Abstract
In this study, three types of treated wastewater were tested for infectious enteroviruses, the enterovirus genome, somatic coliphages, and Bacteroides fragilis phages. The aim of this work was to determine whether the presence of the two types of bacteriophages or of the enterovirus genome was a good indicator of infectious enterovirus contamination. The enterovirus genome was detected by reverse transcription-polymerase chain reaction. Infectious enteroviruses were quantified by cell culturing (BGM cells), and the bacteriophages were quantified by plaque formation on the host bacterium (Escherichia coli or B. fragilis) in agar medium. Forty-eight samples of treated wastewater were analyzed. Sixteen samples had been subjected to a secondary treatment for 8 to 12 h (A), 16 had been subjected to a secondary treatment for 30 h (B1), and 16 had been subjected to both secondary and tertiary treatments (B2). The mean concentrations of somatic coliphages were 4.9 × 104 PFU · liter−1 for treatment line A, 9.8 × 103 PFU · liter−1 for B1, and 1.4 × 103 PFU · liter−1 for B2, with all the samples testing positive (100%). The mean concentrations of B. fragilis phages were 1.7 × 103 PFU · liter−1 for A (100% positive samples), 17 to 24 PFU · liter−1 for B1 (44% positive samples), and 0.8 to 13 PFU · liter−1 for B2 (6% positive samples). The mean concentrations of infectious enteroviruses were 4 most probable number of cytopathogenic units (MPNCU) · liter−1 for A (31% positive samples) and <1 MPNCU · liter−1 for B1 and B2 (0% positive samples). The percentages of samples testing positive for the enterovirus genome were 100% for A, 56% for B1, and 19% for B2. The percentages of samples testing positive for the enterovirus genome were significantly higher than those for infectious enteroviruses. This finding may have been due to the presence of noninfectious enteroviruses or to the presence of infectious enteroviruses that do not multiply in BGM cell cultures. However, under our experimental conditions, nondetection of the genome implies the absence of infectious viruses. There was a significant correlation between the concentration of somatic coliphages or B. fragilis phages and the presence of infectious enteroviruses or the presence of the enterovirus genome. However, the somatic coliphage concentration did not lead to fluctuations in the infectious enterovirus concentration, whereas the B. fragilis phage concentration did.
Water in the environment may be contaminated by more than 140 serotypes of viruses via wastewater. Hepatitis A virus, caliciviruses, adenoviruses, rotavirus, and enteroviruses have the greatest effect on public health. A large number of epidemics due to the presence of these viruses in the environment have been reported (2, 3, 24). It is thus necessary to monitor the levels of these viruses in the aqueous environment, particularly in wastewater discharged into surface water.
The microbiological quality of water is currently evaluated by use of indicators of fecal contamination (fecal coliforms, Escherichia coli, and fecal streptococci). Such fecal contamination may lead to the presence in the water environment of organisms that cause diseases in humans. Some studies have shown that these indicators do not provide adequate information about viruses, particularly in terms of their fate in the environment and their resistance to treatment (6, 9, 17). Thus, studies have been directed toward identifying more specific indicators of viral contamination. Two approaches are possible. One involves the detection of enteroviruses (poliovirus, coxsackievirus groups A and B, and echovirus) by cell culture or molecular biology techniques. The other involves looking for particular types of bacteriophages commonly found in human feces.
The isolation of enteroviruses by cell culturing is the reference method because it is the only way in which the infectious nature of the virus isolated can be determined. However, it is time-consuming (1 to 2 weeks) and difficult to perform, and not all viral serotypes can be detected. Molecular biology techniques, such as reverse transcription-polymerase chain reaction (RT-PCR), can be used for the sensitive, specific, and rapid (24 to 48 h) detection of the enterovirus genome (13). Certain areas of the 5′ noncoding region of the enterovirus genome are highly conserved among all serotypes. Primers binding to these areas can be used to amplify sequences common to most enteroviruses. Thus, the detection of the enterovirus genome by RT-PCR is a valuable alternative to cell culturing for evaluating the virological status of the water environment. However, the detection of the viral genome by itself does not provide any information about the infectivity of the viruses detected.
Three types of bacteriophages have also been proposed as specific indicators of viral contamination: the somatic coliphages (18), the F-specific RNA phages (8), and the Bacteroides fragilis phages (12). The size, structure, and survival rate in the environment of these bacteriophages are similar to those of enteroviruses. The B. fragilis HSP40 phages seem to be specific indicators of human fecal contamination, whereas the other two types of phages may be indicators of both human and nonhuman animal fecal contamination (8, 12).
