Abstract
Introduction
Patients with end-stage renal disease receiving hemodialysis (HD) have a high morbidity and mortality rate associated with pulmonary hypertension (PH). A nomogram was developed to predict all-cause mortality in HD patients with PH. In this study, we aimed to validate the usefulness of this nomogram.
Methods
A total of 274 HD patients with PH were hospitalized at the Affiliated Hospital of Xuzhou Medical University between January 2014 and June 2019 and followed up for 3 years. Echocardiography detected PH when the peak tricuspid regurgitation velocity (TRV) was more than 2.8 m/s. To evaluate the all-cause mortality for long-term HD patients with PH, Cox regression analysis was performed to determine the factors of mortality that were included in the prediction model. Next, the area under the receiver-operating characteristic curve (AUC-ROC) was used to assess the predictive power of the model. Calibration plots and decision curve analysis (DCA) were used to assess the accuracy of the prediction results and the clinical utility of the model.
Results
The all-cause mortality rate was 29.20% throughout the follow-up period. The nomogram comprised six commonly available predictors: age, diabetes mellitus, cardiovascular disease, hemoglobin, left ventricular ejection fraction, and TRV. The 1-year, 2-year, and 3-year AUC-ROC values were 0.842, 0.800, and 0.781, respectively. The calibration curves revealed excellent agreement with the nomogram, while the DCA demonstrated favorable clinical practicability.
Conclusion
The first developed nomogram for predicting all-cause mortality in HD patients with PH could guide clinical decision-making and intervention planning.
Keywords: Hemodialysis, Pulmonary hypertension, Nomogram, Prognosis, Mortality
Introduction
Chronic kidney disease (CKD) is a leading cause of death worldwide and thus a major public health issue [1]. In 2017, the prevalence of CKD increased, affecting an estimated 843.6 million people globally [2] and causing death to 1.2 million people [3]. The number of patients with end-stage renal disease (ESRD) requiring renal replacement therapy is anticipated to rise from 2.5 million to 5.4 million by 2030 [4].
Pulmonary hypertension (PH) is characterized by an increase in pulmonary artery pressure caused by a cardiac, respiratory, or systemic disease, and its prevalence has recently risen in patients with ESRD from 19% to 56% [5]. Furthermore, PH is a risk factor for mortality in ESRD [6]. In hemodialysis (HD) patients, PH is a separate predictor of cardiovascular morbidity and all-cause mortality [7–10]. The mortality rate is four times greater in HD patients with PH than in those without PH [7]. Most HD patients with PH have impaired left ventricular systolic function, which increases the risk of developing heart failure [11]. In renal transplant recipients, patients with PH are more likely to experience perioperative hypotension and delayed graft function [12]. Given the dismal prognosis for PH in dialysis patients, the risk factors for death must be identified to plan strategies for early intervention. Moreover, a model for predicting morbidity risk should be developed to inform clinical treatment modalities and intervention planning.
Right heart catheterization (RHC) and direct pressure measurement are the gold standards for PH diagnosis [13]. However, these modalities are not routinely used due to their invasiveness and complications. Echocardiography has been recommended as a noninvasive alternative for screening and evaluating PH [14–17]. In echocardiography, pulmonary pressure is estimated by measuring the peak tricuspid regurgitation velocity (TRV) in order to screen for PH in susceptible or high-risk populations [13, 18, 19].
Nomograms are a type of prediction tool that visualizes the outcomes of a multifactorial regression model to assess the likelihood that clinical events will occur, resulting in a straightforward graphical depiction of a statistical prediction model. Nomograms have been proven to be most effective in predicting the survival rates and cardiovascular events in HD patients, resulting in the reduction of risk unfavorable outcomes [20, 21]. However, no study has focused on constructing the nomogram to predict the mortality of HD patients with PH. Therefore, this study was aimed at exploring the risk factors and creating a unique nomogram that could predict the all-cause mortality for HD patients with PH in order to guide clinical decision-making and intervention planning.
Materials and Methods
Study Participants
This single-center retrospective observational cohort study included 274 ESRD patients between January 2014 and June 2019 from the Affiliated Hospital of Xuzhou Medical University. Data were obtained from electronic medical records of the hospital’s in-patient system. Patients older than 18 years who underwent maintenance HD therapy were included for analysis. The Medical Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University granted approval for this study (approval number XYFY2022-KL093), which was carried out in accordance with the principles of the Declaration of Helsinki. Since it was a single-center retrospective study, the Investigational Review Board waived the requirement for written informed consent.
The inclusion criteria were (1) older than 18 years old, (2) HD for more than 3 months with an average of 2.5–3.5 sessions per week lasting more than 4 h per session, and (3) measured TRV >2.8 m/s by echocardiogram. The exclusion criteria were (1) dialysis duration <3 months; (2) chronic peritoneal dialysis; (3) chronic obstructive pulmonary disease, congenital cardiac anomalies, pulmonary emboli; (4) complicated with malignant tumor; (5) history of transplantation; and (6) incomplete essential data. Our endpoint was all-cause mortality throughout the 3-year follow-up period. Patients were followed up for 3 years via phone, interviews, or evaluation of medical records.
