Abstract
Background
Nicotine dependence is a key factor influencing the diversity of gut microbiota, and targeting gut microbiota may become a new approach for the prevention and treatment of nicotine dependence. However, the causal relationship between the two is still unclear. This study aims to investigate the causal relationship between nicotine dependence and gut microbiota.
Methods
A two-sample bidirectional Mendelian randomization (MR) study was conducted using the largest existing gut microbiota and nicotine dependence genome-wide association studies (GWAS). Causal relationships between genetically predicted nicotine dependence and gut microbiota abundance were examined using inverse variance weighted, MR-Egger, weighted median, simple mode, weighted mode, and MR-PRESSO approaches. Cochrane’s Q test, MR-Egger intercept test, and leave-one-out analysis were performed as sensitivity analyses to assess the robustness of the results. Multivariable Mendelian randomization analysis was also conducted to eliminate the interference of smoking-related phenotypes. Reverse Mendelian randomization analysis was then performed to determine the causal relationship between genetically predicted gut microbiota abundance and nicotine dependence.
Results
Genetically predicted nicotine dependence had a causal effect on Christensenellaceae (β: -0.52, 95% CI: -0.934–0.106, P = 0.014). The Eubacterium xylanophilum group (OR: 1.106, 95% CI: 1.004-1.218), Lachnoclostridium (OR: 1.118, 95% CI: 1.001-1.249) and Holdemania (OR: 1.08, 95% CI: 1.001-1.167) were risk factors for nicotine dependence. Peptostreptococcaceae (OR: 0.905, 95% CI: 0.837-0.977), Desulfovibrio (OR: 0.014, 95% CI: 0.819-0.977), Dorea (OR: 0.841, 95% CI. 0.731-0.968), Faecalibacterium (OR: 0.831, 95% CI: 0.735-0.939) and Sutterella (OR: 0.838, 95% CI: 0.739-0.951) were protective factor for nicotine dependence. The sensitivity analysis showed consistent results.
Conclusion
The Mendelian randomization study confirmed the causal link between genetically predicted risk of nicotine dependence and genetically predicted abundance of gut microbiota. Gut microbiota may serve as a biomarker and offer insights for addressing nicotine dependence.
Keywords: gut microbiota, Mendelian randomization, nicotine dependence, causality, genetics
Introduction
The use of tobacco products is responsible for the deaths of nearly 8 million people worldwide each year (1) and is a significant contributor to lung cancer and cardiovascular disease (2). Although many people are aware of the detrimental effects associated with smoking, the presence of nicotine, a highly addictive substance in tobacco products makes it difficult for individuals to quit (3, 4). Nicotine is known to reinforce smoking and tobacco use behaviors that establish and sustain nicotine dependence. The majority of smokers require some form of assistance to quit, as only approximately 4% of smokers are able to quit on their own successfully (1). Nicotine dependence often presents with physical manifestations, including an increase in tolerance, withdrawal symptoms, and reduced ability to control behavior.
Nicotine is quickly absorbed via the oral mucosa and respiratory tract (5), thereby increasing the risk of related cardiovascular, respiratory, and gastrointestinal diseases (6). Smoking also increases the likelihood of developing gastrointestinal diseases, notably inflammatory bowel disease (7), irritable bowel syndrome (8), peptic ulcer disease (9), and gastrointestinal cancer (10). In addition, smoking heightens the risk of gastrointestinal infections (11), including Helicobacter pylori (12).
Gut microbiota comprises a diverse array of microorganisms that colonize the mammalian gut, including bacteria, fungi, archaea, viruses, and parasites (13). The two-way communication between gut microbes and their hosts may influence many immunity- and metabolism-related biological systems, thereby impacting host health (14). Enhancing host immunity is an important function of the gut microbiota (15). The gut microbiota competes for limited nutrients and regulates host immunity to suppress the colonization of exogenous pathogenic microorganisms (16–18). Additionally, the effects of these immune reactions can extend to almost all parts of the human body (15). When there is an imbalance in the gut microbiota and impaired intestinal barrier function, an increase in harmful pathogenic microorganisms may further induce the occurrence and development of diseases, such as hypertension (19), autoimmune hepatitis (20), cancer (21), and others.
Smoking can modify the microbiome in several regions (22), including the periodontal, intestinal, and respiratory tracts, and augments the mechanisms whereby changes in mucosal immune responses, fluctuations in intestinal cytokine levels, alteration in intestinal permeability, and epigenetic modification alter gene expression (23, 24). Prebiotics are undigestible food elements that can selectively promote the growth and function of the colonic microbiota, ultimately improving host health (25). Supplementation of probiotics and the reconstruction of a healthy microbiota in the gut are now considered effective strategies for treating diseases caused by gut microbiota dysbiosis (26–28). Therefore, using appropriate prebiotics to target specific microbial communities may be an effective approach for preventing and treating nicotine dependence. However, the causal relationship and mechanisms between gut microbiota and nicotine dependence are still unclear, which poses obstacles to the prevention and treatment of nicotine dependence. Thus, it is imperative to study the causal link between the gut microbiota and nicotine dependence.
The Mendelian randomization (MR) method is an epidemiological technique (29) that employs genetic variation as an instrumental variable to explore the putative causal effects of exposure on the onset of disease. Building upon the recent large-scale genome-wide association studies (GWAS) on the gut microbiota (30–32) and disease, we employed the Mendelian randomization approach to investigate the causal link between the gut microbiota and the risk of nicotine dependence in this study. This study aims to explore the impact of genetic prediction of nicotine dependence on the gut microbiota, and elucidate the role of the gut microbiota in the pathogenesis of nicotine dependence through genetic prediction. Furthermore, it aims to uncover the potential of genetic prediction of the gut microbiota to aid in the development of novel preventive strategies.
Methods
Study design
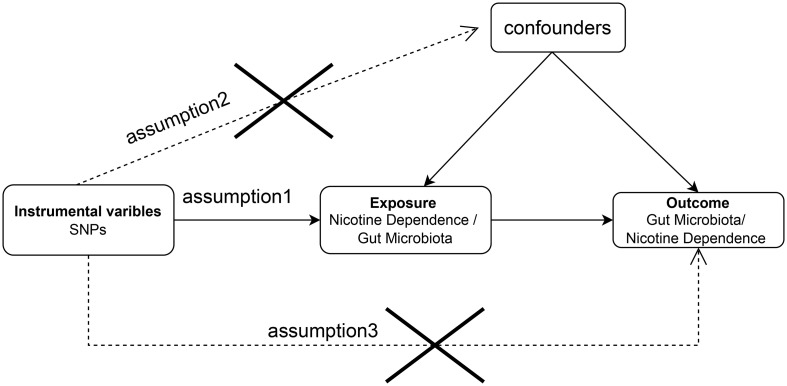
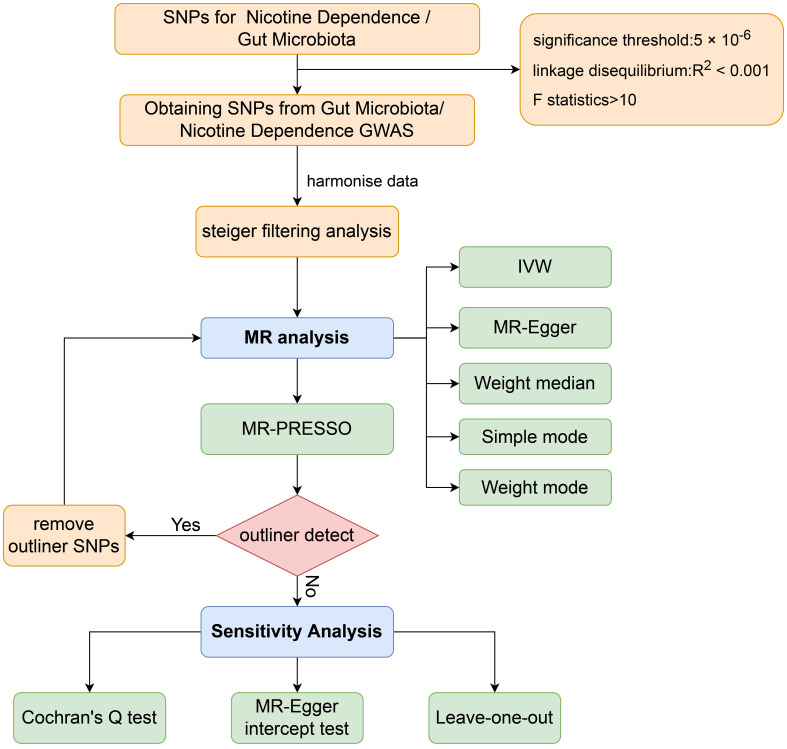
The aim of this study was to evaluate the causal relationship between genetically predicted nicotine dependence risk and genetically predicted abundance of gut microbiota using a Mendelian randomization method. The Mendelian randomization design consisted of three components. Firstly, the selection of genetic variants as instrumental variables for nicotine dependence. Secondly, the acquisition of a summary dataset for genetic instruments derived from a genome-wide association study of nicotine dependence, and finally, obtaining a summary dataset for single nucleotide polymorphism results. These results were used to investigate the impact of GWAS genetic instruments on gut microbiota. Figure 1 outlines the design of the Mendelian randomization study, while Figure 2 presents an overview of the investigation along with a flow chart. The research design of this study follows the reporting guidelines of STROBE-MR (33), and the supplementary files include the checklist based on STROBE-MR and the checklist based on Critical Appraisal Checklist for evaluating Mendelian randomization studies (34). The checklist is elaborated in detail in the supplementary materials.
