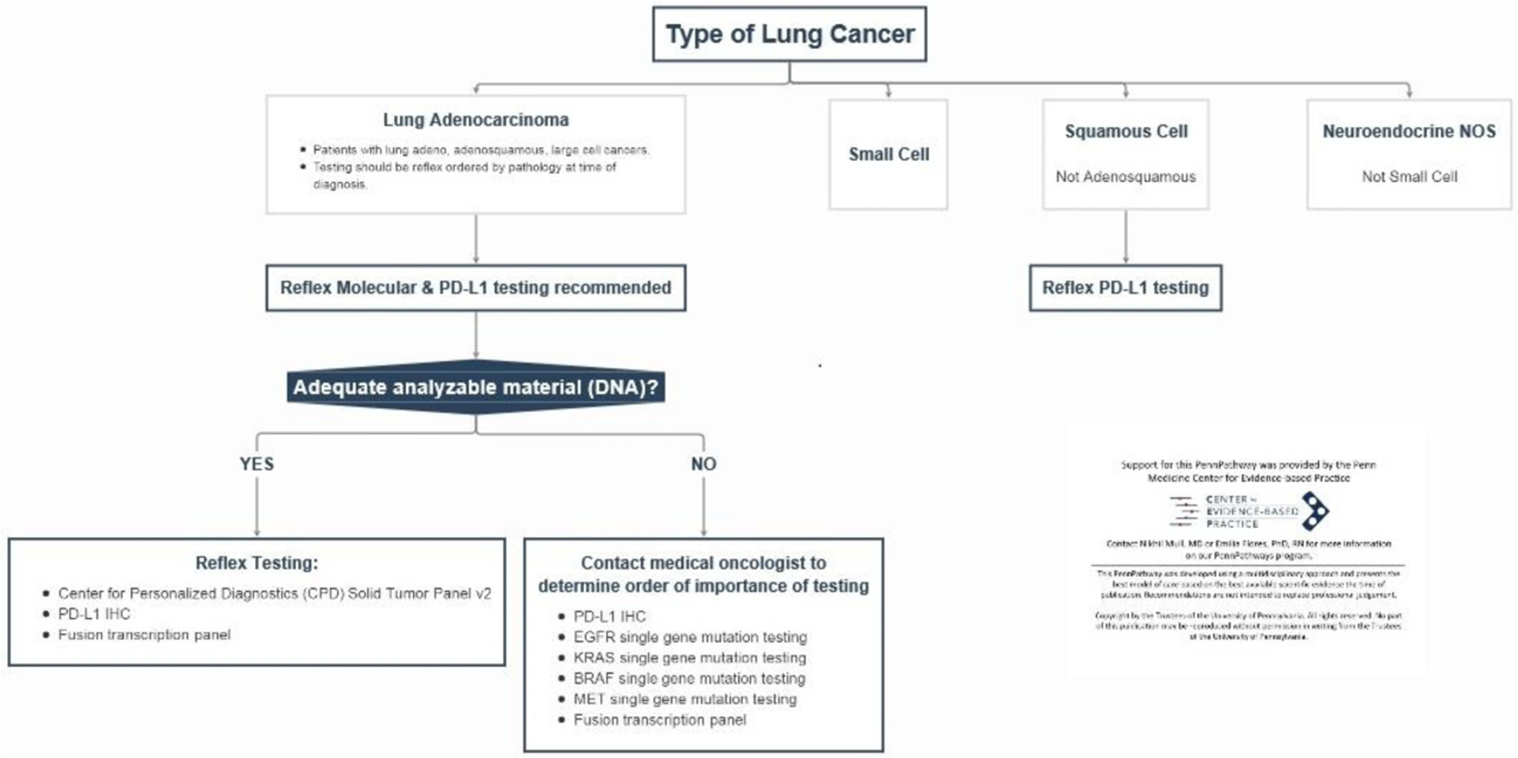
NSCLC molecular analysis and IHC pathway at University of Pennsylvania Hospital.
Programmed death-ligand 1 (PD-L1) expression was evaluated by IHC on either cytological or histologic pathology samples and was internally validated. Echinoderm microtubule-associated protein-like 4 - anaplastic lymphoma kinase (EML4-ALK) and proto-oncogene tyrosine-protein kinase 1 (ROS1) were evaluated by IHC and/or fluorescence in situ hybridization (FISH) before RNA NGS was available.
