Abstract
Introduction
The utility of arithmetic product of urinary tissue metalloproteinase inhibitor 2 (TIMP2) and insulin-like growth factor-binding protein 7 (IGFBP7) concentrations has been widely accepted on early diagnosis of acute kidney injury (AKI). However, which organ is the main source of those two factors and how the concentration of IGFBP7 and TIMP2 changed in serum during AKI still remain to be defined.
Methods
In mice, gene transcription and protein levels of IGFBP7/TIMP2 in the heart, liver, spleen, lung, and kidney were measured in both ischemia-reperfusion injury (IRI)- and cisplatin-induced AKI models. Serum IGFBP7 and TIMP2 levels were measured and compared in patients before cardiac surgery and at inclusion (0 h), 2 h, 6 h, and 12 h after intensive care unit (ICU) admission, and compared with serum creatinine (SCr), blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), and serum uric acid (UA).
Results
In mouse IRI-AKI model, compared with the sham group, the expression levels of IGFBP7 and TIMP2 did not change in the kidney, but significantly upregulated in the spleen and lung. Compared with patients who did not develop AKI, the concentration of serum IGFBP7 at as early as 2 h after ICU admission (sIGFBP7-2 h) was significantly higher in patients who developed AKI. The relationships between sIGFBP7-2 h in AKI patients and log2 (SCr), log2 (BUN), log2 (eGFR), and log2 (UA) were statistically significant. The diagnostic performance of sIGFBP7-2 h measured by the macro-averaged area under the receiver operating characteristic curve was 0.948 (95% CI, 0.853–1.000; p < 0.001).
Conclusion
The spleen and lung might be the main source of serum IGFBP7 and TIMP2 during AKI. The serum IGFBP7 value demonstrated good predictive accuracy for AKI following cardiac surgery within 2 h after ICU admission.
Keywords: Acute kidney failure, Cardiac surgery, Insulin-like growth factor-binding protein 7, Serum biomarker, Tissue metalloproteinase inhibitor 2
Introduction
Acute kidney injury (AKI) complicates recovery from cardiac surgery in up to 40% of patients and is associated with an increased risk of death during hospitalization [1]. An accurate assessment of an individual patient’s risk provides an opportunity to directly influence clinical decision-making and potentially prevent AKI. Strategies aimed at optimizing hemodynamics and fluid status and avoiding hyperglycemia and nephrotoxic exposures have been shown to be effective at preventing moderate or severe AKI in high-risk patients after cardiac surgery. Thus, it is widely acknowledged that these patients should be identified as early as possible. A major limitation in clinical practice is the lack of reliable tools to complete this task in a timely and practical manner. Although novel kidney biomarkers are effective and included in some guidelines, these injury biomarkers are not universally available [2].
In the last few years, “NephroCheck®,” the combination of urinary insulin-like growth factor-binding protein 7 (IGFBP7) and tissue metalloproteinase inhibitor 2 (TIMP2) (IGFBP7*TIMP2), has been shown to be predictive of AKI in large diverse cohorts of critically ill patients [3–7]. Despite its widespread acceptance, it remains unclear where the biomarker described above comes from. In both experimental and clinical AKI studies, AKI-induced urinary IGFBP7/TIMP2 elevations are not due to stress-induced gene transcription in kidney [8–11]. Rather, increased filtration, decreased tubule reabsorption, and proximal tubule cell IGFBP7/TIMP2 urinary leakage seem to be the most likely mechanisms [11]. If so, the expression of IGFBP7/TIMP2 in the source organs and then their protein levels in serum should be changed earlier than that in urine. In this study, the source organs of IGFBP7/TIMP2 were investigated in mice AKI models, and we also investigated the use of serum IGFBP7 (sIGFBP7) and serum TIMP2 (sTIMP2) for prediction of AKI in a cohort of adults undergoing cardiac surgery.
Materials and Methods
Mouse Experiments
All experiments were conducted with male C57BL/6 mice (6–8 week old) maintained under routine vivarium conditions. Free food and water access were supplied throughout. All surgeries were performed under deep pentobarbital anesthesia. Two different models of AKI were used as detailed below.
Ischemia-Reperfusion Injury
Mice were randomly assigned to the experimental groups. Mice were deeply anesthetized with pentobarbital (1% w/v, in PBS, 2 mL/kg) and subjected to a midline abdominal excision. Both renal pedicles were visualized and occluded for 20 min or 35 min with microvascular clamps. After clamp removal, uniform reperfusion was confirmed by return of normal kidney color. The abdominal incisions were then sutured in two layers. Body temperature was maintained at approximately 37°C throughout. At 0, 2, 4, 6, or 24 h after reperfusion, mice were reanesthetized, respectively (n = 4–6 at each time point/group), and the abdominal incisions were opened; plasma, heart, liver, spleen, lung, or kidney was obtained. The data obtained were compared with those in corresponding normal controls.
Cisplatin-Induced Nephrotoxic AKI
Eight mice were randomly assigned to the experimental groups. AKI was induced by intraperitoneal injection of a single-dose saline or 30 mg/kg cisplatin (479306, Sigma). Seventy-two hours later, mice were deeply anesthetized, and the abdominal cavities were opened to obtain blood, heart, liver, spleen, lung, and kidney. The results were compared with those in four normal controls.
mRNA Analyses
Total RNAs were extracted from heart, liver, spleen, lung, or kidney using TRIzol (Sigma, T9424) following the manufacturer’s instruction. The concentration and the purity of RNA were measured spectrophotometrically using NanoDrop (NanoDrop Technologies). Complementary DNA was synthesized (PrimeScript RT Master Mix, RR036B, Takara, Japan), and real-time quantitative PCRs were performed using TB Green Master Mix (RR820B, Takara). All samples were analyzed in duplicate, and all quantitative data from qRT-PCR were normalized to ACTB (ACTIN). Sequences of primers are given in online supplementary Table S1 (for all online suppl. material, see https://doi.org/10.1159/000531489).