The aim of this work was to determine whether B. fragilis phages or somatic coliphages and the enterovirus genome are reliable indicators of contamination of wastewater by infectious enteroviruses. We therefore tested for infectious enteroviruses, the enterovirus genome, somatic coliphages, and B. fragilis phages in various treated-wastewater samples.
MATERIALS AND METHODS
Sampling.
Forty-eight samples of treated wastewater were taken from two wastewater treatment plants. The first treatment line (A) included a pretreatment step (sand and oil removal), primary settling, activated sludge treatment, and secondary settling. The treatment time was between 8 and 12 h. The sampling site was situated at the plant outlet before the point at which treated wastewaters were discharged into the environment. Sixteen samples were taken. The second treatment line included two phases. The first phase included a pretreatment step (sand and oil removal), primary settling, biological treatment (alternating aeration and anoxia), and secondary settling. The second phase involved tertiary phosphate removal and enhanced settling. The treatment time was about 30 h. Two sampling sites were used: one at the outlet of the secondary treatment (B1) and the other at the outlet of the tertiary treatment (B2). Sixteen samples were taken at B1, and 16 samples were taken at B2. The 48 samples were taken between 18 March and 7 May 1997.
Enterovirus and enterovirus genome concentration.
The enteroviruses were concentrated by adsorption-elution on glass wool (23). Twenty liters of water was filtered at a rate of 20 liters · h−1 through 50 g of sodocalcic glass wool (type 725; Rantigny, Saint-Gobain, France) compacted (0.4 g · cm3) in a stainless steel cartridge (Sartorius SM 16249). The viruses adsorbed to the glass wool were eluted with 300 ml of 0.05 M glycine buffer–3% beef extract–0.0005% phenol red at pH 9.5. The eluate was rapidly neutralized to pH 7.2 to produce the concentrate.
B. fragilis phage concentration.
B. fragilis phages were concentrated on an inorganic membrane (Whatman 6809-5022) as described by Lucena et al. (15). The membrane was saturated with protein by filtration of 3 ml of a solution containing 3% beef extract. Water (100 ml) was filtered through the membrane to facilitate phage adsorption. The membrane was removed and ground in 5 ml of 0.05 M glycine buffer (pH 9.5) to elute the bacteriophages. The eluate was rapidly neutralized to pH 7.2 to produce the concentrate.
Sample decontamination.
Fungi and bacteria were removed from the water and concentrate samples by filtration through a 0.22-μm-pore-size Millex GV membrane (Millipore) saturated with fetal calf serum. The decontaminated samples were the filtrates.
Infectious enterovirus quantification.
Analysis of the samples was carried out directly and after concentration. Water or concentrate (25 μL) was added to a well containing 175 μl of a suspension of 7.5 × 104 BGM (monkey kidney cell line) cells ml−1 in Eagle’s minimal essential medium (Eurobio) containing 2% fetal calf serum. Four hundred wells were used for each sample (five plates each containing 80 wells), so that 10 ml of each sample was tested. Sixteen wells per plate contained only the 175 μl of cells to serve as controls.
The plates were incubated for 6 days at 37°C in a 5% CO2–95% air atmosphere. The plates were then examinated under a light microscope, and wells in which there was a cytopathogenic effect (CPE) on the cells were identified. Two subcultures were made for each well with the same protocol. The number of wells with a CPE were counted, and the most probable number of cytopathogenic units (MPNCU) was calculated (16). The results are expressed as MPNCU · liter−1.
Viral genome detection.
Analysis of the samples was carried out directly and after concentration.
(i) Extraction.
Viral genome RNA was extracted as described by Chomczynski and Sacchi (4). Sample aliquots (100 μl) were treated with 500 μl of extraction solution (4 M guanidine thiocyanate, 25 mM sodium citrate [pH 7], 0.5% N-lauroylsarcosine, 0.1 M β-mercaptoethanol). Fifty microliters of 2 M sodium acetate (pH 5.2) was added, followed by 600 μl of phenol-chloroform-isoamyl alcohol (25:24:1). The RNA in the aqueous phase after centrifugation (10,000 × g for 20 min at 4°C) was precipitated with 1 volume of absolute ethanol in the presence of 20 μg of glycogen ml−1 for 1 h at −20°C. A pellet obtained by centrifugation (10,000 × g for 10 min at 4°C) was suspended in 300 μl of extraction solution–600 μl of absolute ethanol. A second precipitation (1 h at −20°C) was carried out, followed by centrifugation. The pellet was washed with 1 ml of 70% ethanol and dried, and then the resulting RNA precipitate was dissolved in 20 μl of diethyl pyrocarbonate-treated deionized water.