Figure 1.
Bidirectional two-sample Mendelian randomization between nicotine dependence and gut microbiota abundance outcomes. Directed acyclic graph (DAG) of the causal relationship between nicotine dependence and gut microbiota abundance.
Figure 2.
Workflow of Mendelian randomization study revealing causality between gut microbiota abundance and risk of nicotine dependence. SNP, single-nucleotide polymorphism; IVW, inverse variance weighted; MR, Mendelian randomization; MR-PRESSO, MR Pleiotropy Residual Sum and Outlier.
GWAS summary data for nicotine dependence
This study used the genome-wide association meta-analysis data from Hancock DB et al. to investigate nicotine dependence risk (35). The authors conducted a genome-wide meta-analysis on 38,602 former smokers of European and African American descent with mild (N = 17,796; 46.1%), moderate (N = 13,527; 35%), or severe (N = 7,279; 18.9%) nicotine dependence. Genotyping was performed on various genome-wide platforms, and after quality control, 1000G genomic interpolation was used to analyze the genotype data. Linear regression was carried out on the data and adjusted for age, sex, pedigree principal components, and cohort-specific covariates. The Genome-wide association study (GWAS) results were combined using METAL with fixed-effects inverse variance weighting meta-analysis, across all studies with FTND data to maximize statistical power. More than 99% of the former smokers were over 18 years old, and in case of the presence of relatives, family structure was adjusted. Additional information can be found in Table S1 .
GWAS summary data for gut microbiota
Data on the composition of human gut microbiota were obtained from the MiBioGen consortium through a large-scale multi-ethnic GWAS study (36). This study involved 18,340 participants from 24 cohorts in countries such as the United States, Canada, Israel, Korea, Germany, Denmark, the Netherlands, Belgium, Sweden, Finland, and the UK. The participants’ 16S ribosomal RNA gene sequences and genotyped data were analyzed to investigate the relationship between human autosomal genetic variation and gut microbial communities. The study included 211 taxa comprising 35 families, 20 orders, 16 phyla, 9 orders, and 131 genera.
GWAS summary data for smoking-related phenotypes
The GWAS data for smoking-related phenotypes were obtained from a meta-analysis of GWAS summary association data from 1,232,091 individuals predominantly of European ancestry (37). The smoking phenotypes included age of smoking initiation, smoking initiation, cigarettes per day, and smoking cessation. The authors applied extensive genetic quality control and filtering to the summary statistics provided by each cohort. Imputed variants with an imputation quality below 0.3 (estimated squared correlation between imputed and true dosage) were subsequently removed. Then, the allele labels and allele frequencies of each study were compared with those of the imputation reference panel, and discrepancies were either removed or harmonized. Finally, a meta-analysis was conducted using the software package rareGWAMA based on a fixed-effect model.
Selection of instrumental variables
This study aimed to explore the causal relationship between nicotine dependence and gut microbiota through the Mendelian Randomization analysis of instrumental variables. First, single nucleotide polymorphisms (SNPs) with a genome-wide significance threshold (5 × 10-8) were selected as instrumental variables (IVs) relating to nicotine dependence. After linkage disequilibrium analysis (R2 < 0.001, clumping distance = 10,000 kb), only one SNP was retained. To ensure a satisfactory number of IVs, a significance threshold of 5 × 10-6 for SNP versus nicotine-dependent phenotypes and a minor allele frequency (MAF) threshold of 0.01 were set. Additional linkage disequilibrium analysis (R2 < 0.001, clumping distance = 10,000 kb) was conducted on the European 1000 Genomes Project data to screen out instrumental variables that could cause biased results.
To assess the potential causal influence of gut microbiota on nicotine dependence, we analyzed genome-wide association data of gut microbes at five taxa levels: order, class, family, genus, and phylum, defining each taxon as a trait. We implemented quality control steps to select the most suitable instrument and ensure the reliability and accuracy of conclusions regarding the causal relationship between the gut microbiome and nicotine risk. Firstly, we selected snps with significance below the genome-wide statistical threshold (5 × 10-8), but this provided few eligible IVs. Therefore, we lowered the threshold to P<5×10-6, which is more comprehensive. We then used a MAF threshold of 0.01 for variants of interest and performed an LD analysis (R2 < 0.001, with a clumping distance of 10,000 kb) to evaluate LD among the included snps.
We evaluated the strength of instrumental variables by computing the F-statistic as F = R2 × (N - 2)/(1 - R2), where R2 represents the proportion of variation in the exposure factor clarified by each instrumental variable while N represents the sample size for the GWAS that relates to the exposure (38). In turn, R2 is calculated as (2 x EAF x (1 - EAF) x beta2)/[(2 x EAF x (1 - EAF) x beta2) + (2 x EAF x (1 - EAF) x N x SE(beta)2)], where EAF is the effect allele frequency, beta is the estimated genetic effect of the exposure factor, N is the GWAS sample size for the SNP-exposure correlation, and SE (beta) refers to the genetic effect’s standard error (39). Instrumental variables having a F-statistic <10 for weak instruments may suggest a possible bias and need to be removed. Meanwhile, those having a F-statistic >10 are included for further analysis.
Steiger filtering analysis (40) was further used to determine the directional effect of individual instrumental variable SNPs on the outcome. A “TRUE” result predicts the expected direction of association. SNPs that are shown as “False” in the Steiger filtering analysis will be excluded and not included in the subsequent Mendelian randomization analysis.
Statistics analysis
Statistical analyses were performed using R software version 4.2.2, utilizing the R packages “TwoSampleMR” (v.0.5.6) (40), “MRPRESSO” (v.1.0) (41), and “MendelianRandomization” (42) (v.0.7.0) in order to carry out a Mendelian randomization (MR) analysis on the causal relationship between nicotine dependence and gut microbiota. Statistical significance was determined at p<0.05 to establish causality.