Western Blot Analysis
Protein was obtained from tissues using a chilled lysis buffer containing protease inhibitor and phosphatase inhibitor. Lysates were centrifuged at 12,000 g for 10 min at 4°C. Equal amounts of sample proteins (40 μg–80 μg) mixed with loading buffer were separated in 10% (wt/vol) SDS/PAGE gels. Afterward, proteins were transferred from SDS/PAGE gels to 0.45 μm polyvinylidene fluoride membranes. Unspecific antibody binding was prevented by blocking for 1 h at room temperature with 5% nonfat milk powder in triethanolamine-buffered saline/Tween 20 (TBST). Blots were incubated with primary antibodies overnight at 4°C. The primary antibodies used in this study were rabbit anti-IGFBP7 (abs136651, Absin, 1:1,000), rabbit anti-TIMP2 (ab180630, Abcam, 1:1,000), and mouse anti-β-actin (100166-MM10, Sino Biological, 1:5,000). After three times of washing in TBST, the blots were incubated with HRP-conjugated goat anti-rabbit antibody (A0208, Beyotime) or HRP-conjugated goat anti-mouse antibody (A0216, Beyotime) diluted 1:5,000 in TBST for 1 h. Chemiluminescent signal was detected using chemiluminescence substrate (Tanon, Shanghai). After chemiluminescent detection, β-actin was performed on the same blots as housekeeping protein. The density of the bands was measured using ImageJ software and normalized to healthy controls.
Online Data Collection
Single-nucleus ATAC and RNA sequencing (snRNA-seq) data of human normal kidney were achieved from http://humphreyslab.com/SingleCell/ [12]. Protein expression of IGFBP7 and TIMP2 in renal tubule segment was analyzed by online tools/database (https://esbl.nhlbi.nih.gov/KTEA/) [13].
Study Participants
The study included adults (18 years and older) who underwent selective cardiac surgery at Zhongshan Hospital in Shanghai, China. Patients who were receiving long-term dialysis, required preoperative dialysis (up to 6 months prior to surgery), or had preoperative serum creatinine (SCr) values of 4 mg/dL or higher were excluded. Serum samples for measurement of IGFBP7 and TIMP2 were collected prior to surgery (presurgery), 0 h, 2 h, 6 h, and 12 h after intensive care unit (ICU) admission. Clinical data for the study were abstracted from hospital records and included patient demographics, medical history, surgical procedure, left ventricular ejection fraction and extracorporeal circulation time, surgical risk score (EuroSCORE), SCr, blood urea nitrogen (BUN), estimated glomerular filtration rate (eGFR), and uric acid (UA) for AKI assessment.
Clinical Endpoints
The primary endpoints were AKI and moderate-to-severe AKI within 7 days following surgery. The study used a modified KDIGO definition [14] for AKI diagnosis by using the most recent preoperative SCr as baseline.
sIGFBP7 and TIMP7 Measurement
Plasma samples were drawn and stored at −80°C. IGFBP7 and TIMP2 protein levels were measured in plasma using commercially available ELISAs (Human IGFBP7-rp1/IGFBP7, R&D System, DY1334-05), Ancillary Reagent Kit 3 (R&D System, DY009), and Human TIMP2 (R&D System, DTM200) at presurgery, 0 h, 2 h, 6 h, and 12 h after ICU admission. All samples were run on first freeze-thaw cycles in a blinded fashion (to treatment arm or visit) and during the same period, thereby minimizing interassay variations.
Statistical Methods
Data were presented as mean ± standard deviation continuous variables or N (%) for categorical variables. Comparisons were performed using t test for continuous variables and χ2 test for categorical variables, respectively. Log transformation was used to normalize the values of concentration of plasma biomarkers. Receiver operator characteristics (ROC) curves were employed to test the predictive ability of sIGFBP7 or/and sTIMP2 for the primary endpoints, with statistical analyses of area under the ROC curve (AUC), sensitivity, and specificity. Pearson correlation analyses were used to evaluate the associations of biomarker with continuous SCr, BUN, eGFR, or UA. A 2-sided p value <0.05 was used as the threshold of statistically significant. GraphPad Prism (version 9.3.0) was used for statistical analyses.
Results
The Different Expression Profiles of IGFBP7 and TIMP2 in Ischemia-Reperfusion or Cisplatin-Injured Kidney
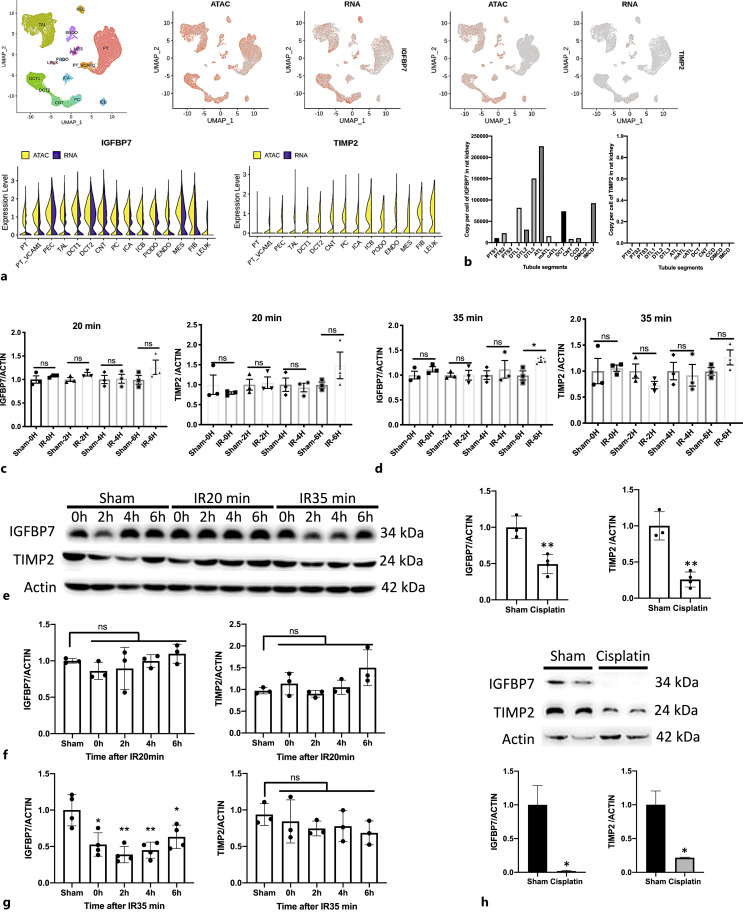
Databases of single-nucleus ATAC and snRNA-seq of adult human normal kidney were used for analyzing the baseline expression level of renal IGFBP7 and TIMP2, and we found that all nephron tubular segments showed greater chromatin accessibility in IGFBP7, but much lower chromatin accessibility in TIMP2 (shown in Fig. 1a). Similarly, in the snRNA-seq datasets, there was a high IGFBP7 but rare TIMP2 transcription in all nephron tubular segments (shown in Fig. 1a). Moreover, based on quantitative proteomics of all renal tubule segments in rat, the IGFBP7 protein can be detected, while the TIMP2 protein was undetectable in nearly all nephron segments (shown in Fig. 1b). As a result, IGFBP7 and TIMP2 have different expression profiles in kidney; the former was highly expressed, while the latter was rarely expressed.