Outcomes and Covariates
The success of DNA and RNA NGS assays using EBUS-TBNA samples were considered the co-primary outcomes. When the NGS report or genetic mutation report was available, the NGS assay was considered successful. If sequencing report showed no mutations, it was still considered as successful, as the internal validation process suggested this meant the cancer did not carry mutations that were included in the panel. The success rate was calculated at the sample level, given that some procedures had more than 1 EBUS-TBNA samples sent for NGS if the previous run was unsuccessful. The denominator used to calculate the success rate was the number of samples that actually underwent NGS analysis. We analyzed factors that could be associated with a higher success rate of NGS. Computed tomography(CT) and positron emission tomography (PET) scans obtained within 1-month of each biopsy procedure were reviewed. N stage was further categorized into 2 groups - primary tumor and N1 node versus N2 and N3 nodes. Fluorodeoxyglucose(FDG) avidity on PET scan was categorized in to low intensity (maximum standardized uptake value or SUVmax 2.5–5), moderate (SUVmax 5–10), intense (SUVmax 10–15) and very intense (SUVmax > 15).17 Procedural and cytological factors were also included in the analysis. Age, gender, and smoking status were not considered sample-level variables, and therefore, did not enter the analysis.
Statistical Analysis
We used descriptive statistics to evaluate patient baseline characteristics. Median and interquartile range(IQR) were used to describe continuous variables, while frequencies and percentages for categorical variables. We used the univariable logistic regression to evaluate factors associated with assay success. Statistically significant factors from univariable analysis entered multivariable logistic regression analysis. All statistical tests were 2-sided, and a P < .05 was considered statistically significant. All analyses were conducted using Stata/BE 17.0.
Results
Patients
During the study period, 2048 patients underwent 2129 EBUS procedures. A total of 292 patients had pathologically confirmed mediastinal metastasis of non-squamous NSCLC. Patient characteristics are shown in Table 1. More than half of the patients were female. The majority of individuals identified was white. Over 80% of the patients formerly or currently smoked.
Table 1.
Baseline Characteristics of Patients With Non-Squamous NSCLC Diagnosed by EBUS-TBNA
| Patients (N=292) No. of patients (%) or Median(IQR) | |
|---|---|
| Age, y | 68(61–75) |
| Sex | |
| Female | 168 (57.5%) |
| Male | 124 (42.5%) |
| Race/ethnicity | |
| White | 194 (66.4%) |
| Black | 70 (24.0%) |
| Asian | 11 (3.8%) |
| Other | 10 (3.4%) |
| Missing | 7 (2.4%) |
| Smoking status | |
| Current | 29 (9.9%) |
| Former | 217 (74.3%) |
| Never | 41 (14.0%) |
| Missing | 5 (1.7%) |
Abbreviations: IQR = interquartile range.
Overall Success Rates for NGS and IHC
A total of 300 EBUS procedures were performed on the afore-mentioned 292 patients. DNA NGS was ordered in 271 out of 300 EBUS procedures, while RNA NGS was ordered in 139 EBUS procedures. Detailed information on ordering status of NGS panel can be found in Table 2. Twenty-two procedures had DNA NGS ordered but did not generate any sample with tumor cellularity > 10%, therefore, no DNA NGS was performed. Similarly, 19 procedures had RNA NGS ordered without any sample of > 10% tumor cellularity. There were 2 procedures that sent more than 1 sample for DNA NGS. Overall, a total of 251 samples from 249 procedures(249/271 = 91.9%) and 120 samples from 120 procedures(120/139 = 86.3%) were sent for DNA and RNA NGS respectively. The majority of the samples used for NGS panels were EBUS-TBNA samples. The other sample types included peripheral lung nodule biopsy (including FNA, brushing or TBBx), lymph node core biopsy (which was considered as a pathology sample instead of a cytology sample) or EBBx. Success rates for NGS and IHC are reported in Table 3. There were 234 procedures (234/271 = 86.3%) that resulted in successful DNA NGS and 110 (110/139 = 79.1%) for RNA NGS. The success rates of DNA and RNA NGS using EBUS-TBNA samples with > 10% tumor cellularity, were 92.5% and 91.5% respectively. For successfully sequenced tumors, only 1.9% had normal sequencing with no genetic alteration by DNA NGS panel using EBUS-TBNA samples, while the majority of the cases (92.8%) reported no fusion gene detected by RNA NGS panel. The performance of EBUS-TBNA samples on IHC staining was high (close to 100% success) and similar to IHC stains performed on surgical pathology samples.