(ii) Primers.
The primers corresponded to the 5′ noncoding region (14) and were selected because they corresponded to sequences present in many enterovirus serotypes: primer 2 (nucleotides 164 to 184), primer 3 (nucleotides 584 to 603), and primer f2 (nucleotides 516 to 530).
(iii) cDNA synthesis.
cDNA was synthesized from the extracted RNA by use of oligo (dT)15 (Promega). We used a reaction volume of 20 μl containing 4 μl of 5× reverse transcription buffer (250 mM Tris-HCl [pH 8.4], 50 mM MgCl2, 350 mM KCl, 15 mM dithiothreitol, 2.5 mM spermidine), 40 U of RNase inhibitor (Promega), 0.25 mM each deoxynucleoside triphosphate (Perkin-Elmer Cetus), 1 mM oligo(dT)15, 10 U of avian myeloblastosis virus reverse transcriptase (Promega), 6 μl of diethyl pyrocarbonate-treated H2O, and 5 μl of RNA extract. Reverse transcription was performed at 42°C for 30 min. RNA-DNA hybrids were denatured, and reverse transcriptase was inactivated by heating to 95°C for 5 min. The resulting cDNA was then amplified by PCR.
(iv) PCR amplification.
PCR was carried out with 5 μl of cDNA mixed with 95 μl of PCR mix, containing 10 μl of 10× PCR buffer (500 mM KCl, 100 mM Tris-HCl [pH 8.3], 15 mM MgCl2, 0.001% [wt/vol] gelatin), 0.25 mM each deoxynucleoside triphosphate, 0.1 mM each primer (primers 2 and 3), and 2.5 U of Taq polymerase (Perkin-Elmer Cetus). Thirty cycles of amplification were performed as follows: 30 s at 96°C for denaturation, 45 s at 50°C for hybridization, and 60 s at 72°C for elongation. A fragment of 439 bp was amplified.
(v) Seminested PCR.
One microliter of the first PCR amplification product was mixed with 99 μl of PCR mix as described above, except that primers 2 and f2 were used. The temperature cycles were as described above. A 366-bp fragment was amplified. The amplified fragment was separated by electrophoresis on a 2% agarose gel containing 0.5 μg of ethidium bromide ml−1 and examined under UV light.
Phage quantification.
B. fragilis HSP40 grown on Bacteroides phage recovery medium was used in the quantification of B. fragilis phages. Phages contained in 1 ml of wastewater or concentrated wastewater were quantified by the double-agar-layer method (21). E. coli C strain ATCC 13706 grown in modified Scholten’s broth was used in the quantification of somatic coliphages. Phages contained in 1 ml of wastewater were quantified by the single-agar-layer method (10). The results for both phages are expressed in PFU · liter−1.
RESULTS
Sixteen samples from treatment line A, 16 samples from treatment line B1, and 16 samples from treatment line B2 were tested for somatic coliphages, infectious enteroviruses, the enterovirus genome, and B. fragilis phages.
Preliminary study.
A preliminary study was carried out to determine whether the two types of bacteriophages and the enteroviruses were present in sufficient amounts to be detected directly in the samples or whether concentration of the samples was necessary. The 16 samples from each treatment line (A, B1, and B2) were analyzed directly without concentration (Table 1): somatic coliphages were found in all 48 samples, B. fragilis phages were detected in 6 of the 48 samples, the enterovirus genome was detected in 9 of the 48 samples, and no infectious enteroviruses were isolated. The small number of samples testing positive by direct analysis showed that preliminary concentration was necessary for B. fragilis phage and enterovirus detection. The high density of somatic coliphages present in all samples made direct detection possible for these phages.
TABLE 1.
Number of samples containing somatic coliphages, B. fragilis phages, infectious enteroviruses, and the enterovirus genome, as detected by direct water analysis
| Treatment line (no. of samples) | No. of samples containing:
|
|||
|---|---|---|---|---|
| Somatic coliphages | B. fragilis phages | Infectious enteroviruses | Enterovirus genome | |
| A (16) | 16 | 6 | 0 | 7 |
| B1 (16) | 16 | 0 | 0 | 2 |
| B2 (16) | 16 | 0 | 0 | 0 |
| Total (48) | 48 | 6 | 0 | 9 |
The levels of somatic coliphages were determined directly from water samples, those of the infectious enteroviruses and the enterovirus genome were determined after concentration from 20 liters of water, and those of B. fragilis phages were determined after concentration from 100 ml of water.
Water from line A.