Fig. 1.
Expression of insulin-like growth factor-binding protein 7 (IGFBP7) and tissue metalloproteinase inhibitor 2 (TIMP2) in normal or AKI kidneys. a Distribution of mRNA and cell type-specific chromatin accessible regions of IGFBP7 and TIMP2 in human normal kidney at single cell levels. b The expression of IGFBP7 and TIMP2 protein in rat normal renal tubule segments. c, d The expression levels of IGFBP7 and TIMP2 mRNA were normalized using ACTIN. e–h The expression levels of IGFBP7 and TIMP2 protein were quantified by densitometry and normalized using ACTIN. Data are mean ± SEM. *p < 0.05, **p < 0.01; ns, no significant difference compared with sham groups.
To determine whether the urinary IGFBP7 and TIMP2 were derived from kidney during AKI, we performed short- (20 min) and long-time (35 min) ischemia-reperfusion kidney injury in mice models. The expressions of renal IGFBP7 and TIMP2 were measured 0 h, 2 h, 4 h, and 6 h after reperfusion, respectively. In short-time ischemia-reperfusion injury (IRI)-AKI mice, the expressions of IGFBP7 and TIMP2 were remained at control levels at all time points after reperfusion in both mRNA and protein levels (shown in Fig. 1c, e, f). In the case of long-time IRI-AKI, only a modest increase in IGFBP7 mRNA was observed at 6 h after reperfusion (shown in Fig. 1d), but IGFBP7 protein was dramatically decreased after ischemia-reperfusion from 0 h to 6 h compared with sham groups (shown in Fig. 1e, g). However, in cisplatin-AKI mice model, by 72 h after induction, decreased protein levels of IGFBP7 and TIMP2 were observed concomitant with decreased mRNA levels in kidney (shown in Fig. 1h).
The Spleen and Lung Expressed More IGFBP7 and TIMP2 than Other Organs in IRI-AKI Mice Model
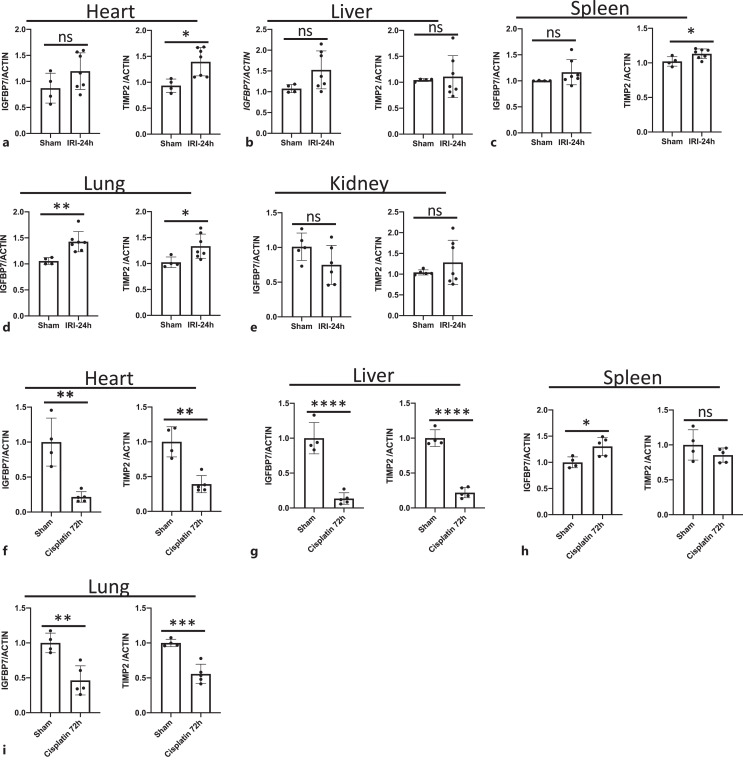
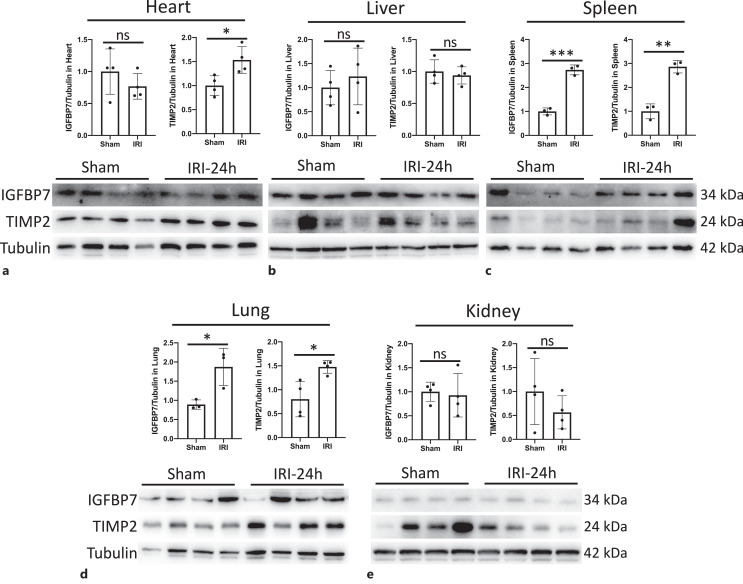
As the source of IGFBP7 and TIMP2 in the urine has not been well described, we performed mRNA and protein analysis in the heart, liver, spleen, lung, and kidney obtained from IRI-AKI mice. We found that IGFBP7 mRNA was only markedly increased in the lung (shown in Fig. 2d), and TIMP2 mRNA was significantly elevated in the heart, spleen, and lung at 24 h post-IRI (shown in Fig. 2a, c, d). In protein levels, IGFBP7 was increased dramatically in the spleen and lung (shown in Fig. 3c, d). The elevated expression of TIMP2 protein was observed in the heart, spleen, and lung (shown in Fig. 3a, c, d). Moreover, we also compared the baseline levels of IGFBP7 and TIMP2 mRNA expression among those five organs, the result showed that the expressive abundance of IGFBP7 was highest in the kidney, and there was no significant difference among the heart, liver, spleen, and lung (shown in online suppl. Fig. S1a). The heart and lung expressed more TIMP2 than the liver, spleen, and kidney (shown in online suppl. Fig. S1b). Thus, these findings suggested that the spleen and lung might be the main sources of sIGFBP7, and the heart, lung, and spleen were the main contributors of sTIMP2 during AKI.
Fig. 2.
Expression of IGFBP7 and TIMP2 mRNA in the heart, liver, spleen, lung, and kidney in IRI- or cisplatin-AKI mouse models. Expression levels of IGFBP7 and TIMP2 in the heart (a, f), liver (b, g), spleen (c, h), lung (d, i), and kidney (e) were normalized using ACTIN. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, no significant difference compared with sham groups.
