Abstract
This review article provides a concise guide to interpreting receiver operating characteristic (ROC) curves and area under the curve (AUC) values in diagnostic accuracy studies. ROC analysis is a powerful tool for assessing the diagnostic performance of index tests, which are tests that are used to diagnose a disease or condition. The AUC value is a summary metric of the ROC curve that reflects the test’s ability to distinguish between diseased and nondiseased individuals. AUC values range from 0.5 to 1.0, with a value of 0.5 indicating that the test is no better than chance at distinguishing between diseased and nondiseased individuals. A value of 1.0 indicates perfect discrimination. AUC values above 0.80 are generally consideredclinically useful, while values below 0.80 are considered of limited clinical utility. When interpreting AUC values, it is important to consider the 95% confidence interval. The confidence interval reflects the uncertainty around the AUC value. A narrow confidence interval indicates that the AUC value is likely accurate, while a wide confidence interval indicates that the AUC value is less reliable. ROC analysis can also be used to identify the optimal cutoff value for an index test. The optimal cutoff value is the value that maximizes the test’s sensitivity and specificity. The Youden index can be used to identify the optimal cutoff value. This review article provides a concise guide to interpreting ROC curves and AUC values in diagnostic accuracy studies. By understanding these metrics, clinicians can make informed decisions about the use of index tests in clinical practice.
Keywords: Area under the curve, diagnostic study, receiver operating characteristic analysis, receiver operating characteristic curve
Introduction
Diagnostic accuracy studies are a cornerstone of medical research. When evaluating novel diagnostic tests or repurposing existing ones for different clinical scenarios, physicians assess test efficacy, which is referred to as index tests in diagnostic accuracy analyses. Index tests can encompass a variety of elements, such as serum markers derived from blood samples, radiological imaging, specific clinical findings, or clinical decision rules. Diagnostic studies assess the index test’s diagnostic performance by reporting specific metrics, such as sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and accuracy. These metrics are compared to the gold standard reference test. Diagnostic ability encompasses not only the index test’s diagnostic prowess (specificity, PPV, and PLR) but also its ability to distinguish healthy individuals from those with the targeted condition (sensitivity, NPV, and NLR).[1-4]
Two Types of Diagnostic Studies
There are two main types of diagnostic studies in medicine: two-by-two tables and receiver operating characteristic (ROC) analysis. The choice between these depends on whether the index test yields dichotomous or continuous results.
Diagnostic Accuracy Studies with Dichotomous Index Test Results
The two-by-two table is used when both the index test and reference test results are dichotomous. As shown in Table 1, sensitivity, specificity, PPV, NPV, PLR, and NLR are calculated based on the data in the table’s four cells. True positive fraction (TPF) and False positive fraction (FPF) are two other important parameters that have a diagnostic character in cases where the index test is positive. TPF reflects the index test’s accuracy in detecting disease (and is equivalent to sensitivity), while FPF gauges the index test’s positivity in nondiseased individuals (and is equivalent to 1 – specificity).[5] In cases where the reference test is also dichotomous, but the index test yields continuous numerical results, the diagnostic study method used is the ROC analysis.[6-8] While the ROC curve and the resultant area under the curve (AUC) offer a concise summary of the index test’s diagnostic utility, clinicians may encounter challenges in interpreting these values. This concise review aims to guide clinicians through the interpretation of ROC curves and AUC values when presenting findings from their diagnostic accuracy studies.
Table 1.
Two-by-two table and calculating parameters of diagnostic test performance
| Index test | Reference test | Total | |
|---|---|---|---|
|
| |||
| Diseased | Nondiseased | ||
| Positive | a (true positive) | b (false positive) | a + b |
| Negative | c (false negative) | d (true negative) | c + d |
| Total | a + c | b + d | |
Summary parameters of diagnostic test performance: Sensitivity=a/(a + c), specificity=d/(b + d), PPV=a/(a + b), NPV=d/(c + d), TPF=Sensitivity=a/(a + c), FPF=1 − specificity=d/(b + d). PPV: Positive predictive value, NPV: Negative predictive value, TPF: True positive fraction, FPF: False-positive fraction
Diagnostic Accuracy Studies with Numerical Index Test Results
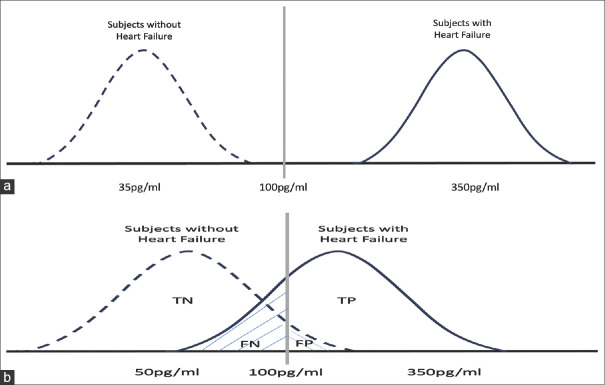
In cases where the index test yields a dichotomous outcome (a single cutoff value), the two-by-two table is sufficient, as discussed earlier. However, when the index test generates continuous (or occasionally ordinal) outcomes, multiple potential cutoff values emerge. Selecting the optimal cutoff value, especially for novel diagnostic tests, poses challenges. With continuous numerical outcomes, diagnostic accuracy studies yield distinct distributions of test results for both diseased and nondiseased groups.[9] For example, a diagnostic accuracy study evaluating B-type natriuretic peptide (BNP) blood levels in diagnosing heart failure could yield the following distributions:
An ideal diagnostic test would yield sensitivity and specificity of 100%, resulting in nonoverlapping BNP distribution graphs for individuals with and without heart failure [Figure 1a]
However, real-world scenarios tend to involve overlapping distributions [Figure 1b].