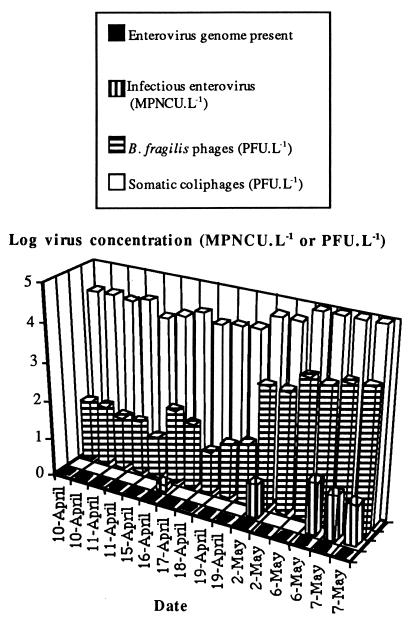
The results obtained for the four types of samples are shown in Fig. 1. Somatic coliphages were detected in the 16 samples analyzed at concentrations of 1.1 × 104 to 9.7 × 104 PFU · liter−1. The mean somatic coliphage concentration for the first 10 samples (10 April to 19 April) was 1.9 × 104 PFU · liter−1, and the mean concentration for the last six samples (2 May to 7 May) was 7.9 × 104 PFU · liter−1. There was a significant difference (P < 0.01; Mann-Whitney test) between these two series of samples, which differed in somatic coliphage content by a factor of 4. B. fragilis phages were found in the 16 samples analyzed at concentrations of 1.3 × 101 to 5.1 × 103 PFU · liter−1. There was an increase in phage contamination between the first 10 samples (10 April to 19 April) and the last 6 samples (2 May to 7 May). The mean concentrations of B. fragilis phages were 3.5 × 101 PFU · liter−1 between 10 April and 19 April and 3.4 × 103 PFU · liter−1 between 2 May and 7 May. There was a significant difference (P < 0.01; Mann-Whitney test) between these two series of samples, which showed a 97-fold increase in B. fragilis phage content.
FIG. 1.
Qualitative detection of the enterovirus genome and concentrations of infectious enteroviruses, B. fragilis phages, and somatic coliphages in treatment line A water. L, liter.
Infectious enteroviruses were isolated from 5 of the 16 samples analyzed at concentrations of 1.5 to 22.5 MPNCU · liter−1. The mean concentration was between 3.5 and 4.5 MPNCU · liter−1. The lower value of this interval was the average concentration calculated assuming that the samples testing negative had a virus concentration of zero, and the upper value was that calculated assuming that the samples testing negative had a virus concentration equal to the detection threshold (1 MPNCU · liter−1). Dissociation of the two sample series described above showed that only 1 of 10 samples was positive between 10 April and 19 April. It contained 1.5 MPNCU · liter−1 (mean concentration, 0.15 to 1 MPNCU · liter−1). Four of six samples were positive between 2 May and 7 May (mean concentration, 9.5 to 10 MPNCU · liter−1). Thus, the concentration of infectious enteroviruses increased by factors of 10 to 67.
The viral genome was present in all of the samples analyzed.
Water from line B1.
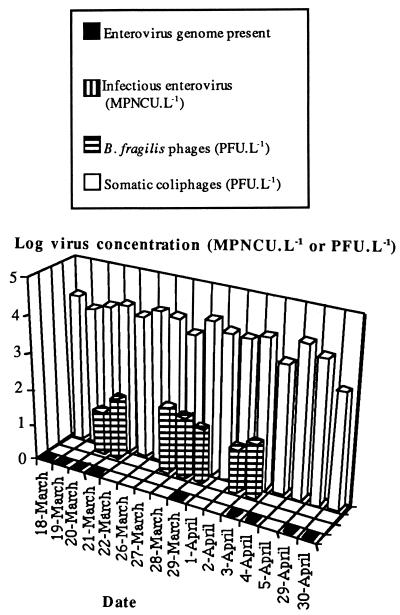
The results for B1 are shown in Fig. 2. Somatic coliphages were present in all 16 samples analyzed at concentrations of 6.5 × 103 to 1.7 × 104 PFU · liter−1, with a mean concentration of 9.8 × 103 PFU · liter−1. B. fragilis phages were present in 7 of the 16 samples analyzed at concentrations of 1.7 × 101 to 6.7 × 101 PFU · liter−1, with a mean concentration between 1.7 × 101 and 2.4 × 101 PFU · liter−1. No infectious enteroviruses were detected, although the enterovirus genome was detected in 9 of the 16 samples analyzed. There was no significant difference between the number of samples testing positive for B. fragilis phages and the number testing positive for the enterovirus genome (P > 0.1). However, the number of samples testing positive for somatic coliphages was significantly higher than that testing positive for the enterovirus genome or for B. fragilis phages (P = 0.01).