Fig. 3.
IGFBP7 and TIMP2 were upregulated in the spleen and lung. Expression levels of IGFBP7 and TIMP2 protein in the heart (a), liver (b), spleen (c), lung (d), and kidney (e) were quantified by densitometry and normalized using ACTIN. Data are mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001; ns, no significant difference.
The expression profiles of IGFBP7 and TIMP2 in all five organs from cisplatin-induced AKI were also examined, and we found that both IGFBP7 and TIMP2 mRNAs were significantly decreased in heart, liver, lung, and kidney at 72 h after cisplatin induction (shown in Fig. 2f, g, i, 1h). IGFBP7 mRNA only significantly upregulated in spleen (shown in Fig. 2h). The results suggested that unlike IRI-AKI, cisplatin cannot evoke IGFBP7 or TIMP2 expression in all of the five organs.
Patient Characteristics
Based on the above results, we hypothesized that the sIGFBP7 and sTIMP2 levels might be changed earlier than that in the urine. Clinical serum samples were collected and analyzed to prove this assumption. In total, 20 selective cardiac surgery patients were included. Among them, 60% (12/20) of patients developed AKI within 7 days after surgery, and 10% (2/20) of patients developed stage 2–3 AKI. Compared with patients who did not develop AKI (no-AKI group), patients in AKI group were more likely to be older, with higher proportions of males and hypertension, and lower levels of eGFR (shown in Table 1). Moreover, the left ventricular ejection fraction, cardiopulmonary bypass time, and EuroSCORE were significantly higher in AKI group compared with no-AKI group.
Table 1.
Baseline characteristics for all patients and by AKI status
| All (n = 20) | AKI (n = 12) | No-AKI (n = 8) | p value | |
|---|---|---|---|---|
| Male, n (%) | 16 (80.00) | 10 (83.33) | 6 (75.00) | 0.648 |
| Age, mean ± SD, years | 58.05±14.18 | 66.33±6.01 | 45.63±14 | <0.001 |
| Comorbidities | ||||
| Hypertension, n (%) | 6 (30.00) | 6 (50.00) | 0 (0.00) | 0.017 |
| Diabetes mellitus, n (%) | 3 (15.00) | 3 (25.00) | 0 (0.00) | 0.125 |
| Cerebral infarction, n (%) | 3 (15.00) | 3 (25.00) | 0 (0.00) | 0.125 |
| Chronic renal insufficiency, n (%) | 1 (5.00) | 1 (8.33) | 0 (0.00) | 0.402 |
| Preoperative SCr, μmol/L | 98.30 (23.10) | 103.42 (25.95) | 90.623 (16.68) | 0.235 |
| Preoperative serum BUN, mg/dL | 6.99 (2.66) | 7.74 (3.11) | 5.85 (1.24) | 0.122 |
| Preoperative eGFR, mL/min per 1.73 m2 | 73.75 (18.48) | 66.33 (15.66) | 84.875 (17.48) | 0.023 |
| Preoperative serum UA, μmol/L | 410.25 (127.21) | 428.25 (160.23) | 383.25 (46.76) | 0.453 |
| Procedures | ||||
| Mitral valve replacement, n (%) | 5 (25.00) | 3 (25.00) | 2 (25.00) | >0.99 |
| Aortic valve replacement, n (%) | 4 (20.00) | 4 (33.33) | 0 (0.00) | 0.068 |
| Coronary artery bypass graft, n (%) | 2 (10.00) | 2 (16.67) | 0 (0.00) | 0.224 |
| Valvuloplasty, n (%) | 12 (60.00) | 7 (58.33) | 5 (62.50) | 0.852 |
| Cardiopulmonary bypass, n (%) | 18 (90.00) | 10 (83.33) | 8 (100.00) | 0.224 |
| LVEF, mean ± SD, % | 60.7±10.29 | 57.08±11.75 | 66.13±3.80 | 0.051 |
| CPB, mean ± SD, min | 88.67±43.69 | 108.40±49.71 | 64.00±5.08 | 0.027 |
| EuroSCORE, mean ± SD | 4.45±2.72 | 5.91±2.61 | 2.25±0.46 | 0.001 |
Qualitative variables are shown as n (%).
AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; BUN, blood urea nitrogen; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass time; SD, standard deviation.
sIGFBP7 Concentration at 2 h after ICU Admission Correlated Well with AKI Occurrence in Patients Undergoing Cardiac Surgery
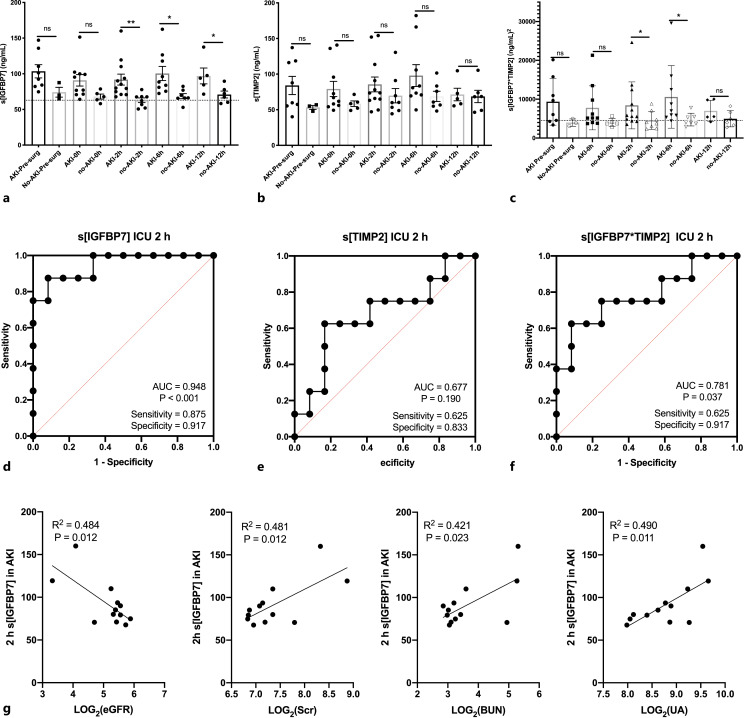
Compared to presurgery, relative sIGFBP7 level in patients who developed AKI (AKI group) has no significant difference either at 0 h, 2 h, 6 h, or 12 h after ICU admission (shown in online suppl. Fig. S2a). While a decrease of sIGFBP7 was observed in no-AKI group at 6 h after ICU admission, the difference still exists at 12 h (shown in online suppl. Fig. S2b). At presurgery and 0 h after ICU, no difference in absolute sIGFBP7 value was observed between AKI and no-AKI groups (shown in Fig. 4a). Interestingly, the sIGFBP7 values in no-AKI group were significantly lower than those in AKI groups at 2 h (62.86 ± 9.25 ng/mL vs. 91.84 ± 26.68 ng/mL), 6 h (68.56 ± 9.38 ng/mL vs. 100.43 ± 29.68 ng/mL), and 12 h (70.87 ± 11.75 ng/mL vs. 96.93 ± 24.78 ng/mL) after ICU admission (shown in Fig. 4a). ROC curves confirmed that the sIGFBP7 at 2 h after ICU admission had the potential ability to predict AKI cases in this study (AUC = 0.948; p < 0.001, sensitivity = 0.875, specificity = 0.917, shown in Fig. 4d; online suppl. Fig. S2c).
Fig. 4.
Predictive value of serum IGFBP7 (sIGFBP7) or/and TIMP2 (sTIMP2 in developing AKI after cardiac surgery. a, b sIGFBP7 or sTIMP2 was compared between AKI patients and no-AKI patients at different time points after cardiac surgery. c sIGFBP7*sTIMP2 values were compared between AKI patients and no-AKI patients at different time points after cardiac surgery. ROC curves for prediction of AKI using sIGFBP7 (d), sTIMP2 (e), and sIGFBP7*sTIMP2 (f). g Linear regression and correlation between sIGFBP7 2 h after ICU admission and eGFR, SCr, BUN, and UA. Log conversion was performed due to marked variations among the different groups. Data are mean ± SEM. *p < 0.05, **p < 0.01; ns, no significant difference.
We further analyzed the relationship of sIGFBP7 value of presurgery (presurgery sIGFBP7) or 2 h after ICU (sIGFBP7-2 h) with SCr, BUN, eGFR, or UA, respectively, in AKI groups. As shown in Figure 4g, sIGFBP7-2 h strongly correlated with SCr (r = 0.475; p = 0.012), BUN (r = 0.649; p = 0.023), and UA (r = 0.700; p = 0.011), and inversely correlated with eGFR (r = −0.696; p = 0.012). However, in the no-AKI groups, both sIGFBP7-2 h and presurgery sIGFBP7 were not correlated with any of those factors (shown in online suppl. Fig. S2d–g).