A multiple test significance threshold was set at 0.05/n (where n represents the number of independent bacterial taxa at the corresponding taxonomic level) due to the numerous comparisons that took place at each character level, such as phylum, class, order, family, and genus. Significance values that fell between the multiple test significance threshold and 0.05 were considered potentially significant.
Two-sample Mendelian randomization
The primary analysis used inverse variance-weighted (IVW) to explore the potential causal relationship between gut microbiota abundance and nicotine dependence. The IVW method is widely applied in Mendelian randomization studies and provides reliable causal estimates in the absence of horizontal pleiotropy. IVW method, namely the meta-analysis of the variant-specific Wald ratios of each variant (i.e., the beta coefficient of the exposure SNP divided by the beta coefficient of the outcome SNP) (43), is used to provide a combined estimate of the causal estimates for each SNP in each potential direction of effect. The IVW method assumes independence of genetic variation and serves as an effective tool for instrumental variable analysis. However, it may ignore the mediating effects of other risk factors or potential pleiotropy, and bias may occur when there is horizontal pleiotropy between instrumental SNPs (44). In addition to the exposure, it may also affect the outcome of interest through causal pathways, resulting in a violation of the instrumental variable assumption of Mendelian randomization. Therefore, we additionally applied the methods of weighted median, MR-Egger, simple mode, and weighted mode. Based on the assumption of Instrument strength independent of direct effect (InSIDE), the MR-Egger regression method conducts weighted linear regression to generate consistent causal effect estimates independent of IV effectiveness (44). However, the MR-Egger regression method has relatively poor accuracy and is susceptible to the influence of peripheral genetic variation (45). The weighted median method can achieve unbiased estimation of the effect, which does not rely on the InSIDE assumption and thus holds significant advantages over the MR-Egger regression method (46). Specifically, it is an excellent alternative method that allows stable estimation of the causal effect when the weight of the causal effect calculated by effective instrumental variables exceeds 50%, while providing lower Type I error. Finally, the weighted mode method was employed to assess the overall causal effect of a large number of genetic instruments. In many cases, this method yields lower Type I error, less bias, and lower computational complexity compared to the primary methods (47).
Sensitivity analysis
We conducted several sensitivity analyses consisting of tests such as Cochran’s Q statistic, funnel plots, leave-one-out analysis, and the MR-Egger intercept test. Cochran’s Q test revealed heterogeneity in the instrumental variables in case the p-value was lower than 0.05. The “leave-one-out” method was applied to validate the causal relationship between nicotine dependency and gut microbiota abundance. The fluctuations observed in results before and after SNP removal demonstrate the stability of the causal association between the exposure variable and the outcome. In the MR-Egger intercept test, a non-zero intercept reflects the presence of directional pleiotropy and represents the mean pleiotropic effect of genetic variation (44).
For detecting and correcting pleiotropic outliers, we employed the mendelian randomized pleiotropic residuals and outliers (MR-PRESSO) method (48). The method tested for overall heterogeneity through regressing SNP-outcome associations on SNP-exposure associations. The observed distance of each SNP from the regression was then matched with the expected distance under the original hypothesis of no pleiotropy. Upon detecting outliers in the MR-PRESSO analysis, we removed them and repeated the Mendelian randomization analysis mentioned above.
Multivariable Mendelian randomization
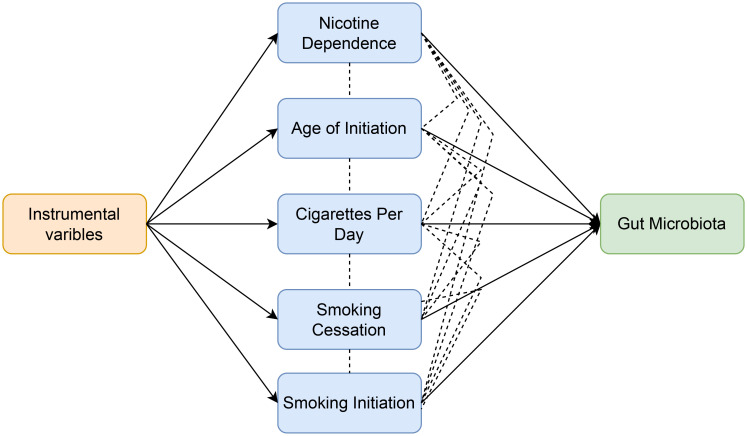
In order to evaluate the moderating effect of smoking-related phenotypes on the causal relationship between nicotine dependence and gut microbiota, SNPs related to smoking-related phenotypes and nicotine dependence were extracted and selected as instrumental variables (IVs) for Multivariable Mendelian randomization ( Figure 3 ). The GWAS p-value threshold between SNPs and phenotypes was set at 5 × 10−6. A block window of 10,000 kb and r2 = 0.001 were chosen to remove linkage disequilibrium. Cross-instrumental variables were harmonized with the outcome to obtain adjusted assessments for causal effects. A multivariable random-effect IVW model and MR-egger model were constructed in Multivariable Mendelian randomization. Statistical significance was determined at p<0.05 to establish causal relationships.
Figure 3.
Workflow of multivariable Mendelian randomization study revealing causal effect of nicotine dependence on gut microbiota abundance by evaluating the moderating effect of smoking-related phenotypes.
Reverse Mendelian randomization analysis
To investigate whether gut microbiota abundance is associated with the risk of nicotine dependence, we conducted a reverse Mendelian randomization (MR) analysis using SNPs related to gut microbiota abundance as instrumental variables (where gut microbiota is the exposure and nicotine dependence risk is the outcome).
Standard protocol approval, registration and patient consent
The GWAS data used in this study were all from publicly available databases. The summary statistics of nicotine dependence, smoking-related phenotypes and gut microbiota abundance do not contain any personal information, and each GWAS has received ethical approval from the relevant ethical review board.
Results
Causal effect of nicotine dependence on gut microbiota
17 SNPs met the instrumental variable screening criteria for nicotine dependence, and all had an F-statistic >10 indicating no weak instrumental bias ( Table S2 ). The F-statistic for the instrumental variable lies between 20.91 and 62.95. In addition, Steiger filtering analysis helped to exclude SNPs with reverse causal directions (three from genus Victivallis and one from genus Prevotella9) ( Table S3 ). As the summary data for SNP results were not extracted for the genera Erysipelotrichaceae UCG003, Lachnospira and Blautia, a mendelian randomization analysis was carried out for the outcomes, including a combined total of 208 gut microbiota classifications to investigate the relationship between nicotine dependence and gut microbiota ( Table S4 ), namely 16 classes, 128 genera, 35 families, 20 orders, and 9 phyla.
The significance thresholds for multiple comparisons at different taxon levels were set at: phylum (p = 5.560×10-3), class (p = 3.125×10-3), order (p = 2.500×10-3), family (p = 1.429×10-3), and genus (p = 3.906×10-4), with adjusted P-values based on Bonferroni correction.