Table 2.
Ordering Status of NGS Panels
| DNA NGS panel No. of EBUS procedures | RNA NGS panel No. of EBUS procedures | |
|---|---|---|
| NGS panel ordered | 271 | 139 |
| Samples from procedures other than EBUS | 9 | 1 |
| Not ordered by proceduralist | 11 | 16 |
| Not resulted for unclear reason | 9 | 14 |
| Not sent prior to being available | NA | 130 |
Abbreviations: EBUS = endobronchial ultrasound; NGS = next generation sequencing.
Table 3.
Tissue Adequacy for NGS and IHC
| Success rate, % | |
|---|---|
| (No. of success run/Total No.) | |
| Success run of NGS on EBUS-TBNA samples | |
| DNA NGS | 92.5% (209/226) |
| RNA NGS | 91.5% (97/106) |
| Success run of NGS on any sample type | |
| DNA NGS | 93.3% (234/251) |
| RNA NGS | 91.7% (110/120) |
| Negative rates for NGS panels | |
| No mutation reported by DNA NGS panel using EBUS samples | 1.9% (4/209) |
| No fusion gene reported by RNA NGS panel using EBUS samples | 92.8% (90/97) |
| Successful PD-L1 staining on | |
| Cytology sample | 99.2% (124/125) |
| Surgical pathology sample | 100.0% (96/96) |
| Successful EML4-ALK staining on | |
| Cytology samples | 98.5% (66/67) |
| Surgical pathology sample | 100.0% (40/40) |
| Successful ROS1 staining on | |
| Cytology samples | 98.5% (64/65) |
| Surgical pathology sample | 100.0% (41/41) |
Abbreviations: EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration; EML4-ALK = Echinoderm microtubule-associated protein-like 4 - anaplastic lymphoma kinase; IHC = immunohistochemistry stains; NGS = next generation sequencing; PD-L1 = programmed death-ligand 1; ROS1 = proto-oncogene tyrosine-protein kinase 1.
Factors Associated With Success run of NGS Using EBUS-TBNA Samples
Imaging, procedural and cytological characteristics were analyzed. The results of univariable logistic regression are summarized in Table 4.
Table 4.
Univariable Logistic Regression Analysis of Imaging, Procedural and Cytological Characteristics Associated With Success run of NGS Using EBUS-TBNA Samples
| DNA NGS (N=226) | RNA NGS (N=106) | |||
|---|---|---|---|---|
| Success rate, % (No. of Success Run / total No.) | OR (95% CI) | Success rate, % (No. of Success Run / total No.) | OR (95% CI) | |
| CT short-axis diameter, mm | ||||
| >5 to <10 | 92.0% (46/50) | 1.3 (0.3 – 4.7) | 90.5% (19/21) | 1.4 (0.2 – 9.3) |
| >10 to <20 | 93.6% (88/94) | 1.4 (0.3–6.0) | 93.2% (41/44) | 1.1 (0.2 – 6.9) |
| >20 | 94.3% (66/70) | 90.9% (30/33) | ||
| FDG avidity, SUV | ||||
| >2.5 to <5 | 84.6% (22/26) | 2.0 (0.5 – 7.7) | 92.3% (12/13) | 0.8 (0.1 – 8.5) |
| >5 to <10 | 91.7% (66/72) | 6.2 (0.6 – 59.0) | 90.6% (29/32) | / |
| >10 to <15 | 97.1% (34/35) | 2 (0.3 – 12.1) | 100.0% (17/17) | 0.5 (0.0 – 5.8) |
| > 15 | 91.7% (22/24) | 84.6% (11/13) | ||
| Tissue source | ||||
| Primary tumor+N1 | 86.7% (52/60) | 3.5 (1.2 – 10.0) | 93.3% (28/30) | 0.7 (0.1 – 3.4) |
| N2+N3 | 95.7% (157/164) | 90.3% (65/72) | ||
| Number of passes, n | ||||
| <3 | 94.3% (116/123) | 0.8 (0.3 – 2.3) | 87.7% (50/57) | 3.2 (0.6 – 16.3) |
| >3 | 92.9% (92/99) | 95.8% (46/48) | ||
| Tumor cellularity, % | ||||
| 10–25 | 88.2% (75/85) | 3.4 (1.1 – 10.4) | 86.4% (38/44) | 3.1 (0.7 – 13.2) |
| >25 | 96.3% (129/134) | 95.2% (59/62) | ||
Abbreviations: 95% CI = 95% confidence interval; EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration; FDG = fluorodeoxyglucose; NGS = next generation sequencing; OR = Odds Ratio; SUVmax = maximum standardized uptake value.