Relative sTIMP2 Concentration Elevated at Inclusion after ICU Admission in the AKI Groups but Cannot Predict AKI Occurrence
The dynamic changes of sTIMP2 were also examined in AKI and no-AKI groups, respectively. The results showed that relative fold changes of sTIMP2 in AKI groups significantly increased at inclusion (0 h) after ICU admission compared with those in presurgery, but the difference disappeared at 2–12 h after ICU admission (shown in online suppl. Fig. S3a). In no-AKI groups, no changes in relative sTIMP2 concentration were observed at any time point after ICU admission (shown in online suppl. Fig. S3b). The sTIMP2 value also showed no significant difference between AKI and no-AKI groups at presurgery or at any time point after ICU admission (shown in Fig. 4b). ROC curves confirmed that the sTIMP2 had poor capacity to identify AKI even at 2 h after ICU admission (AUC: 0.677; p = 0.19; shown in Fig. 4e; online suppl. Fig. S3c). In no-AKI groups, the sTIMP2 value showed no significant correlation with either SCr, BUN, eGFR or UA (shown in online suppl. Fig. S3d–f). However, the statistical correlation between sTIMP2 and UA was observed at presurgery in AKI groups (R2 = 0.701; p = 0.01, shown in online suppl. Fig. S3g).
The Combination of sIGFBP7 and sTIMP2 Did Not Perform Well in Predicting AKI
Because these markers in urinary appeared to have additive predictive value when used together in the discovery cohort, we also evaluated the combination of sIGFBP7 and sTIMP2 (sIGFBP7*sTIMP2) in AKI predicting performance. Compared to presurgery, the absolute sIGFBP7*sTIMP2 value did not change significantly after ICU admission in either AKI or no-AKI groups (shown in online suppl. Fig. S4a, b), while the sIGFBP7*sTIMP2 value was higher at 2 h after ICU admission in AKI than in no-AKI groups (shown in Fig. 4c). This difference still existed at 6 h but disappeared at 12 h after ICU admission. ROC curves of sIGFBP7*sTIMP2 values exhibited an AUC of 0.781 (p = 0.037) and 0.873 (p = 0.013) for development of AKI (stage 1 or 2) at 2 h and 6 h after ICU admission, respectively (shown in Fig. 4f; online suppl. S4c). There was no correlation between sIGFBP7*sTIMP2 values and SCr in either AKI or no-AKI groups at presurgery and 2 h after ICU admission (shown in online suppl. Fig. S4d). Similarly, sIGFBP7*sTIMP2 values were also not associated with eGFR in both groups at presurgery and 2 h after ICU admission (shown in online suppl. Fig. S4f). Of note, the sIGFBP7*sTIMP2 values at 2 h after ICU admission only correlated with BUN (R2 = 0.407, p = 0.035, shown in online suppl. Fig. S4e) in AKI groups. Moreover, the sIGFBP7*sTIMP2 values at presurgery only correlated with UA in AKI groups (R2 = 0.575, p = 0.029, shown in online suppl. Fig. S4g).
Discussion
The source of urinary IGFBP7 and TIMP2 has been investigated in previous studies [11, 15] whether kidney is the main organ secreting IGFBP7 and TIMP2 still controversial. In this study, IGFBP7 was highly expressed but TIMP2 was extremely low expressed in both human and rat kidneys. IGFBP7 and TIMP2 obviously upregulated in the spleen and lung in ischemia-induced AKI. However, in cisplatin-induced AKI mice model, the upregulation of those two factors could not be observed in any of those organs. That might be a clue to explain why urinary TIMP2*IGFBP7 has not been shown definitively to be of benefit in the detection of nephrotoxicity in humans [16–18]. High sIGFBP7 concentration is an independent risk factor of heart failure [19, 20] and lung injury [21, 22]. However, there are very limited studies to investigate the expression of IGFBP7 in the spleen, except that one study ever reported an obvious downregulation of IGFBP7 in tumorous spleens [23]. Whether the heart, spleen, or lung is the source of plasma IGFBP7 and TIMP2 in patient following cardiac surgery was rarely reported.
In our previous studies, the combination of urinary TIMP2 and IGFBP7 4 h after postoperative ICU admission identified patients at risk for developing AKI [24] and this combination also identified patients with AKI at increased risk for mortality or receipt of RRT over the next 9 months [6]. Recently, metabolic panel-based models showed very good discrimination for moderate-to-severe AKI within 72 h and 14 days after the surgical procedure, with an AUC of 0.876 within 72 h and 0.854 within 14 days, and for AKI requiring dialysis within 72 h and 14 days after the surgical procedure, with an AUC of 0.916 within 72 h and 0.900 within 14 days [25]. In our study, the absolute sIGFBP7 value in patients at as early as 2 h after ICU admission was predictive of both stage 1 and stage 2–3 AKI. Moreover, we also observed that, although with no statistic significance, the average sIGFBP7 at presurgery and 0 h after ICU admission in AKI group was higher than in no-AKI group, similar to previous studies in which subjects with higher SCr developed acute or chronic kidney dysfunction after surgery [24, 26, 27]. As the cell-cycle arrest biomarker [4, 28], a decrease in sIGFBP7 and sTIMP2 might evoke organ repair after injury. In no-AKI group, IGFBP7 serum level has a significant decrease at 6 h after ICU admission compared to presurgery that might be a positive sight of tissue repair, while, as to TIMP2 serum level, a significant increase was observed at 0 h after ICU admission in AKI group.
Having identified sIGFBP7 2 h after ICU admission as a predictor of AKI, we determined the correlation between this value and log2 (SCr), log2 (BUN), log2 (eGFR), and log2 (UA). Strikingly, sIGFBP7 at 2 h after ICU admission was correlated well with all of these factors in AKI groups. This is the first known investigation of the association between sIGFBP7 and SCr, BUN, eGFR, or UA. Moreover, log2 (UA) at presurgery was strongly correlated with both sTIMP2 value and sIGFBP7*sTIMP2 in AKI groups. The association of UA levels with the development of AKI has been reported in various clinical settings [29–32]. Together, the results of these studies highlight sIGFBP7 as a potential risk factor of AKI and further work is needed on this subject.