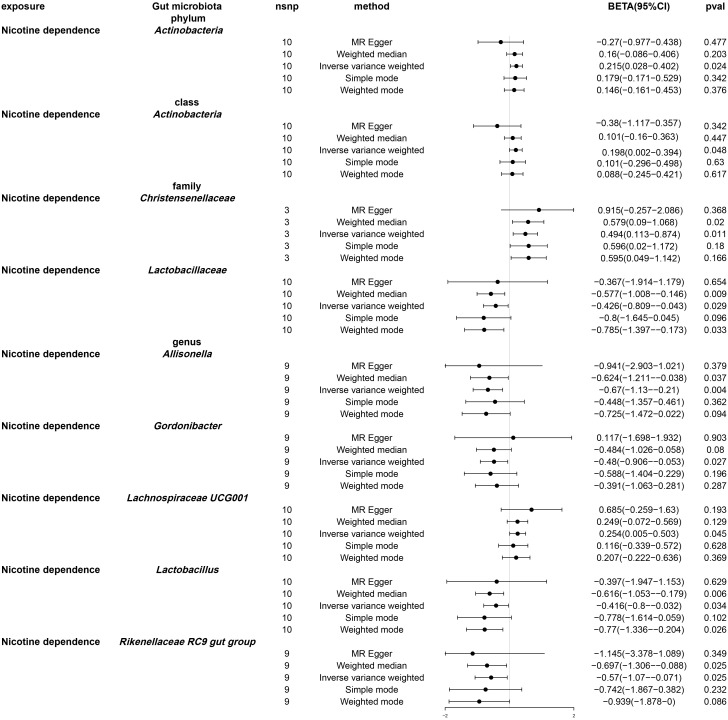
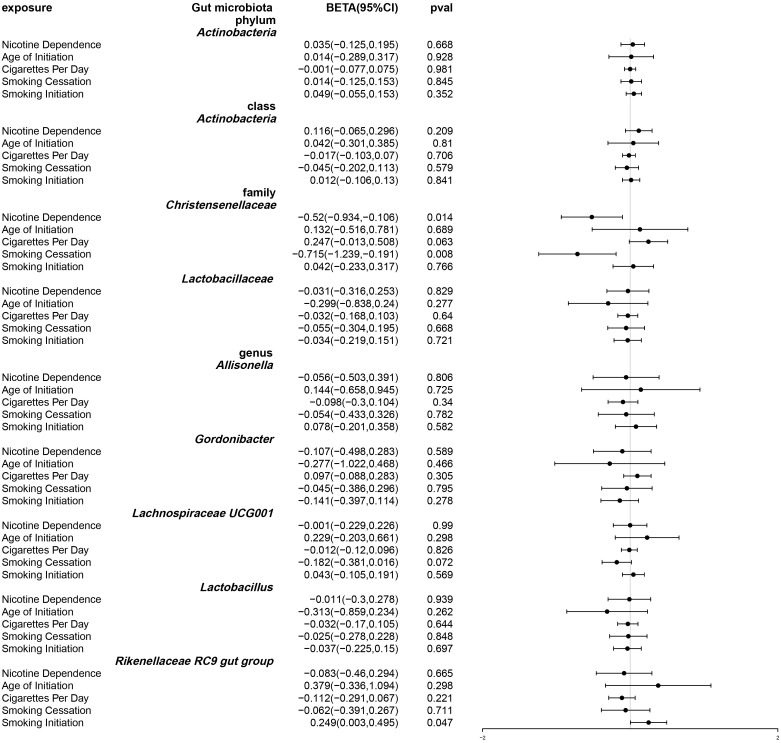
Following univariable Mendelian randomization analysis, a potential causal effect of nicotine dependence on the abundance of five genera, two families, one phylum, and one class was found ( Figure 4 ). According to the results of mendelian randomization analysis based on the IVW method, nicotine dependence caused a causal effect on the abundance of Actinobacteria, Christensenellaceae (beta: 0.494, 95% CI: 0.113-0.874, P = 0.011) and Lachnospiraceae UCG001 (beta. 0.254, 95% CI: 0.005-0.503, P =0.045) increased in abundance, where the causal effect of nicotine dependence on Actinobacteria was consistent at both the phylum (beta: 0.215, 95% CI: 0.028-0.402, P = 0.024) and class (beta: 0.198, 95% CI: 0.002-0.394, 0.048) levels. Nicotine dependence was simultaneously induced in Lactobacillaceae (beta: -0.426, 95% CI: -0.809-0.043, P = 0.029), Allisonella (beta: -0.670, 95% CI: -1.130-0.210, P = 0.004), Gordonibacter (beta: -0.480, 95% CI: -0.906-0.053, P =0.027), Lactobacillus (beta: -0.416, 95% CI: -0.800-0.032, P =0.034), Rikenellaceae RC9 gut group (beta: -0.570, 95% CI: -1.070-0.071, P =0.025). The causal effects of nicotine dependence on Lactobacillaceae were consistent at the family and genus levels. For the phylum Actinobacteria, class Actinobacteria and genus Gordonibacter, MR-Egger method yielded results in the opposite direction to the IVW method, whereas weighted median, simple mode, and weighted mode methods produced analysis results consistent with the IVW method. However, for family Christensenellaceae, family Lactobacillaceae, genus Lachnospiraceae UCG001, genus Lactobacillus, genus Allisonella, and genus Rikenellaceae RC9, MRegger, weighted median, simple mode, and weighted mode methods provided effect directions consistent with the IVW method. The scatter plot and forest plot were shown in Figures S1 and S2 .
Figure 4.
Forest plot of Mendelian randomization of two samples as a result of gut microbiota abundance. Causal effects of five Mendelian randomization methods with nicotine dependence as exposure and gut microbiota abundance as outcome. Effect estimates are expressed as effect size (BETA) with 95% confidence intervals (CI). SNP, single nucleotide polymorphism.
After using multivariable Mendelian randomization to adjust for smoking-related phenotypes (age of smoking initiation, smoking initiation, cigarettes per day, and smoking cessation), nicotine dependence was found to have a causal impact only on Christensenellaceae (β: -0.52, 95% CI: -0.934–0.106, P = 0.014). ( Table S5 and Figure 5 ) The detailed information about the instrumental variables used for each covariate in the multivariate Mendelian randomization analysis is recorded in Table S6 . Furthermore, conditional F statistics of the instrumental variables for each covariate in the multivariable Mendelian randomization were all greater than 10, indicating no weak instrumental bias ( Table S7 ).
Figure 5.
Forest plot of multivariable Mendelian randomization. Causal effects of IVW methods with nicotine dependence and four smoking related phenotypes as exposure while gut microbiota abundance as outcome. Effect estimates are expressed as effect size (BETA) with 95% confidence intervals (CI).
Causal effects of gut microbiota on nicotine dependence
In the first step, 1425 SNPs, which were associated with gut microbiota in phylum, class, order, family, and genus, were identified, excluding Christensenellaceae since they did not have suitable instrumental variables. The F statistic of each SNP exceeded 10, ranging from 17.17 to 88.41, indicating no weak instrumental bias ( Table S8 ). Furthermore, Steiger filtering analysis did not identify any SNPs with opposite causal directions ( Table S9 ). We extracted 115 genera, 29 families, 16 orders, and 5 phyla for our instrumental variables. The range of number of IVs from each classification ranged from 3-13.
The significance thresholds for multiple comparisons were set based on the Bonferroni correction: phylum (p = 5.560 × 10-3), class (p = 3.125 × 10-3), order (p = 2.778 × 10-3), family (p = 1.562 × 10-3), and genus (p = 4.310 × 10-4).