FIG. 2.
Qualitative detection of the enterovirus genome and concentrations of infectious enteroviruses, B. fragilis phages, and somatic coliphages in treatment line B1 water. L, liter.
Water from line B2.
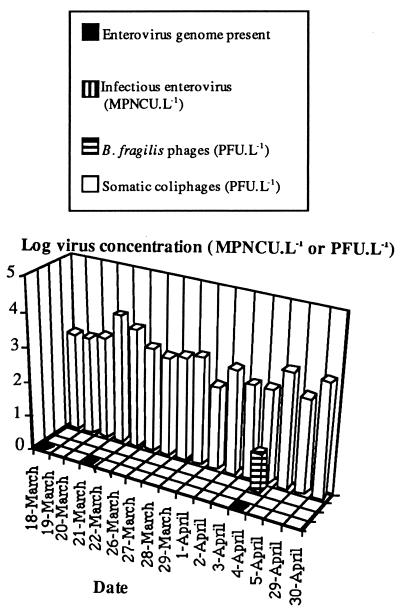
The results for B2 are shown in Fig. 3. Coliphages were present in the 16 samples analyzed at concentrations of 2.5 × 102 to 5.5 × 103 PFU · liter−1, the mean concentration being 1.4 × 103 PFU · liter−1. B. fragilis phages were present in only one sample, at a concentration of 13 PFU · liter−1, for a mean concentration between 0.8 and 13 PFU · liter−1. No infectious enteroviruses were detected, although the enterovirus genome was detected in 3 of the 16 samples analyzed. As so few samples tested positive for B. fragilis phages and the enterovirus genome, statistical comparison was not possible. However, the number of samples testing positive for somatic coliphages was significantly higher than that testing positive for the enterovirus genome or for B. fragilis phages (P < 0.01).
FIG. 3.
Qualitative detection of the enterovirus genome and concentrations of infectious enteroviruses, B. fragilis phages, and somatic coliphages in treatment line B2 water. L, liter.
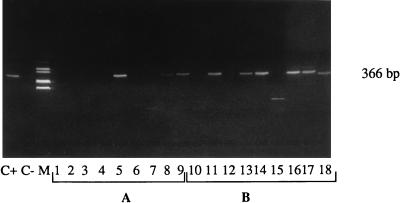
Figure 4 shows some results obtained for the detection of the enterovirus genome in three unconcentrated wastewater samples from each treatment line (A, B1, and B2) and the same three wastewater samples after concentration. The three samples (3, 4, and 5 April) taken from treatment line B2 were negative for the enterovirus genome before concentration on glass wool (A1, A2, and A3 in Fig. 4), but one sample became positive after concentration (B10, B11, and B12 in Fig. 4). For treatment line B1, one of three samples (3, 4, and 5 April) was positive before concentration (A4, A5, and A6 in Fig. 4), and two of three samples were positive after concentration (B13, B14, and B15 in Fig. 4). Finally, for treatment line A, two of three samples (15, 16, and 17 April) were positive before concentration (A7, A8, and A9 in Fig. 4), and all three samples were positive after concentration (B16, B17, and B18 in Fig. 4).
FIG. 4.
Detection of the enterovirus genome in unconcentrated (A) and 67-fold-concentrated (B) treated wastewaters. The seminested RT-PCR products were analyzed by gel electrophoresis. Lanes: C+, positive control (deionized water with 10 MPNCU of poliovirus 1 ml−1); C−, negative control (deionized water); 1, 2, and 3, unconcentrated treatment line B2 wastewater (3, 4, and 5 April); 4, 5, and 6, unconcentrated treatment line B1 wastewater (3, 4, and 5 April); 7, 8, and 9, unconcentrated treatment line A wastewater (15, 16, and 17 April); 10 to 18, samples from lanes 1 to 9 concentrated (67-fold) by adsorption-elution on glass wool; M, markers.
Correlation between phage concentration and the presence of enteroviruses.
We used the results obtained for the three categories of treated wastewater to evaluate the possible relationships between bacteriophage concentration and the presence of enteroviruses (infectious or genome).
There was a significant correlation (t test) between the concentration of somatic coliphages and the presence of infectious enteroviruses (P = 0.0014) and between the somatic coliphage concentration and the presence of the enterovirus genome (P < 0.0001) (Table 2). Infectious enteroviruses were isolated from 22% of the samples in which the somatic coliphage concentration was greater than 104 PFU · liter−1, whereas the enterovirus genome was detected in 87% of these samples. No enteroviruses were isolated when the somatic coliphage concentration was between 102 and 104 PFU · liter−1, and the enterovirus genome was detected in only 29 to 33% of these samples.