Figure 1.
Two different BNP distribution graphs of the subjects groups with and without heart failure. TN: true negative, TP: true positive, FN: false negative, FP: false positive (a) An ideal diagnostic test would yield sensitivity and specificity of 100%, resulting in non-overlapping BNP distribution graphs for individuals with and without heart failure. (b) real-world scenarios tend to involve overlapping distributions; sensitivity and specificity values are not 100%
Receiver Operating Characteristic Analysis and Receiver Operating Characteristic Curve
ROC analysis involves dichotomizing all index test outcomes into positive (indicative of disease) and negative (nondisease) based on each measured index test value. For instance, if a measured BNP result is 235 pg/ml, ROC analysis would classify all values exceeding 235 as positive and the rest as negative. Relevant diagnostic performance metrics (sensitivity, specificity, PPV, NPV, PLR, and NLR) are then calculated, mirroring the two-by-two table methodology. This process is repeated for all measured values within the ROC analysis. This approach enables the presentation and examination on of these metrics as a table, followed by 33 the graphical depiction of this table, termed the ROC 34 curve [Figure 2].[10-12] The ROC curve plots TPF (sensitivity) and FPF (1 – specificity) values for each index test outcome on an x-y coordinate graph. This curve results from combining coordinate points from each outcome. The diagonal reference line at a 45° angle signifies the diagnostic test’s discriminative power akin to random chance. The upper left corner corresponds to perfect discriminatory power, represented by a TPF of 1 and an FPF of 0 (where sensitivity and specificity both attain 100%).
Figure 2.
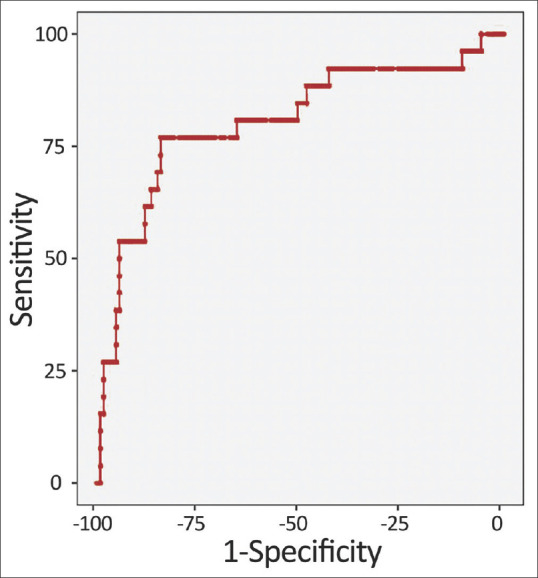
Receiver Operating Characteristic (ROC) Curve
Area under the Curve Value and Interpretation
The AUC value is a widely used metric in clinical studies, succinctly summarizing index test diagnostic performance. The AUC value signifies the likelihood that the index test will categorize a randomly selected subject from a sample as a patient more accurately than a nonpatient. AUC values range from 0.5 (equivalent to chance) to 1 (indicating perfect discrimination).[13]
AUC values serve as a gauge for the index test’s ability to distinguish disease. An AUC value of 1 signifies flawless discernment, while an AUC of 0.5 indicates performance akin to random chance. New researchers often make errors when interpreting the AUC value in diagnostic accuracy studies. This is usually due to an overestimation of the clinical interpretation of the AUC value. For example, an AUC value of 0.65, calculated in a study of the diagnostic performance of an index test, means that the test is not clinically adequate. However, some researchers make the inference that the test is a clinically useful diagnostic test by only looking at statistical significance. In diagnostic value studies, AUC values above 0.90 are interpreted as indicating a very good diagnostic performance of the test, while AUC values below 0.80, even if they are statistically significant, are interpreted as indicating a very limited clinical usability of the test. The classification table of AUC values and their clinical usability is presented in Table 2.