Several limitations of our clinical study should be noted. First, we measured sIGFBP7 at a multiple time point, and therefore, this sample is too small to parse out these associations at each single time point. Second, all the study participants were inpatients who received cardiac surgery, which might reduce the generalizability of our results.
Overall, spleen and lung, but not kidney, might be the main sources of serum or urinary IGFBP7 and TIMP2 after AKI induction. The sIGFBP7 value might be a valuable blood index to identify patients at risk for developing AKI as early as 2 h after postoperative ICU admission. In the future, more attention should be paid on the changes of sIGFBP7, especially for the expression of IGFBP7 and TIMP2 in the spleen and lung, which will get more valuable clues to predict AKI before cardiac surgery.
Statement of Ethics
The protocols used in the mouse experiment were approved by the Animal Care and Use Committee of Fudan University and were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory. All enrolled patients provided written informed consent. The protocol of serum collection and the serum usage were reviewed and approved by Ethics Committee of Zhongshan Hospital Fudan University, approval number (B2018-175).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by the National Natural Science Foundation of China (81800596), the Shanghai Municipal Key Clinical Specialty (shslczdzk02501), Shanghai Federation of Nephrology Project supported by Shanghai Shenkang Hospital Development Center (SHDC2202230), Shanghai “Science and Technology Innovation Plan” Yangtze River Delta Scientific and Technological Innovation Community project (21002411500), Shanghai “Science and Technology Innovation Plan” technical standard project (19DZ2205600), and Shanghai Sailing Program (20YF1406000).
Author Contributions
Yimei Wang, Bo Shen, and Xuesen Cao: data curation, formal analysis, investigation, software, resources, and writing – original draft. Zhihui Lu: data curation, investigation, and resources. Yang Zhang and Weidong Zhang: investigation and methodology. Bowen Zhu: data curation, methodology, and software. Yiqin Shi and Jialin Wang: data curation, formal analysis, and writing – review and editing. Yi Fang, Nana Song, Yang Li, and Xialian Xu: review and editing. Xiaoqiang Ding: conceptualization, project administration, supervision, and writing – review and editing. Shuan Zhao: conceptualization, data curation, formal analysis, funding acquisition, project administration, and writing – original draft, review, and editing.
Data Availability Statement
All data were shown in the results, figures, and tables of this manuscript, and in the supplemental materials.
Funding Statement
This work was supported by the National Natural Science Foundation of China (81800596), the Shanghai Municipal Key Clinical Specialty (shslczdzk02501), Shanghai Federation of Nephrology Project supported by Shanghai Shenkang Hospital Development Center (SHDC2202230), Shanghai “Science and Technology Innovation Plan” Yangtze River Delta Scientific and Technological Innovation Community project (21002411500), Shanghai “Science and Technology Innovation Plan” technical standard project (19DZ2205600), and Shanghai Sailing Program (20YF1406000).
Supplementary Material
References
- 1. Nadim MK, Forni LG, Bihorac A, Hobson C, Koyner JL, Shaw A, et al. Cardiac and vascular surgery-associated acute kidney injury: the 20th international consensus conference of the ADQI (acute disease quality initiative) group. J Am Heart Assoc. 2018 Jun 1;7(11):e008834. 10.1161/JAHA.118.008834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engelman DT, Ben Ali W, Williams JB, Perrault LP, Reddy VS, Arora RC, et al. Guidelines for perioperative Care in cardiac surgery: enhanced recovery after surgery society recommendations. JAMA Surg. 2019 Aug 1;154(8):755–66. 10.1001/jamasurg.2019.1153. [DOI] [PubMed] [Google Scholar]
- 3. Kashani K, Al-Khafaji A, Ardiles T, Artigas A, Bagshaw S, Bell M, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17(1):R25. 10.1186/cc12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bihorac A, Chawla L, Shaw A, Al-Khafaji A, Davison D, Demuth G, et al. Validation of cell-cycle arrest biomarkers for acute kidney injury using clinical adjudication. Am J Respir Crit Care Med. 2014;189(8):932–9. 10.1164/rccm.201401-0077OC. [DOI] [PubMed] [Google Scholar]
- 5. Hoste E, McCullough P, Kashani K, Chawla L, Joannidis M, Shaw A, et al. Derivation and validation of cutoffs for clinical use of cell cycle arrest biomarkers. Nephrol Dial Transplant. 2014;29(11):2054–61. 10.1093/ndt/gfu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koyner J, Shaw A, Chawla L, Hoste E, Bihorac A, Kashani K, et al. Tissue inhibitor metalloproteinase-2 (TIMP-2)-IGF-Binding protein-7 (IGFBP7) levels are associated with adverse long-term outcomes in patients with AKI. J Am Soc Nephrol. 2015;26(7):1747–54. 10.1681/ASN.2014060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn P, et al. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313(21):2133–41. 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 8. Mar D, Gharib S, Zager R, Johnson A, Denisenko O, Bomsztyk K. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int. 2015;88(4):734–44. 10.1038/ki.2015.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Knafl D, Müller M, Pajenda S, Genc Z, Hecking M, Wagner L. The urine biomarker panel IGFBP7xTIMP-2 (NephroCheck® parameter) does not correlate with IGFBP7 and TIMP-2 gene expression in urinary sediment. PLoS One. 2017;12(11):e0188316. 10.1371/journal.pone.0188316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu K, Rosenstiel P, Paragas N, Hinze C, Gao X, Huai Shen T, et al. Unique transcriptional programs identify subtypes of AKI. J Am Soc Nephrol. 2017;28(6):1729–40. 10.1681/ASN.2016090974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson A, Zager R. Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephrol. 2018;29(8):2157–67. 10.1681/ASN.2018030265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muto Y, Wilson PC, Ledru N, Wu H, Dimke H, Waikar SS, et al. Single cell transcriptional and chromatin accessibility profiling redefine cellular heterogeneity in the adult human kidney. Nat Commun. 2021;12(1):2190. 10.1038/s41467-021-22368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Limbutara K, Chou C, Knepper M. Quantitative proteomics of all 14 renal tubule segments in rat. J Am Soc Nephrol. 2020;31(6):1255–66. 10.1681/ASN.2020010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zarbock A, Küllmar M, Ostermann M, Lucchese G, Baig K, Cennamo A, et al. Prevention of cardiac surgery-associated acute kidney injury by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI-multicenter randomized controlled trial. Anesth Analg. 2021;133(2):292–302. 10.1213/ANE.0000000000005458. [DOI] [PubMed] [Google Scholar]
- 15. Emlet DR, Pastor-Soler N, Marciszyn A, Wen X, Gomez H, Humphries WH, et al. Insulin-like growth factor binding protein 7 and tissue inhibitor of metalloproteinases-2: differential expression and secretion in human kidney tubule cells. Am J Physiol Renal Physiol. 2017 Feb 1;312(2):F284–96. 10.1152/ajprenal.00271.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, et al. Clinical use of the urine biomarker TIMP-2 × IGFBP7 for acute kidney injury risk assessment. Am J Kidney Dis. 2016 Jul;68(1):19–28. 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schanz M, Hoferer A, Shi J, Alscher MD, Kimmel M. Urinary TIMP2-IGFBP7 for the prediction of platinum-induced acute renal injury. Int J Nephrol Renovasc Dis. 2017;10:175–81. 10.2147/IJNRD.S135271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toprak Z, Cebeci E, Helvaci SA, Toprak ID, Kutlu Y, Sakin A, et al. Cisplatin nephrotoxicity is not detected by urinary cell-cycle arrest biomarkers in lung cancer patients. Int Urol Nephrol. 2017 Jun;49(6):1041–7. 10.1007/s11255-017-1556-4. [DOI] [PubMed] [Google Scholar]
- 19. Kalayci A, Peacock WF, Nagurney JT, Hollander JE, Levy PD, Singer AJ, et al. Echocardiographic assessment of insulin-like growth factor binding protein-7 and early identification of acute heart failure. ESC Heart Fail. 2020 Aug;7(4):1664–75. 10.1002/ehf2.12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vaduganathan M, Sattar N, Xu J, Butler J, Mahaffey K, Neal B, et al. Stress cardiac biomarkers, cardiovascular and renal outcomes, and response to canagliflozin. J Am Coll Cardiol. 2022;79(5):432–44. 10.1016/j.jacc.2021.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie X, Zhao J, Xie L, Wang H, Xiao Y, She Y, et al. Identification of differentially expressed proteins in the injured lung from zinc chloride smoke inhalation based on proteomics analysis. Respir Res. 2019 Feb 15;20(1):36. 10.1186/s12931-019-0995-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen C, Tian X, Zhao X, Ren L. Clinical study of serum IGFBP7 in predicting lymphatic metastasis in patients with lung adenocarcinoma. Curr Probl Cancer. 2020 Dec;44(6):100584. 10.1016/j.currproblcancer.2020.100584. [DOI] [PubMed] [Google Scholar]
- 23. Lian L, Qu LJ, Sun HY, Chen YM, Lamont SJ, Liu CJ, et al. Gene expression analysis of host spleen responses to Marek’s disease virus infection at late tumor transformation phase. Poult Sci. 2012 Sep;91(9):2130–8. 10.3382/ps.2012-02226. [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Zou Z, Jin J, Teng J, Xu J, Shen B, et al. Urinary TIMP-2 and IGFBP7 for the prediction of acute kidney injury following cardiac surgery. BMC Nephrol. 2017;18(1):177. 10.1186/s12882-017-0592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Demirjian S, Bashour CA, Shaw A, Schold JD, Simon J, Anthony D, et al. Predictive accuracy of a perioperative laboratory test-based prediction model for moderate to severe acute kidney injury after cardiac surgery. JAMA. 2022 Mar 8;327(10):956–64. 10.1001/jama.2022.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thakar C, Arrigain S, Worley S, Yared J, Paganini E. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16(1):162–8. 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 27. Esposito C, De Mauri A, Vitulo P, Oggionni T, Cornacchia F, Valentino R, et al. Risk factors for chronic renal dysfunction in lung transplant recipients. Transplantation. 2007;84(12):1701–3. 10.1097/01.tp.0000295989.63674.53. [DOI] [PubMed] [Google Scholar]
- 28. Dusse F, Edayadiyil-Dudásova M, Thielmann M, Wendt D, Kahlert P, Demircioglu E, et al. Early prediction of acute kidney injury after transapical and transaortic aortic valve implantation with urinary G1 cell cycle arrest biomarkers. BMC Anesthesiol. 2016 Sep 8;16:76. 10.1186/s12871-016-0244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaushik M, Choo JC. Serum uric acid and AKI: is it time? Clin Kidney J. 2016 Feb;9(1):48–50. 10.1093/ckj/sfv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Srivastava A, Palsson R, Leaf D, Higuera A, Chen M, Palacios P, et al. Uric acid and acute kidney injury in the critically ill. Kidney Med. 2019;1(1):21–30. 10.1016/j.xkme.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Özgür Y, Akın S, Yılmaz NG, Gücün M, Keskin Ö. Uric acid albumin ratio as a predictive marker of short-term mortality in patients with acute kidney injury. Clin Exp Emerg Med. 2021 Jun;8(2):82–8. 10.15441/ceem.20.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Puti E, Rasyid H, Tandean P, Sanusi H, Kasim H, Bakri S, et al. High uric acid level increases the risk of acute kidney injury in acute coronary syndrome patients. Caspian J Intern Med. 2021 Apr;12(3):323–6. 10.22088/cjim.12.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data were shown in the results, figures, and tables of this manuscript, and in the supplemental materials.