TABLE 2.
Relationship between the concentration of somatic coliphages and the presence of enteroviruses (infectious or genome)
| Somatic coliphage concn (PFU · liter−1) | No. of samples | % Detection of:
|
|
|---|---|---|---|
| Infectious enteroviruses | Enterovirus genome | ||
| >104 | 23 | 22 | 87 |
| 103–104 | 18 | 0 | 33 |
| 102–103 | 7 | 0 | 29 |
There was a significant correlation (t test) between the concentration of B. fragilis phages and the presence of infectious enteroviruses (P = 0.0004) and between the B. fragilis phage concentration and the presence of the enterovirus genome (P = 0.0001) (Table 3). Infectious enteroviruses were isolated from 71% of the samples in which the B. fragilis phage concentration was greater than 102 PFU · liter−1, and the enterovirus genome was detected in 100% of these samples. No enteroviruses were isolated at phage concentrations of 101 to 102 PFU · liter−1, although the enterovirus genome was detected in 75% of these samples. At phage concentrations below the detection threshold, no infectious enteroviruses were isolated, but the enterovirus genome was detected in 29% of the samples.
TABLE 3.
Relationship between the concentration of B. fragilis phages and the presence of enteroviruses (infectious or genome)
| B. fragilis phage concn (PFU · liter−1) | No. of samples | % Detection of:
|
|
|---|---|---|---|
| Infectious enteroviruses | Enterovirus genome | ||
| >102 | 7 | 71 | 100 |
| 10–102 | 17 | 0 | 75 |
| <10 | 24 | 0 | 29 |
DISCUSSION
This study evaluated the value of a concentration step for the detection of bacteriophages, enteroviruses, and the enterovirus genome in treated wastewater. It also investigated whether somatic coliphages, B. fragilis phages, and the enterovirus genome could be used as indicators of enterovirus contamination. The concentration step was necessary for all of the samples tested except for the somatic coliphages. Enough somatic coliphages were present in treated wastewater for direct analysis.
The number of samples testing positive after concentration was significantly higher than that before concentration for the infectious enteroviruses (5 versus 0), the enterovirus genome (28 versus 9), and B. fragilis phages (24 versus 6). The requirement for a concentration step is consistent with some studies of infectious enteroviruses (20) and the enterovirus genome (5). However, it conflicts with the genome detection results of other studies (1, 13) showing the inhibition of RT-PCR after concentration of surface water on electropositive filters. Simultaneous concentration of both the viral genome and substances inhibiting RT-PCR may occur during the virus concentration step. The concentration of inhibitors may depend on the support medium used; there may be less coconcentration of inhibitors with glass wool than with electropositive filters.
Based on the number of positive samples, the concentration of somatic coliphages (P < 0.01) was significantly higher than that of B. fragilis phages, which were present at a frequency similar (P > 0.1) to that of the enterovirus genome. The enterovirus genome was present in significantly more samples (P < 0.01) than infectious enteroviruses. This classification is consistent with that of Tartera et al. (22), who showed that the somatic coliphage concentration is, on average, 100 times higher than the B. fragilis phage concentration, which is 10 times higher than the concentration of enteroviruses.
The number of samples testing positive for the enterovirus genome was significantly higher than the number of samples containing infectious enteroviruses (28 versus 5). Free viral RNA is rapidly broken down in wastewater (13), so the difference in the rates of detection of these two factors may have been due to the presence of noninfectious viral capsids containing the genome or to the presence of infectious enteroviruses that do not have a CPE on BGM cell cultures, such as particular coxsackie virus group A serotypes. Thus, the presence of the enterovirus genome may be regarded only as an indicator of more or less recent viral contamination. It is not possible to determine the relationship between the presence of the enterovirus genome and infectious enterovirus concentration unless experiments are carried out under conditions optimal for the isolation of all types of enteroviruses, which would mean the inoculation of a large number of cell culture systems. However, the genome may be a useful indicator because it is rapidly detected by this sensitive method and, in this study, no infectious enteroviruses were detected when the enterovirus genome was not detected.