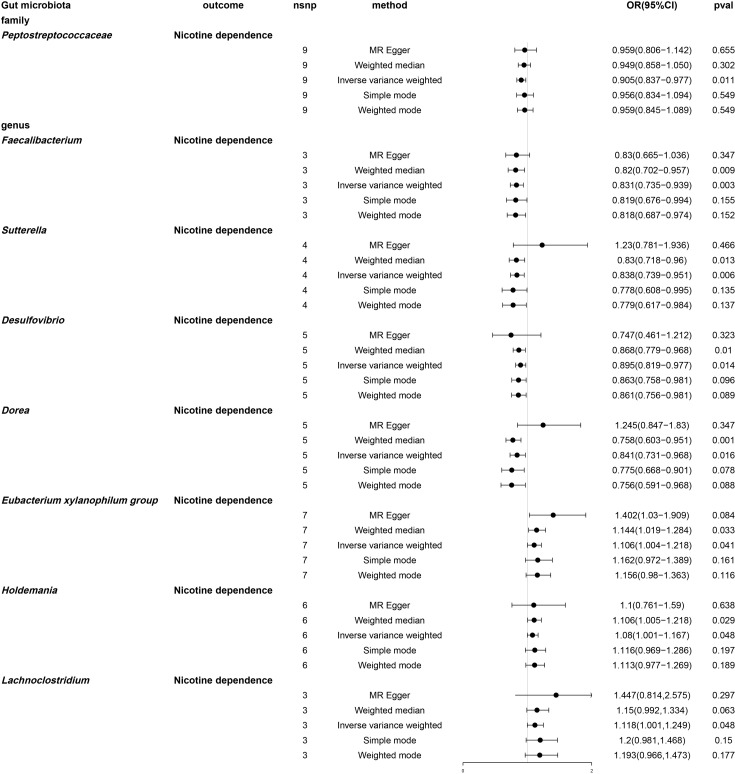
By using MR analysis ( Table S10 ), we combined the SNP effects from the gut microbiota and found that one family and seven genera have a potential causal influence on nicotine dependence ( Figure 6 ). According to the IVW approach, the Eubacterium xylanophilum group (OR: 1.106, 95% CI: 1.004-1.218, P = 0.041), Lachnoclostridium (OR: 1.118, 95% CI: 1.001-1.249, P = 0.048) and Holdemania (OR: 1.08, 95% CI: 1.001-1.167, P =0.048) were risk factors for nicotine dependence. Of these, Lachnoclostridium had the smallest value of OR. Peptostreptococcaceae (OR: 0.905, 95% CI: 0.837-0.977, P =0.011), Desulfovibrio (OR: 0.014, 95% CI: 0.819-0.977, P =0.895), Dorea (OR: 0.841, 95% CI. 0.731-0.968, P =0.016), Faecalibacterium (OR: 0.831, 95% CI: 0.735-0.939, P =0.003) and Sutterella (OR: 0.838, 95% CI: 0.739-0.951, P =0.006) were protective factor for nicotine dependence, with Faecalibacterium having the smallest value of OR. According to the results of other Mendelian analysis methods, for genus Sutterella and genus Dorea, MR-Egger method yielded results in the opposite direction to the IVW method, while weighted median, simple mode, and weighted mode methods produced analysis results consistent with the IVW method. For the family Peptostreptococcaceae, genus Eubacterium xylanophilum group, genus Lachnoclostridium, genus Holdemania, genus Lachnoclostridium, and genus Desulfovibrio, MR-Egger, weighted median, simple mode, and weighted mode provided effect directions consistent with the IVW method. The scatter plot and forest plot were shown in Figures S3 and S4 .
Figure 6.
Forest plot of two-sample Mendelian randomization with nicotine dependence as an outcome. Causal effects of five Mendelian randomization methods with gut microbiota abundance as exposure and nicotine dependence as outcome. Effect estimates are expressed as odds ratios (OR) with 95% confidence intervals (CI). SNP, single nucleotide polymorphism.
Sensitivity analysis
No evidence was found for horizontal pleiotropy when using the MR-Egger regression intercept method on the gut microbiota and nicotine-dependent instrumental variables ( Tables S11 – S13 ). We screened and removed any outliers in the MR-PRESSO analysis and found no horizontal pleiotropy for the gut microbiota or nicotine-dependent instrumental variables ( Tables S14 and S15 ). Furthermore, the majority of Cochrane Q statistics did not show significant heterogeneity (p > 0.05) as shown in the Supplementary Material ( Tables S16 – S18 ). In cases where heterogeneity was found to be significant, we used a random-effects model with an IVW model. The results of leave-one-out sensitivity and funnel plot were shown in Figures S5 – S8 .
Discussion
Our study employed a bidirectional Mendelian randomization approach to assess the causality between nicotine dependence and gut microbial abundance. To our knowledge, this is the first mendelian randomization study to examine the causal relationship between nicotine dependence and gut microbial abundance.
Smoking is a complex behavior that encompasses several stages, including initiation, regular smoking, nicotine dependence, cessation, and relapse. While some individuals may maintain low levels of smoking without experiencing dependence (49), others may become heavily dependent, which escalates the challenges associated with stopping smoking and augments the risk of relapse (50, 51). Furthermore, while smoking behavior broadly encompasses various stages, nicotine dependence has a strong genetic component with high heritability rates (up to 75%) coupled with environmental factors (52). Nicotine dependence is a significant predictor of the severity of tobacco withdrawal (53), response to treatments (54), and smoking-related health outcomes (55, 56), which impede smoking cessation success.
Previous studies have found that smokers are often accompanied by alterations in gut microbiota composition. Proposed mechanisms to explain the impact of smoking on the gut microbiota include enhanced oxidative stress (57), alterations in intestinal tight junctions and gut mucin composition (58), and changes in acid-base homeostasis (59). Smoking leads to changes in the composition of the gut microbiota, showing an increased abundance of certain bacteria such as Prevotella, Veloperae, Anaplasma, Acidophilus, and Helicobacter oxysporum, and a decrease in the abundance of other bacteria such as Thiotrichales and Helicobacter Lachesis (60). The use of nicotine products leads to known health consequences, but may also be a major cause of intestinal ecological disorders and increased intestinal permeability (14). Smoking cessation partially reversed these microbial alterations, resulting in increased microbial diversity and abundance in certain phyla. In healthy individuals who quit smoking, significant changes were observed in the fecal microbiota, including an increase in the relative abundance of thick-walled bacteria and actinomycetes and a decrease in anaplasma and amoebae (61). Quitting smoking resulted in significant changes in the fecal microbiota of healthy individuals. Although studies have investigated the effects of tobacco use on gut microbiota, previous studies are limited by small sample sizes and inaccurate assessment of tobacco use. Patient inclusion is often based solely on self-reported smoking history, with unstable criteria for assessing smoking frequency, which renders studies susceptible to confounding factors that may not be excluded.
While a prior mendelian randomization study has investigated the relationship between smoking associated phenotypes and gut microbiota (62), no study has examined the relationship between nicotine dependence and gut microbiota. Given the aforementioned limitations of previous studies on smoking and gut microbiota, further research is needed to address the relationship between nicotine dependence and gut microbiota. Therefore, in this study, we used genome-wide association data from patients with nicotine dependence based on FTND scores to investigate the causal relationship between nicotine dependence and gut microbiota using mendelian randomization.
Studies have shown that smoking leads to a higher abundance of Streptococcus spp. and Desulfovibri in the digestive tract (63), as well as a higher abundance of Streptococcus spp. in the upper small intestinal mucosa of smokers (64). Additionally, smokers show a significantly lower abundance of Faecalibacterium in the gut (65). In this study, we found that genetically dependent nicotine dependence can lead to a decrease in the gut abundance of Christensenellaceae. Furthermore, even after smoking cessation, the effect of decreased Christensenellaceae abundance persists. Christensenellaceae, belonging to the Firmicutes phylum, is widely present in the human gut and mucosa (66–69), and it plays a crucial role in host health. Previous studies have found a negative correlation between the relative abundance of Christensenellaceae and host body mass index in different populations and multiple research studies (70). It is also closely associated with glucose metabolism (71, 72) and inflammatory bowel disease (73). Christensenellaceae may serve as probiotics to improve health status (74), but further research is needed to elucidate the underlying mechanisms. Previous studies have shown that smoking reduces the abundance of Firmicutes in the gut (60). Our study suggests a causal effect between genetically predicted nicotine dependence and Christensenellaceae. Further investigation is needed to explore the potential of Christensenellaceae in improving symptoms of nicotine dependence in patients and preventing diseases associated with nicotine dependence.