The success rate in each subgroup was high regardless of the size of the lymph node on CT or FDG avidity on PET. The number of TBNA passes per lymph node (> 3 vs. ≤ 3 passes) did not make a difference to the success rate of NGS panels. The 19G needle was only used in 20 procedures (6.7%) while miniforceps in 6 procedures (2.0%). Given the limited use and both being pathology samples, logistic regression was not performed.
There was a significant increase in the success rate of DNA NGS in the high tumor cellularity group (> 25%) compared to the low tumor cellularity group, as well as the N2/N3 lymph nodes compared to the primary tumor or N1 nodes.
Lymph node station(N2/N3 versus N1/primary tumor) and tumor cellularity(> 25% versus ≤25%) were entered into a multi-variable logistic regression analysis for DNA NGS. The results are shown in Table 5. Samples from higher N stage (N2 or N3 nodes) had 3.1 times the odds of success run of DNA NGS than that from lower N stage (primary tumor and N1 lymph node), however, the effect of higher tumor cellularity became borderline in the multi-variable analysis. Given no factor was found to be significantly associated with the success run of RNA NGS, multivariable analysis was not performed.
Table 5.
Multi-Variable Logistic Regression Analysis of Factors Associated With Success run of NGS Using EBUS-TBNA samples
| DNA NGS (N=226) OR (95% CI) | |
|---|---|
| Tissue source | |
| Primary tumor+N1 | 3.1 (1.1 – 9.2) |
| N2+N3 | |
| Tumor cellularity, % | |
| 10–25 | 3.1 (1.0 – 9.5) |
| >25 |
Abbreviations: 95% CI = 95% confidence interval; EBUS-TBNA = endobronchial ultrasound-guided transbronchial needle aspiration; FDG = fluorodeoxyglucose; NGS = next generation sequencing; OR = odds ratio; SUVmax = maximum standardized uptake value.
Discussion
In this single-center, retrospective cohort study, we demonstrated > 90% success rate for both DNA and RNA NGS panels using EBUS-TBNA samples in the setting of advanced stage non-squamous NSCLC. This is the first study to our knowledge that analyzed imaging, procedural and cytological factors that were associated with the success of both DNA and RNA NGS. Higher lymph node N stage (N2 +N3) and higher tumor cellularity (> 25%) were associated with better performance of DNA NGS. The effect of tumor cellularity became borderline significant after entering multivariable logistic regression.
Our study reflected the performance of DNA and RNA NGS performance in a real-world, tertiary care center with NGS testing performed locally. A recently published meta-analysis with a total of 1,175 patients reported the pooled proportion of adequate EBUS-TBNA samples for DNA NGS was 86.5% (95% CI: 80.9% to 91.4%).18 Given that the success rate for DNA NGS can decrease as the number of genes tested increases,19 the similar performance of the larger DNA NGS panel in our study was encouraging. Our study included an RNA NGS panel of 55 fusion genes. Previously reported RNA NGS assays validated the technique with a smaller sample size and smaller gene panel.14 , 20 , 21 Despite the assay size, the success rate of RNA NGS panel in our study was higher than most previously published studies.21
The high performance for both NGS panels in our study likely relates the laboratory preparation to some degree. Once NGS was validated on cytology specimens in 2015, the cytology laboratory changed processing protocols to routinely make a molecular vial, as described in the methods, at the time of routine processing, which shortens turnaround time. Importantly, our laboratory has found that the rinse material/molecular vial as described in the methods, yielded improved quantity and quality of DNA compared to paraffin embedded tissue (PET) material due to more intact cells and, the alcohol fixation rather than formalin fixation which can cause DNA cross-linking and fragmentation.16 Immediate fixation of RNA also makes it less degradable as RNA can be labile.