As the level of treatment increased among treatment line A (biological treatment, 8 to 12 h), treatment line B1 (biological treatment with alternating phases of aeration and anoxia, approximately 30 h), and treatment line B2 (secondary and tertiary treatments), the concentrations of infectious enteroviruses, somatic coliphages, and B. fragilis phages decreased, as did the number of samples testing positive for the enterovirus genome. There was a significant correlation between infectious enterovirus concentration and that of somatic coliphages and between infectious enterovirus concentration and B. fragilis phage concentration for all treatments. This correlation made it possible to define bacteriophage concentration thresholds over which enteroviruses were detected in the samples. These thresholds were 104 PFU · liter−1 for somatic coliphages and 102 PFU · liter−1 for B. fragilis phages. Above these concentrations, infectious enteroviruses were detected in 22% (coliphages) and 71% (B. fragilis phages) of the samples, whereas below these concentrations, no infectious enteroviruses were isolated. Similar correlations were found between these phages and the enterovirus genome. At somatic coliphage concentrations between 102 and 103 PFU · liter−1 and B. fragilis phage concentrations of <10 PFU · liter−1, the enterovirus genome was detected in almost 30% of the samples.
The study of various markers is particularly useful in cases of failure of a treatment line. There was a destabilization of the bacterial flora involved in biological treatment on treatment line A between 2 May and 7 May. It was accompanied by a decrease in the yield from the secondary settling step. This treatment failure resulted in increases in the levels of suspended solids from 12.5 mg · liter−1 to 22 mg · liter−1, in the 5-day biochemical oxygen demand from 19.5 mg · liter−1 to 24 mg · liter−1, and in the chemical oxygen demand from 67 mg · liter−1 to 83 mg · liter−1, whereas these factors were constant at the plant inlet over the entire sampling period. During this period, the concentration of enteroviruses increased by factors of 10 to 67. The results obtained during this treatment failure showed that the detection of the enterovirus genome cannot be used to evaluate changes in the concentration of infectious enteroviruses. This result could only be achieved by adapting existing genome quantification protocols to wastewater.
B. fragilis phages were good indicators during treatment failure. The phage concentration increase (by a factor of 97) was higher than that for enteroviruses (by factors of 10 to 67). This strong correlation between enterovirus and B. fragilis phage concentrations has been observed in other environments, such as marine sediments (11). Changes in somatic coliphage concentration were not related to changes in enterovirus concentration because somatic coliphage concentration increased by a factor of only 4. This result may have occurred because the maximum percentage of adsorbed suspended solids in secondary effluents is about 24% for somatic coliphages, whereas it may be as high as 100% for enteroviruses (7). The significant increase in the levels of suspended solids at the treatment line outlet therefore may have caused a larger increase in the enterovirus concentration than in the coliphage concentration. Somatic coliphages may simply indicate fecal contamination in the broad sense of the term, as suggested by Nieuwstad et al. (19), who observed a strong correlation between the concentrations of these phages and fecal coliform bacteria.
Thus, in the three different types of wastewater tested, B. fragilis phages were good indicators of enterovirus contamination. Despite a significant correlation between somatic coliphage concentration and the presence of infectious enteroviruses, this factor was a poor indicator of fluctuations in enterovirus concentration. The enterovirus genome could be used to evaluate viral contamination of treated wastewater if nucleic acid quantification is carried out and a threshold for the presence of infectious enteroviruses is determined.
ACKNOWLEDGMENTS
We thank Lyonnaise des Eaux (CIRSEE) for financial support. This study was carried out within the framework of the Franco-Israeli cooperation (AFIRST) water management program.