From the perspective of the effect of gut microbiota on nicotine dependence, a recent study has shown that colonization of Bacteroides xylanisolvens, a human gut bacteria, can effectively degrade intestinal nicotine, providing a new target for the treatment of patients with non-alcoholic fatty liver disease (75). This suggests that gut microbiota abundance may influence nicotine metabolism and further impact the disease progression of nicotine dependence. However, there is insufficient evidence from previous studies to assess whether gut microbiota abundance has a preventive or promotive effect on nicotine dependence. Our study found that Peptostreptococcaceae, Desulfovibrio, Dorea, Faecalibacterium, and Sutterella decrease the risk of nicotine dependence, while the Eubacterium xylanophilum group and Holdemania increase the risk of nicotine dependence. These findings have not been widely reported previously, indicating a potential contribution of this study to the existing literature.
There are several advantages in our study. Specifically, we employed a biodirectional mendelian randomization analysis to establish the causal association between gut microbiota and nicotine dependence. This approach allowed us to control for confounding factors and minimize the risk of reverse causation. The gut microbiota and nicotine dependence genome-wide association data were retrieved from the largest available GWAS meta-analysis to ensure the statistical robustness of the instrumental variables used in the Mendelian randomization analysis. To minimize the potential impact of weak IV bias, we employed a suitable threshold for the genomic correlation of instrumental variables (p = 5e-06). This threshold was chosen based on the availability of a sufficient number of SNPs with adequate statistical power for most gut flora, effectively avoiding confounding. In contrast, previous studies used a p-value cut-off of 1e-05 (36, 76) or 1e-06 (62) resulting in only a few gut flora receiving 3 or more SNPs for the Mendelian randomization analysis. Consequently, the power of the previous studies might have been insufficient, introducing false negatives. In addition, the previous study ended up including only 41 gut microbiota (62), which may have circumvented the inclusion of a larger number of flora and led to false positives when performing the FDR test for p-value (FDR = p*n/rank). The phenotypes utilized in the prior Mendelian randomization analyses of smoking initiation, lifetime smoking, and daily cigarette consumption, were not clinically practical for age-specific interventions. In contrast, our study of the FTND scale for diagnosing nicotine dependence as a phenotype may offer clinical guidance to those who smoke, but do not meet the diagnostic criteria for nicotine dependence. Employing the FTND scale may help prevent the development of nicotine dependence.
Although our study has several strengths, we acknowledge some limitations. Notably, the p-values in our findings are not robust to the Bonferroni method adjusted for significance. However, it is important to note that our study is hypothesis-driven, based on strong physiological evidence that supports the epidemiologically established link between gut flora and nicotine dependence. To strengthen the results further, future studies may need to include a larger sample size of nicotine-dependent patients. Additionally, the use of multiple comparisons to adjust p-values may increase the risk of false negatives due to the high number of microbial taxa and the multilevel structure (correlation between abundance and microbial strains) and the correlation between nicotine dependence. Therefore, caution should be exercised when interpreting negative results or potentially significant p-values. As with the previous point, future GWAS studies could benefit from increasing the sample size of patients with gut flora and nicotine dependence to reduce the likelihood of such biases. Third, as the majority of participants in the Nicotine Dependent GWAS were of European ancestry, the external validity of our findings to other ethnic groups may be constrained. Given that smoking is more prevalent among men, a disproportionately high number of male patients were included in the nicotine-dependent phenotype. Moreover, gender differences may exist in the composition of the gut microbiota. In our study, out of the nicotine dependence GWAS data employed, 53.2% of the participants were male, and the relatively even sex ratio could alleviate the potential gender bias to some extent. Nevertheless, the summary data from genome-wide association analyses limited our capacity to conduct further subgroup analyses to explore gender-specific discrepancies.
Conclusion
In conclusion, our investigation confirms the causal link between genetically predicted nicotine dependence and gut microbiota, underscoring the interactive impacts of nicotine dependence on gut microbes that might act as novel biomarkers and yield revelations for addressing and avoiding nicotine dependence.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The GWAS meta-analysis results on nicotine dependence were provided by the dbGaP study of PubMed (study_id=phs001532) and can be downloaded from http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001532.v1.p1. The summary data on gut microbiota is from MiBioGen consortium, which can be obtained from the IEU GWAS database (https://gwas.mrcieu.ac.uk/) (GWAS ID: ebi-a-GCST90016908– ebi-a-GCST90017118).The summary data on smoking related phenotypes is from GSCAN consortium, which can be obtained from the University Digital Conservancy Home (https://conservancy.umn.edu/handle/11299/201564).
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
All authors have been involved in the preparation of the manuscript and approved its submission. Conceptualization: LL, ZC and JZ. Formal analysis: YC, MZ, KJ and JL. Funding acquisition: LL, ZC and JZ. Methodology: JL, SW and LL. Writing original draft: YC, MZ and KJ. Writing-review and editing: YC, MZ, KJ, JL, SW, LL, ZC and JZ. All authors contributed to the article and approved the submitted version.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by National Natural Science Foundation of China (Grant No. 82174527), Guangzhou Municipal Science and Technology Bureau (Grant No. 202102010505 and 202201011206), the Traditional Chinese Medicine Bureau of Guangdong Province (Grant No. 20211128). These funding organizations did not participate in the study design, data collection, analysis, interpretation or writing of the manuscript or in the decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1244272/full#supplementary-material
References
- 1. Tobacco . Available at: https://www.who.int/news-room/fact-sheets/detail/tobacco (Accessed May 20, 2023a).
- 2. Tobacco . Available at: https://www.who.int/health-topics/tobacco (Accessed May 20, 2023b).
- 3. Rupprecht LE, Smith TT, Schassburger RL, Buffalari DM, Sved AF, Donny EC. Behavioral mechanisms underlying nicotine reinforcement. Curr Top Behav Neurosci (2015) 24:19–53. doi: 10.1007/978-3-319-13482-6_2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicotine Addiction: Practice Essentials, Background, Pathophysiology . (2023). Available at: https://emedicine.medscape.com/article/287555-overview (Accessed May 20, 2023).
