Abstract
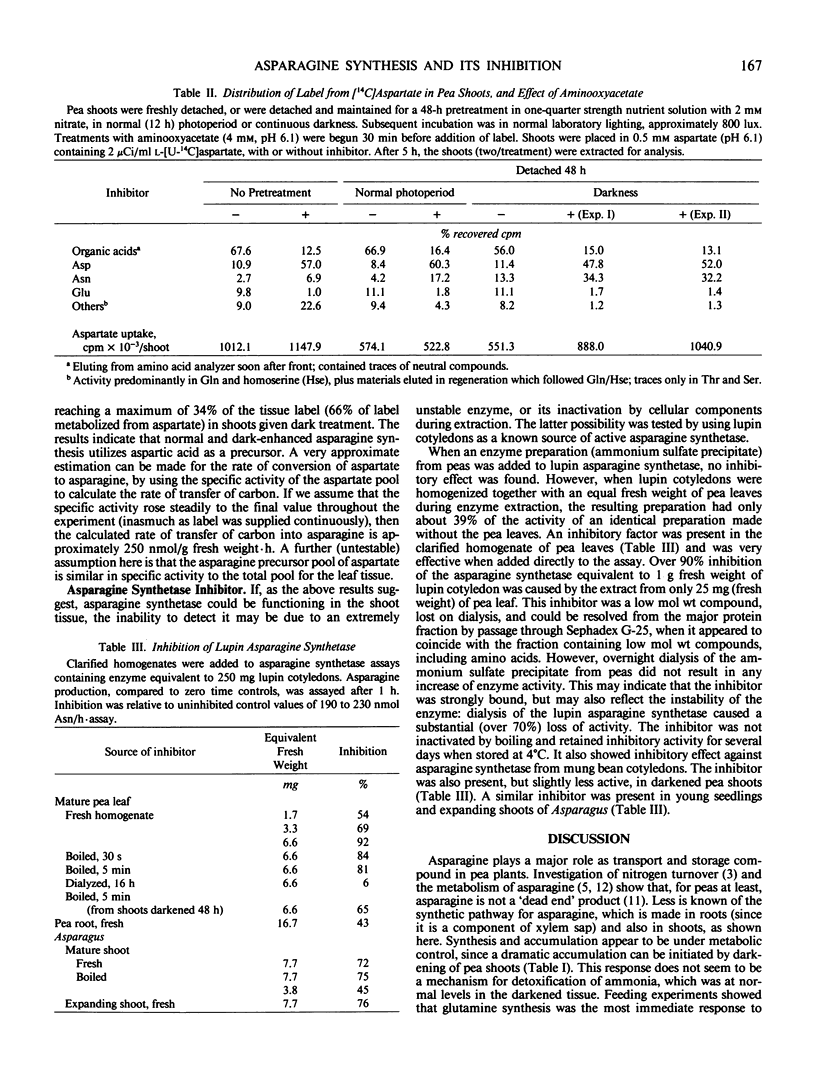
Asparagine is present in the mature leaves of young pea (Pisum sativum cv Little Marvel) seedlings, and is synthesized in detached shoots. This accumulation and synthesis is greatly enhanced by darkening. In detached control shoots, [14C]aspartate was metabolized predominantly to organic acids and, as other workers have shown, there was little labeling of asparagine (after 5 hours, 3.1% of metabolized label). Addition of the aminotransferase inhibitor aminooxyacetate decreased the flow of aspartate carbon to organic acids and enhanced (about 3-fold) the labeling of asparagine. The same treatment applied to darkened shoots resulted in a substantial conversion of [14C]aspartate to asparagine, over 10-fold greater than in control shoots (66% of metabolized label), suggesting that aspartate is the normal precursor of asparagine.
Only traces of glutamine-dependent asparagine synthetase activity could be detected in pea leaf or root extracts; activity was not enhanced by sulfhydryl reagents, oxidizing conditions, or protease inhibitors. Asparagine synthetase is readily extracted from lupin cotyledons, but yield was greatly reduced by extraction in the presence of pea leaf tissue; pea leaf homogenates contained an inhibitor which produced over 95% inhibition of an asparagine synthetase preparation from lupin cotyledons. The inhibitor was heat stable, with a low molecular weight. Presence of an inhibitor may prevent detection of asparagine synthetase in pea extracts and in Asparagus, where a cyanide-dependent pathway has been proposed to account for asparagine synthesis: an inhibitor with similar properties was present in Asparagus shoot tissue.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Sharkey P. J. Asparagine metabolism-key to the nitrogen nutrition of developing legume seeds. Plant Physiol. 1975 Dec;56(6):807–812. doi: 10.1104/pp.56.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Joy K. W., Urquhart A. A. Amino Acid metabolism of pea leaves: labeling studies on utilization of amides. Plant Physiol. 1977 May;59(5):920–924. doi: 10.1104/pp.59.5.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A., Urquhart A. A., Joy K. W. Amino Acid metabolism of pea leaves: diurnal changes and amino Acid synthesis from N-nitrate. Plant Physiol. 1977 May;59(5):915–919. doi: 10.1104/pp.59.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney D. A., Jayaram N., Swengros S. G., Alter S. C., Levine M. The metabolism of L-asparagine in Asparagus officinalis. Int J Biochem. 1980;11(1):69–83. doi: 10.1016/0020-711x(80)90281-5. [DOI] [PubMed] [Google Scholar]
- Kern R., Chrispeels M. J. Influence of the axis on the enzymes of protein and amide metabolism in the cotyledons of mung bean seedlings. Plant Physiol. 1978 Nov;62(5):815–819. doi: 10.1104/pp.62.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees E. M., Farnden K. J., Elliott W. H. Studies on asparagine synthesis and utilization in seedlings. Arch Biochem Biophys. 1968 Aug;126(2):539–546. doi: 10.1016/0003-9861(68)90439-6. [DOI] [PubMed] [Google Scholar]
- Lloyd N. D., Joy K. W. 2-Hydroxysuccinamic acid: a product of asparagine metabolis in plants. Biochem Biophys Res Commun. 1978 Mar 15;81(1):186–192. doi: 10.1016/0006-291x(78)91647-9. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. In vivo and in vitro studies on asparagine biosynthesis in soybean seedlings. Arch Biochem Biophys. 1973 Aug;157(2):613–624. doi: 10.1016/0003-9861(73)90681-4. [DOI] [PubMed] [Google Scholar]
- Ting I. P., Zschoche W. C. Asparagine biosynthesis by cotton roots. Carbon dioxide fixation and cyanide incorporation. Plant Physiol. 1970 Apr;45(4):429–434. doi: 10.1104/pp.45.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart A. A., Joy K. W. Use of Phloem exudate technique in the study of amino Acid transport in pea plants. Plant Physiol. 1981 Sep;68(3):750–754. doi: 10.1104/pp.68.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Morot-Gaudry J. F., Summons R. E., Osmond C. B. Evidence for the Glutamine Synthetase/Glutamate Synthase Pathway during the Photorespiratory Nitrogen Cycle in Spinach Leaves. Plant Physiol. 1982 Nov;70(5):1514–1517. doi: 10.1104/pp.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]