REFERENCES
- 1.Abbaszadegan M, Huber M S, Gerba C, Pepper I L. Detection of enteroviruses in groundwater with the polymerase chain reaction. Appl Environ Microbiol. 1993;59:1318–1324. doi: 10.1128/aem.59.5.1318-1324.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson Y, Strenström A. Waterborne outbreaks in Sweden. Causes and etiology. Water Sci Technol. 1987;19:575–580. [Google Scholar]
- 3.Bosch A, Lucena F, Diez J M, Gajardo R, Blasi M, Jofre J. Waterborne viruses associated with hepatitis outbreak. Res Technol Manag. 1991;3:80–83. [Google Scholar]
- 4.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 5.Gantzer C, Senouci S, Maul A, Levi Y, Schwartzbrod L. Enterovirus genomes in wastewater: concentration on glass wool and glass powder and detection by RT-PCR. J Virol Methods. 1997;65:265–271. doi: 10.1016/s0166-0934(97)02193-9. [DOI] [PubMed] [Google Scholar]
- 6.Geldenhuys J C, Pretorius P D. The occurrence of enteric viruses in polluted water, correlation to indicator organisms and factors influencing their numbers. Water Sci Technol. 1989;21:105–109. [Google Scholar]
- 7.Gerba C P, Stagg C H, Abadie M. Characterization of sewage solid-associated viruses and behavior in natural waters. Water Res. 1978;12:805–812. [Google Scholar]
- 8.Havelaar A H, Furuse K, Hageboom W M. Bacteriophages and indicator bacteria in human and animal faeces. J Appl Bacteriol. 1986;60:252–262. doi: 10.1111/j.1365-2672.1986.tb01081.x. [DOI] [PubMed] [Google Scholar]
- 9.Havelaar A H, Van Olphen M, Drost Y C. F-specific RNA bacteriophages are adequate model organisms for enteric viruses in freshwater. Appl Environ Microbiol. 1993;59:2956–2962. doi: 10.1128/aem.59.9.2956-2962.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Organization for Standardization. Water quality—detection and enumeration of bacteriophages. Part 2. Enumeration of somatic coliphages. ISO/CD publication 10705-2. Geneva, Switzerland: International Organization for Standardization; 1996. [Google Scholar]
- 11.Jofre J, Blasi M, Bosch A, Lucena F. Occurrence of bacteriophages infecting Bacteroides fragilis and other viruses in polluted marine sediments. Water Sci Technol. 1989;21:15–19. [Google Scholar]
- 12.Jofre J, Bosch A, Lucena F, Girones R, Tartera C. Evaluation of Bacteroides fragilis bacteriophages as indicators of the virological quality of water. Water Sci Technol. 1986;18:167–173. [Google Scholar]
- 13.Kopecka H, Dubrou S, Prevot J, Marechal J, Lopez-Pila J M. Detection of naturally occurring enteroviruses in waters by reverse transcription-polymerase chain reaction and hybridization. Appl Environ Microbiol. 1993;59:1213–1219. doi: 10.1128/aem.59.4.1213-1219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leparc I, Fuchs F, Kopecka H, Aymard M. Use of the polymerase chain reaction with a murine model of picornavirus-induced myocarditis. J Clin Microbiol. 1993;31:2890–2894. doi: 10.1128/jcm.31.11.2890-2894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucena F, Muniesa M, Puig A, Araujo R, Jofre J. Simple concentration method for Bacteroides fragilis bacteriophages in drinking water. J Virol Methods. 1995;55:233–239. doi: 10.1016/0166-0934(95)00034-r. [DOI] [PubMed] [Google Scholar]
- 16.Maul A. Aspects statistiques des méthodes de quantification en virologie. In: Schwartzbrod L, editor. Virologie des milieux hydriques. 1991. pp. 143–171. [Google Scholar]
- 17.Merrett H, Pattinson C, Stackhouse C, Cameron S. The incidence of enteroviruses around the Welsh Coast—a one year intensive survey. In: Wheeler D, Richardson M, Bridges J, editors. Watershed 89: the future of water quality in Europe. II. Oxford, England: Pergamon Press; 1989. pp. 345–351. [Google Scholar]
- 18.Morinigo M A, Wheeler D, Berry C, Jones C, Munoz M A, Cornax R, Borrego J J. Evaluation of different bacteriophage groups as faecal indicators in contaminated natural waters in southern England. Water Res. 1992;26:267–271. [Google Scholar]
- 19.Nieuwstad T J, Mulder E P, Havelaar A H, Van Olphen M. Elimination of micro-organisms from wastewater by tertiary precipitation and simultaneous precipitation followed by filtration. Water Res. 1988;22:1389–1397. [Google Scholar]
- 20.Schwartzbrod L, Vilagines P, Schwartzbrod J, Sarrette B, Vilagines R, Collomb J. Evaluation of the viral population in two wastewater treatment plants. Water Res. 1985;19:1353–1356. [Google Scholar]
- 21.Tartera C, Jofre J. Bacteriophages active against Bacteroides fragilis in sewage-polluted waters. Appl Environ Microbiol. 1987;53:1632–1637. doi: 10.1128/aem.53.7.1632-1637.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartera C, Lucena F, Jofre J. Human origin of Bacteroides fragilis bacteriophages present in the environment. Appl Environ Microbiol. 1989;55:2696–2701. doi: 10.1128/aem.55.10.2696-2701.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilagines P, Sarrette B, Husson G, Vilagines R. Glass wool for virus concentration at ambient water pH level. Water Sci Technol. 1993;27:299–306. [Google Scholar]
- 24.Yao G B. Proceedings of the 1988 International Symposium on Viral Hepatitis and Liver Disease. 1989. Clinical and natural history of hepatitis A in an epidemic in Shanghai. [Google Scholar]