- 5. Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Goodman AG. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 9th ed. New York: McGraw-Hill; (1996). [Google Scholar]
- 6. Mishra A, Chaturvedi P, Datta S, Sinukumar S, Joshi P, Garg A. Harmful effects of nicotine. Indian J Med Paediatr Oncol (2015) 36:24–31. doi: 10.4103/0971-5851.151771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. To N, Gracie DJ, Ford AC. Systematic review with meta-analysis: the adverse effects of tobacco smoking on the natural history of Crohn’s disease. Aliment Pharmacol Ther (2016) 43:549–61. doi: 10.1111/apt.13511 [DOI] [PubMed] [Google Scholar]
- 8. Nam SY, Kim BC, Ryu KH, Park BJ. Prevalence and risk factors of irritable bowel syndrome in healthy screenee undergoing colonoscopy and laboratory tests. J Neurogastroenterol Motil (2010) 16:47–51. doi: 10.5056/jnm.2010.16.1.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kato I, Nomura AM, Stemmermann GN, Chyou PH. A prospective study of gastric and duodenal ulcer and its relation to smoking, alcohol, and diet. Am J Epidemiol (1992) 135:521–30. doi: 10.1093/oxfordjournals.aje.a116319 [DOI] [PubMed] [Google Scholar]
- 10. Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA (2008) 300:2765–78. doi: 10.1001/jama.2008.839 [DOI] [PubMed] [Google Scholar]
- 11. Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect (2013) 67:169–84. doi: 10.1016/j.jinf.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 12. Parasher G, Eastwood GL. Smoking and peptic ulcer in the Helicobacter pylori era. Eur J Gastroenterol Hepatol (2000) 12:843–53. doi: 10.1097/00042737-200012080-00003 [DOI] [PubMed] [Google Scholar]
- 13. Whitehead AK, Meyers MC, Taylor CM, Luo M, Dowd SE, Yue X, et al. Sex-dependent effects of inhaled nicotine on the gut microbiome. Nicotine Tob Res (2022) 24:1363–70. doi: 10.1093/ntr/ntac064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez JE, Kahana DD, Ghuman S, Wilson HP, Wilson J, Kim SCJ, et al. Unhealthy lifestyle and gut dysbiosis: A better understanding of the effects of poor diet and nicotine on the intestinal microbiome. Front Endocrinol (Lausanne) (2021) 12:667066. doi: 10.3389/fendo.2021.667066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Montalto M, D’Onofrio F, Gallo A, Cazzato A, Gasbarrini G. Intestinal microbiota and its functions. Digestive Liver Dis Suppl (2009) 3:30–4. doi: 10.1016/S1594-5804(09)60016-4 [DOI] [Google Scholar]
- 16. Kamada N, Seo S-U, Chen GY, Núñez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol (2013) 13:321–35. doi: 10.1038/nri3430 [DOI] [PubMed] [Google Scholar]
- 17. Kaiko GE, Stappenbeck TS. Host-microbe interactions shaping the gastrointestinal environment. Trends Immunol (2014) 35:538–48. doi: 10.1016/j.it.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ubeda C, Djukovic A, Isaac S. Roles of the intestinal microbiota in pathogen protection. Clin Transl Immunol (2017) 6:e128. doi: 10.1038/cti.2017.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang Z, Wang Q, Liu Y, Wang L, Ge Z, Li Z, et al. Gut microbiota and hypertension: association, mechanisms and treatment. Clin Exp Hypertens (2023) 45:2195135. doi: 10.1080/10641963.2023.2195135 [DOI] [PubMed] [Google Scholar]
- 20. Kang Y, Kuang X, Yan H, Ren P, Yang X, Liu H, et al. A novel synbiotic alleviates autoimmune hepatitis by modulating the gut microbiota-liver axis and inhibiting the hepatic TLR4/NF-κB/NLRP3 signaling pathway. mSystems (2023) 8:e0112722. doi: 10.1128/msystems.01127-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Padma S, Patra R, Sen Gupta PS, Panda SK, Rana MK, Mukherjee S. Cell surface fibroblast activation protein-2 (Fap2) of fusobacterium nucleatum as a vaccine candidate for therapeutic intervention of human colorectal cancer: an immunoinformatics approach. Vaccines (Basel) (2023) 11:525. doi: 10.3390/vaccines11030525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Savin Z, Kivity S, Yonath H, Yehuda S. Smoking and the intestinal microbiome. Arch Microbiol (2018) 200:677–84. doi: 10.1007/s00203-018-1506-2 [DOI] [PubMed] [Google Scholar]
- 23. Kaur G, Begum R, Thota S, Batra S. A systematic review of smoking-related epigenetic alterations. Arch Toxicol (2019) 93:2715–40. doi: 10.1007/s00204-019-02562-y [DOI] [PubMed] [Google Scholar]
- 24. Papoutsopoulou S, Satsangi J, Campbell BJ, Probert CS. Review article: impact of cigarette smoking on intestinal inflammation-direct and indirect mechanisms. Aliment Pharmacol Ther (2020) 51:1268–85. doi: 10.1111/apt.15774 [DOI] [PubMed] [Google Scholar]
- 25. Patra R, Mitra S, Das NC, Mukherjee S. Prebiotics as Promising Therapeutics for Treating Gut-Related Disorders: Biochemical and Molecular Perspectives. In: Behera KK, Bist R, Mohanty S, Bhattacharya M, editors. Prebiotics, Probiotics and Nutraceuticals. Singapore: Springer Nature; (2022). p. 133–54. doi: 10.1007/978-981-16-8990-1_8 [DOI] [Google Scholar]
- 26. Hemarajata P, Versalovic J. Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Therap Adv Gastroenterol (2013) 6:39–51. doi: 10.1177/1756283X12459294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Erejuwa OO, Sulaiman SA, Ab Wahab MS. Modulation of gut microbiota in the management of metabolic disorders: the prospects and challenges. Int J Mol Sci (2014) 15:4158–88. doi: 10.3390/ijms15034158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Das NC, Patra R, Dey A, Mukherjee S. Probiotics as Efficacious Therapeutic Option for Treating Gut-Related Diseases: Molecular and Immunobiological Perspectives. In: Behera KK, Bist R, Mohanty S, Bhattacharya M, editors. Prebiotics, Probiotics and Nutraceuticals. Singapore: Springer Nature; (2022). p. 69–93. doi: 10.1007/978-981-16-8990-1_5 [DOI] [Google Scholar]
- 29. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA (2017) 318:1925. doi: 10.1001/jama.2017.17219 [DOI] [PubMed] [Google Scholar]
- 30. Bonder MJ, Kurilshikov A, Tigchelaar EF, Mujagic Z, Imhann F, Vila AV, et al. The effect of host genetics on the gut microbiome. Nat Genet (2016) 48:1407–12. doi: 10.1038/ng.3663 [DOI] [PubMed] [Google Scholar]
- 31. Goodrich JK, Davenport ER, Beaumont M, Jackson MA, Knight R, Ober C, et al. Genetic determinants of the gut microbiome in UK twins. Cell Host Microbe (2016) 19:731–43. doi: 10.1016/j.chom.2016.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Thingholm LB, Skiecevičienė J, Rausch P, Kummen M, Hov JR, et al. Genome-wide association analysis identifies variation in vitamin D receptor and other host factors influencing the gut microbiota. Nat Genet (2016) 48:1396–406. doi: 10.1038/ng.3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Skrivankova VW, Richmond RC, Woolf BAR, Davies NM, Swanson SA, VanderWeele TJ, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomisation (STROBE-MR): explanation and elaboration. BMJ (2021) 375:n2233. doi: 10.1136/bmj.n2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement. JAMA (2021) 326:1614–21. doi: 10.1001/jama.2021.18236 [DOI] [PubMed] [Google Scholar]
- 35. Hancock DB, Guo Y, Reginsson GW, Gaddis NC, Lutz SM, Sherva R, et al. Genome-wide association study across European and African American ancestries identifies a SNP in DNMT3B contributing to nicotine dependence. Mol Psychiatry (2018) 23:1911–9. doi: 10.1038/mp.2017.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kurilshikov A, Medina-Gomez C, Bacigalupe R, Radjabzadeh D, Wang J, Demirkan A, et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat Genet (2021) 53:156–65. doi: 10.1038/s41588-020-00763-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet (2019) 51:237–44. doi: 10.1038/s41588-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burgess S, Thompson SG, CRP CHD Genetics Collaboration . Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol (2011) 40:755–64. doi: 10.1093/ije/dyr036 [DOI] [PubMed] [Google Scholar]
- 39. Shim H, Chasman DI, Smith JD, Mora S, Ridker PM, Nickerson DA, et al. A multivariate genome-wide association analysis of 10 LDL subfractions, and their response to statin treatment, in 1868 Caucasians. PloS One (2015) 10:e0120758. doi: 10.1371/journal.pone.0120758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PloS Genet (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. R package version 1.0; (2018). 50:693–698. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. OYJS . MendelianRandomization: Mendelian Randomization Package. R package version 0.7.0; (2023). Available at: https://CRAN.R-project.org/package=MendelianRandomization [Google Scholar]