During the internal validation process, it was found that samples with low tumor cellularity (< 10%) had high rates of failure. Therefore, only samples with tumor cellularity > 10% are sent for NGS at our center. Our study found that very small portion of the DNA panels (1.9%) reported no mutation using EBUS-TBNA samples. The negative results were likely secondary to the fact that the tumor did not harbor the mutations that were included in the panel, although false negative cannot be ruled out. On the other hand, interestingly, 92.8% of RNA NGS panels reported no fusion genes. RNA NGS panel directly analyses fusion transcripts; hence it is a more accurate way of checking fusion genes compared with DNA panels. Fusions genes are simply not as prevalent in NSCLC, and our results are likely indicative of an expectedly high true negative rate. For example, ALK, ROS1 and rearranged during transfection (RET) genes are a few common fusion genes seen in NSCLC and the prevalence was reported to be 2% to 7%, 1% to 2% and 1% to 2%, respectively.8
Higher N stage lymph nodes (N2 or N3 nodes) were associated with higher success rates of DNA NGS. Similar phenomenon was observed in circulating tumor DNA (ctDNA), as ctDNA concentration in the bloodstream is usually significantly higher in the advanced compared to early-stage lung cancer.22 Future research is needed to look for the mechanism behind the association. Short-axis diameter on CT and FDG-avidity were not associated with an increased success rate of NGS. Uchimura et al reported similar results that there was no significant difference in short-axis diameter of lymph node on CT (14.6 vs. 14.1mm) or FDG-avidity on PET scan(83.8% vs. 69.2% cases with SUVmax ≥2.5) between success and failure cases.20
In general, the recommended minimum tumor cell content is more than 2 times the limit of detection (LOD) in routine mutation testing.23 , 24 The tumor cellularity minimum for NGS in our laboratory is quite low at 10%. Higher tumor cellularity was associated with higher tissue adequacy for DNA NGS panel and the trend was present for RNA NGS, though not statistically significant. Muriana et al commented in their review that tumor cellularity seemed to be the most important factor that influences the yield of NGS.25 Ways to evaluate tumor cellularity immediately, such as rapid on-site cytology or novel cellular imaging at the bedside, are enticing options worthy of future investigation.
Interestingly, our study did not find a difference in the success of samples obtained with > 3 versus ≤3 needle passes. The World Association for Bronchology and Interventional Pulmonology guidelines suggest a total of 4 passes per target be obtained during EBUS whenever molecular testing is planned(Grade 2C recommendation).9 Uchimura and colleagues concluded from their retrospective study that a minimum of 4 passes and 4 pieces of core tissue (defined as thread-like specimen from EBUS-TBNA sampling) were associated with higher success rate of NGS.20 Our data may not have supported the notion of additional needle passes secondary to the high performance of NGS panels and a fairly aggressive agitation protocol. Core tissue was frequently observed, however not documented, and hence not analyzed in the currently study.
Limitations
This is a single center using bronchoscopy specimens that were processed uniformly. The NGS panels were preferably performed on FNA rinse fixed with alcohol. Therefore, the results cannot be generalized to other centers that primarily use formalin-fixed paraffin-embedded (FFPE) samples. The retrospective nature of the study and the fairly uniform procedural technique impacted our ability to analyze more nuanced procedural factors.
Conclusions
The success rates of both DNA and RNA NGS were high using EBUS-TBNA samples. DNA NGS performed better on FNA samples from higher N stage lymph nodes. The effect of tumor cellularity remained borderline statistically significant after multi-variable logistic regression. Other procedural characteristics did not affect NGS success rates.
Clinical Practice Points.
Next generation sequencing (NGS) of a lung cancer specific oncogene panel allows parallel sequencing of hundreds of genetic mutations, requires smaller samples with lower concentrations of malignant cells, and can be finalized more rapidly than sequential multiple single-gene assays.