Table 2.
Area under the curve values and its interpretation
| AUC value | Interpretation suggestion |
|---|---|
| 0.9 ≤ AUC | Excellent |
| 0.8 ≤ AUC < 0.9 | Considerable |
| 0.7 ≤ AUC < 0.8 | Fair |
| 0.6 ≤ AUC < 0.7 | Poor |
| 0.5 ≤ AUC < 0.6 | Fail |
AUC: Area under the curve
Notably, attention to the 95% confidence interval and its width, alongside the AUC value, is pivotal in comprehending diagnostic performance.[13,14] For instance, a BNP marker’s AUC value of 0.81 might be tempered by a confidence interval spanning 0.65–0.95. In this scenario, reliance solely on an AUC value above 0.80 may be unwise, given the potential for outcomes below 0.70. Thus, calculating sample size and mitigating type-2 error risk prove vital prerequisites before undertaking diagnostic studies.[15]
A common mistake made at this point is that when two different index tests are wanted to be compared, the index tests are made by considering only the mathematical differences of the single AUC values from each other. This decision should be made not only with the mathematical difference but also by considering whether this mathematical difference is statistically significant. The most common statistical method used to statistically compare the AUC values of different index tests is the De-Long test.
Determination of Optimal Cutoff Value
ROC analysis also facilitates the identification of an optimal cutoff value, particularly when the AUC value surpasses 0.80. The Youden index, often employed, determines the threshold value that maximizes both sensitivity and specificity. This index, calculated as sensitivity + specificity – 1, aids in selecting a threshold where both metrics achieve their peak. Nonetheless, alternative thresholds might be chosen based on cost-effectiveness or varying clinical contexts, prioritizing either sensitivity or specificity.
Conclusion
Studies employing ROC analysis follow reporting guidelines, such as the Standards for Reporting Diagnostic Accuracy Studies (STARD) guideline. The STARD guideline also states that when reporting the diagnostic performance of an index test, not only sensitivity and specificity parameters should be reported, but also NLR and PLR values.[16] However, certain statistical programs might only report sensitivity and specificity parameters in ROC analysis. Therefore, when an AUC value above 0.80 is attained, generating a two-by-two table based on the chosen optimal threshold and reporting all relevant metrics becomes imperative.
Conflicts of interest
None Declared.
Funding
None.
References
- 1.Knottnerus JA, Buntinx F. 2nd ed. Singapore: Wiley-Blackwell BMJ Books; 2009. The Evidence Base of Clinical Diagnosis: Theory and Methods of Diagnostic Research. [Google Scholar]
- 2.Guyatt G. 3rd ed. New York: McGraw-Hill Education; 2015. Users’Guides to the Medical Literature: A Manual for Evidence-Based Clinical Practice. [Google Scholar]
- 3.Akobeng AK. Understanding diagnostic tests 1: Sensitivity, specificity and predictive values. Acta Paediatr. 2007;96:338–41. doi: 10.1111/j.1651-2227.2006.00180.x. [DOI] [PubMed] [Google Scholar]
- 4.Akobeng AK. Understanding diagnostic tests 2: Likelihood ratios, pre- and post-test probabilities and their use in clinical practice. Acta Paediatr. 2007;96:487–91. doi: 10.1111/j.1651-2227.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 5.Nahm FS. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J Anesthesiol. 2022;75:25–36. doi: 10.4097/kja.21209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–7. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 7.Altman DG, Bland JM. Diagnostic tests 3: Receiver operating characteristic plots. BMJ. 1994;309:188. doi: 10.1136/bmj.309.6948.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar R, Indrayan A. Receiver operating characteristic (ROC) curve for medical researchers. Indian Pediatr. 2011;48:277–87. doi: 10.1007/s13312-011-0055-4. [DOI] [PubMed] [Google Scholar]
- 9.Hajian-Tilaki K. Receiver operating characteristic (ROC) curve analysis for medical diagnostic test evaluation. Caspian J Intern Med. 2013;4:627–35. [PMC free article] [PubMed] [Google Scholar]
- 10.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 11.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 12.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–6. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 13.Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: Clinical example of sepsis. Intensive Care Med. 2003;29:1043–51. doi: 10.1007/s00134-003-1761-8. [DOI] [PubMed] [Google Scholar]
- 14.Tosteson TD, Buonaccorsi JP, Demidenko E, Wells WA. Measurement error and confidence intervals for ROC curves. Biom J. 2005;47:409–16. doi: 10.1002/bimj.200310159. [DOI] [PubMed] [Google Scholar]
- 15.Akoglu H. User's guide to sample size estimation in diagnostic accuracy studies. Turk J Emerg Med. 2022;22:177–85. doi: 10.4103/2452-2473.357348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]