- 43. Smith GD, Ebrahim S. Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32:1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 44. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol (2015) 44:512–25. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol (2017) 32:377–89. doi: 10.1007/s10654-017-0255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol (2016) 40:304–14. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol (2017) 46:1985–98. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C. Characteristics and smoking patterns of intermittent smokers. Exp Clin Psychopharmacol (2012) 20:264–77. doi: 10.1037/a0027546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Breslau N, Johnson EO. Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health (2000) 90:1122–7. doi: 10.2105/ajph.90.7.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Transdisciplinary Tobacco Use Research Center (TTURC) Tobacco Dependence. Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, et al. Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res (2007) 9(Suppl 4):S555–570. doi: 10.1080/14622200701673480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pf S, Ks K. The genetic epidemiology of smoking. Nicotine Tobacco Res (1999) 1(Suppl 2):S51–7. doi: 10.1080/14622299050011811 [DOI] [PubMed] [Google Scholar]
- 53. Baker TB, Piper ME, Schlam TR, Cook JW, Smith SS, Loh W-Y, et al. Are tobacco dependence and withdrawal related amongst heavy smokers? Relevance to conceptualizations of dependence. J Abnorm Psychol (2012) 121:909–21. doi: 10.1037/a0027889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zelman DC, Brandon TH, Jorenby DE, Baker TB. Measures of affect and nicotine dependence predict differential response to smoking cessation treatments. J Consult Clin Psychol (1992) 60:943–52. doi: 10.1037//0022-006x.60.6.943 [DOI] [PubMed] [Google Scholar]
- 55. Gu F, Wacholder S, Kovalchik S, Panagiotou OA, Reyes-Guzman C, Freedman ND, et al. Time to smoke first morning cigarette and lung cancer in a case-control study. J Natl Cancer Inst (2014) 106:dju118. doi: 10.1093/jnci/dju118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Guertin KA, Gu F, Wacholder S, Freedman ND, Panagiotou OA, Reyes-Guzman C, et al. Time to first morning cigarette and risk of chronic obstructive pulmonary disease: smokers in the PLCO cancer screening trial. PloS One (2015) 10:e0125973. doi: 10.1371/journal.pone.0125973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wang H, Zhao J-X, Hu N, Ren J, Du M, Zhu M-J. Side-stream smoking reduces intestinal inflammation and increases expression of tight junction proteins. World J Gastroenterol (2012) 18:2180–7. doi: 10.3748/wjg.v18.i18.2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Allais L, Kerckhof F-M, Verschuere S, Bracke KR, De Smet R, Laukens D, et al. Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut. Environ Microbiol (2016) 18:1352–63. doi: 10.1111/1462-2920.12934 [DOI] [PubMed] [Google Scholar]
- 59. Tomoda K, Kubo K, Asahara T, Andoh A, Nomoto K, Nishii Y, et al. Cigarette smoke decreases organic acids levels and population of bifidobacterium in the caecum of rats. J Toxicol Sci (2011) 36:261–6. doi: 10.2131/jts.36.261 [DOI] [PubMed] [Google Scholar]
- 60. Shapiro H, Goldenberg K, Ratiner K, Elinav E. Smoking-induced microbial dysbiosis in health and disease. Clin Sci (Lond) (2022) 136:1371–87. doi: 10.1042/CS20220175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gomaa EZ. Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek (2020) 113:2019–40. doi: 10.1007/s10482-020-01474-7 [DOI] [PubMed] [Google Scholar]
- 62. Fan J, Zhou Y, Meng R, Tang J, Zhu J, Aldrich MC, et al. Cross-talks between gut microbiota and tobacco smoking: a two-sample Mendelian randomization study. BMC Med (2023) 21:163. doi: 10.1186/s12916-023-02863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang X, Ye P, Fang L, Ge S, Huang F, Polverini PJ, et al. Active smoking induces aberrations in digestive tract microbiota of rats. Front Cell Infect Microbiol (2021) 11:737204. doi: 10.3389/fcimb.2021.737204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Shanahan ER, Shah A, Koloski N, Walker MM, Talley NJ, Morrison M, et al. Influence of cigarette smoking on the human duodenal mucosa-associated microbiota. Microbiome (2018) 6:150. doi: 10.1186/s40168-018-0531-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yan S, Ma Z, Jiao M, Wang Y, Li A, Ding S. Effects of smoking on inflammatory markers in a healthy population as analyzed via the gut microbiota. Front Cell Infect Microbiol (2021) 11:633242. doi: 10.3389/fcimb.2021.633242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Huang YJ, Kim E, Cox MJ, Brodie EL, Brown R, Wiener-Kronish JP, et al. A persistent and diverse airway microbiota present during chronic obstructive pulmonary disease exacerbations. OMICS (2010) 14:9–59. doi: 10.1089/omi.2009.0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moreno-Indias I, Sánchez-Alcoholado L, García-Fuentes E, Cardona F, Queipo-Ortuño MI, Tinahones FJ. Insulin resistance is associated with specific gut microbiota in appendix samples from morbidly obese patients. Am J Transl Res (2016) 8:5672–84. [PMC free article] [PubMed] [Google Scholar]
- 68. Burns MB, Montassier E, Abrahante J, Priya S, Niccum DE, Khoruts A, et al. Colorectal cancer mutational profiles correlate with defined microbial communities in the tumor microenvironment. PloS Genet (2018) 14:e1007376. doi: 10.1371/journal.pgen.1007376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zakrzewski M, Simms LA, Brown A, Appleyard M, Irwin J, Waddell N, et al. IL23R-protective coding variant promotes beneficial bacteria and diversity in the ileal microbiome in healthy individuals without inflammatory bowel disease. J Crohns Colitis (2019) 13:451–61. doi: 10.1093/ecco-jcc/jjy188 [DOI] [PubMed] [Google Scholar]
- 70. Goodrich JK, Waters JL, Poole AC, Sutter JL, Koren O, Blekhman R, et al. Human genetics shape the gut microbiome. Cell (2014) 159:789–99. doi: 10.1016/j.cell.2014.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lim MY, You HJ, Yoon HS, Kwon B, Lee JY, Lee S, et al. The effect of heritability and host genetics on the gut microbiota and metabolic syndrome. Gut (2017) 66:1031–8. doi: 10.1136/gutjnl-2015-311326 [DOI] [PubMed] [Google Scholar]
- 72. Lippert K, Kedenko L, Antonielli L, Kedenko I, Gemeier C, Leitner M, et al. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef Microbes (2017) 8:545–56. doi: 10.3920/BM2016.0184 [DOI] [PubMed] [Google Scholar]
- 73. Mancabelli L, Milani C, Lugli GA, Turroni F, Cocconi D, van Sinderen D, et al. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol Ecol (2017) 93:12–1. doi: 10.1093/femsec/fix153 [DOI] [PubMed] [Google Scholar]
- 74. Chang C-J, Lin T-L, Tsai Y-L, Wu T-R, Lai W-F, Lu C-C, et al. Next generation probiotics in disease amelioration. J Food Drug Anal (2019) 27:615–22. doi: 10.1016/j.jfda.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Chen B, Sun L, Zeng G, Shen Z, Wang K, Yin L, et al. Gut bacteria alleviate smoking-related NASH by degrading gut nicotine. Nature (2022) 610:562–8. doi: 10.1038/s41586-022-05299-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu X, Tong X, Zou Y, Lin X, Zhao H, Tian L, et al. Mendelian randomization analyses support causal relationships between blood metabolites and the gut microbiome. Nat Genet (2022) 54:52–61. doi: 10.1038/s41588-021-00968-y [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: The GWAS meta-analysis results on nicotine dependence were provided by the dbGaP study of PubMed (study_id=phs001532) and can be downloaded from http://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs001532.v1.p1. The summary data on gut microbiota is from MiBioGen consortium, which can be obtained from the IEU GWAS database (https://gwas.mrcieu.ac.uk/) (GWAS ID: ebi-a-GCST90016908– ebi-a-GCST90017118).The summary data on smoking related phenotypes is from GSCAN consortium, which can be obtained from the University Digital Conservancy Home (https://conservancy.umn.edu/handle/11299/201564).