Bronchoscopy with endobronchial ultrasound-guided transbronchial needle aspiration or fine needle aspiration (EBUS-TBNA or FNA) is the standard of care in diagnosing and staging of non-small cell lung cancer (NSCLC).
FNA samples may represent the sole diagnostic material.
It is important to explore and identify factors that are associated with higher success rates for NGS assays. The current study showed both DNA and RNA extended-panel NGS had high performance when using EBUS-TBNA samples in a real-world setting.
Sampling more advanced nodal stations and obtaining samples with higher tumor cellularity were associated with improved success rates of the DNA NGS assay.
Other imaging or procedural characteristics were not found to be associated with better NGS performance.
Interestingly, more EBUS-TBNA passes per station did not result in a higher success rate for NGS. Further investigation into factors that may increase the success rate of DNA and RNA NGS is needed.
Abbreviations:
- NGS
next generation sequencing
- NSCLC
non-small cell lung cancer
- EBUS-TBNA
endobronchial ultrasound-guided transbronchial needle aspiration
- FNA
fine needle aspiration
- ROSE
rapid on-site evaluation
- FB
flexible Bronchoscopy
- EBBx
endobronchial biopsy
- TBBx
transbronchial biopsy
- IHC
immunohistochemistry stains
- STP
solid tumor panel
- FTP
fusion transcription panel
- PD-L1
programmed death-ligand 1
- EML4-ALK
Echinoderm microtubule-associated protein-like 4 - anaplastic lymphoma kinase
- RET
rearranged during transfection
- ROS1
proto-oncogene tyrosine-protein kinase 1
- FISH
fluorescence in situ hybridization
- CT
computed tomography
- PET
positron emission tomography
- FDG
Fluorodeoxyglucose
- SUVmax
maximum standardized uptake value
- IQR
interquartile range
- OR
Odds Ratio
- CI
confidence interval
- ctDNA
circulating tumor DNA
- FFPE
formalin-fixed paraffin-embedded
Footnotes
Disclosure
Drs Haas and DiBardino have received consulting fees from Olympus America, Inc. Dr Morissette has received speaker honoraria from JADPRO, Novartis, NCCN and PER. She is also an unpaid board member of GOAL and owns long-term personal stock from ThermoFisher. There is no disclosure from other authors of this manuscript.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. Ca Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peravali M, Wang H, Kim C, Veytsman I. Combined Inhibition of EGFR and VEGF pathways in patients with EGFR-mutated non-small cell lung cancer: a systematic review and meta-analysis. Curr Oncol Rep. 2020;22:119. doi: 10.1007/s11912-020-00981-0. [DOI] [PubMed] [Google Scholar]
- 4.Jiang L, Meng X, Zhao X, Xing L, Yu J. Perspective on treatment for unresectable locally advanced non-small cell lung cancer with oncogene-driven mutation: a narrative review. Transl Lung Cancer Res. 2020;9:2137–2144. doi: 10.21037/tlcr-20-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lindeman NI, Cagle PT, Aisner DL, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of american pathologists, the international association for the study of lung cancer, and the association for molecular pathology. J Mol Diagn. 2018;20:129–159. doi: 10.1016/j.jmoldx.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Endris V, Penzel R, Warth A, et al. Molecular diagnostic profiling of lung cancer specimens with a semiconductor-based massive parallel sequencing approach: feasibility, costs, and performance compared with conventional sequencing. J Mol Diagn. 2013;15:765–775. doi: 10.1016/j.jmoldx.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Head SR, Komori HK, LaMere SA, et al. Library construction for next-generation sequencing: overviews and challenges. Biotechniques. 2014;56:61–64. doi: 10.2144/000114133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruno R, Fontanini G. Next generation sequencing for gene fusion analysis in lung cancer: a literature review. Diagnostics (Basel). 2020;10:521. doi: 10.3390/diagnostics10080521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Heijden EH, Casal RF, Trisolini R, et al. Guideline for the acquisition and preparation of conventional and endobronchial ultrasound-guided transbronchial needle aspiration specimens for the diagnosis and molecular testing of patients with known or suspected lung cancer. Respiration. 2014;88:500–517. doi: 10.1159/000368857. [DOI] [PubMed] [Google Scholar]
- 10.Dietel M, Bubendorf L, Dingemans AM, et al. Diagnostic procedures for non-small-cell lung cancer (NSCLC): recommendations of the European Expert Group. Thorax. 2016;71:177–184. doi: 10.1136/thoraxjnl-2014-206677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy-Chowdhuri S, Aisner DL, Allen TC, et al. Biomarker testing in lung carcinoma cytology specimens: a perspective from members of the pulmonary pathology society. Arch Pathol Lab Med. 2016;140:1267–1272. doi: 10.5858/arpa.2016-0091-SA. [DOI] [PubMed] [Google Scholar]
- 12.Hagemann IS, Devarakonda S, Lockwood CM, et al. Clinical next-generation sequencing in patients with non-small cell lung cancer. Cancer. 2015;121:631–639. doi: 10.1002/cncr.29089. [DOI] [PubMed] [Google Scholar]
- 13.Turner SR, Buonocore D, Desmeules P, et al. Feasibility of endobronchial ultrasound transbronchial needle aspiration for massively parallel next-generation sequencing in thoracic cancer patients. Lung Cancer. 2018;119:85–90. doi: 10.1016/j.lungcan.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Luca C, Pepe F, Iaccarino A, et al. RNA-based assay for next-generation sequencing of clinically relevant gene fusions in non-small cell lung cancer. Cancers (Basel). 2021:13. doi: 10.3390/cancers13010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest. 2017;151:193–203. [DOI] [PubMed] [Google Scholar]
- 16.Wei S, Lieberman D, Morrissette JJ, Baloch ZW, Roth DB, CJCc McGrath. Using “residual” FNA rinse and body fluid specimens for next-generation sequencing: an institutional experience. Cancer Cytopathology. 2016;124:324–329. doi: 10.1002/cncy.21666. [DOI] [PubMed] [Google Scholar]
- 17.Hofman MS, Hicks RJ. How we read oncologic FDG PET/CT. Cancer Imaging. 2016;16:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao JJ, Chan HP, Soon YY, Huang Y, Soo RA, Kee ACL. A systematic review and meta-analysis of the adequacy of endobronchial ultrasound transbronchial needle aspiration for next-generation sequencing in patients with non-small cell lung cancer. Lung Cancer. 2022;166:17–26. doi: 10.1016/j.lungcan.2022.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Stoy SP, Segal JP, Mueller J, et al. Feasibility of endobronchial ultrasound-guided transbronchial needle aspiration cytology specimens for next generation sequencing in non-small-cell lung cancer. Clin Lung Cancer. 2018;19:e232. doi: 10.1016/j.cllc.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Uchimura K, Yanase K, Imabayashi T, et al. The impact of core tissues on successful next-generation sequencing analysis of specimens obtained through endobronchial ultrasound-guided transbronchial needle aspiration. Cancers (Basel). 2021:13. doi: 10.3390/cancers13235879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murakami S, Yokose T, Nemoto D, et al. Suitability of bronchoscopic biopsy tissue samples for next-generation sequencing. Diagnostics (Basel). 2021:11. doi: 10.3390/diagnostics11030391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pisapia P, Costa JL, Pepe F, et al. Next generation sequencing for liquid biopsy based testing in non-small cell lung cancer in 2021. Crit Rev Oncol Hematol. 2021;161. doi: 10.1016/j.critrevonc.2021.103311. [DOI] [PubMed] [Google Scholar]
- 23.Wong NA, Gonzalez D, Salto-Tellez M, et al. RAS testing of colorectal carcinoma—a guidance document from the association of clinical pathologists molecular pathology and diagnostics group. Practice Guideline. 2014;67:751–757. doi: 10.1136/jclinpath-2014-202467. [DOI] [PubMed] [Google Scholar]
- 24.Li MM, Datto M, Duncavage EJ, et al. Standards and guidelines for the inter-pretation and reporting of sequence variants in cancer: a joint consensus recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diag. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muriana P, Rossetti FJM. The role of EBUS-TBNA in lung cancer restaging and mutation analysis. Mediastinum. 2020;4:23. doi: 10.21037/med-20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