Data Collection
The baseline patient characteristics were obtained from computerized medical records: age, sex, body mass index, and history of smoking, diabetes mellitus (DM), cardiovascular disease (CVD), and hypertension (HTN). CVD included heart failure, stroke, and coronary artery disease. Patients taking oral medications for at least 6 months were considered to have medication information. Venous blood samples were obtained within 1–3 months after the patients’ routine HD achieved dry body mass and after an overnight fast. High-sensitivity C-reactive protein, parathyroid hormone (PTH), albumin, potassium (K), calcium (Ca), chlorine (Cl), serum phosphorus (P), and magnesium (Mg) levels as well as hemoglobin (HGB), platelets, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were also included.
To minimize the impact of volume load, each patient underwent Doppler echocardiography within 12 h after the conclusion of HD. Experienced cardiologists who performed the echocardiography were blinded to all other clinical data. TRV was measured and considered a key parameter in determining the probability of PH, according to the guidelines for echocardiographic evaluation of PH [13]. TRV <2.8 m/s was considered normal. Other parameters such as right atrium diameter, left ventricular posterior wall diameter, aortic root diameter, left ventricular end-diastolic diameter, and left ventricular ejection fraction (LVEF) were also measured.
Statistical Analysis
To ascertain whether continuous variables had a normal distribution, the Shapiro-Wilk test was used. The variable’s data were consistently distributed if p ≥ 0.05. The all-cause mortality and survival groups were then compared using the Student’s t test, and the findings were expressed as mean ± SD. If p < 0.05, the variables were non-normally distributed data. Mann-Whitney U test was used to compare the two groups, and the data were then displayed as the median and interquartile range. The number of instances and percentages were used to show categorical data, and to compare them, the χ2 test was used. The Martingale residuals were used to detect a nonlinear relationship between the covariable and the outcome event. The nonlinear predictor (i.e., age, online suppl. Fig. 1; for all online suppl. material, see https://doi.org/10.1159/000533674) was evaluated using restricted cubic splines with five knots and then classified as a simulation relationship. Additionally, the dataset was randomly split into cohorts at a ratio of 7:3 with no intergroup differences: one for internal validation and the other for development. Predictors of all-cause mortality for HD patients with PH were identified using univariate and multivariate Cox regression techniques. Based on the findings of the multivariable Cox regression analysis, a nomogram was created. The area under the receiver-operating characteristic curve (AUC-ROC) was used to assess the effectiveness of the model in predicting an outcome. Calibration plots and decision curve analysis (DCA) were used to assess the accuracy of the prediction results and the clinical utility of the model. The SPSS software (version 25.0) and R software (version 4.1.0) were used to conduct statistical analyses.
Results
Baseline Characteristics
In total, 274 HD patients with PH participated in the study from January 2014 to June 2019. Depending on whether patients died at the end of follow-up, patients were split into two groups: all-cause mortality (n = 80) and survival (n = 194). Table 1 lists the demographic data and baseline characteristics of HD patients with PH. All patients’ average age was 54.6 ± 12.8 years, and 107 (39.05%) were male. The patients who died were considerably older, and majority of them had obesity, smoking, HTN, and CVD. They also exhibited lower levels of HGB and LVEF, and higher levels of PTH and TRV at baseline than those who survived (all p < 0.05). Furthermore, 80 (29.20%) deaths occurred by the end of follow-up.
Table 1.
Baseline characteristics of all-cause mortality group and survival group
| Variables | Survival group (N = 194) | All-cause mortality group (N = 80) | Significance test |
|---|---|---|---|
| Age, years | 56.50 (47, 63) | 62 (50, 67) | Z = −3.305, p = 0.001 |
| Sex, male, n (%) | 74 (38.14) | 33 (41.25) | χ 2 = 0.230, p = 0.632 |
| BMI, kg/m2 | 22.67 (21.75, 23.15) | 22.97 (21.97, 25,39) | Z = −3.657, p = 0.001 |
| Smoking (yes vs. no) | 45 (23.20) | 33 (41.25) | χ 2 = 9.067, p = 0.030 |
| DM (yes vs. no) | 83 (42.78) | 44 (55) | χ 2 = 3.399, p = 0.065 |
| HTN (yes vs. no) | 135 (69.59) | 70 (87.50) | χ 2 = 9.646, p = 0.020 |
| CVD (yes vs. no) | 41 (21.13) | 37 (46.25) | χ 2 = 17.547, p = 0.001 |
| Dihydropyridine CCBs | 109 (56.2) | 50 (62.5) | χ 2 = 0.927, p = 0.336 |
| β-receptor blocker | 51 (26.2) | 26 (32.5) | χ 2 = 1.082 p = 0.298 |
| Statins | 36(18.5) | 18 (22.5) | χ 2 = 0.952, p = 0.329 |
| HGB, g/L | 90 (75, 102) | 79 (70.25, 90.75) | Z = −2.950, p = 0.003 |
| ALB, mg/L | 33 (29.98, 37.18) | 32.9 (28.18, 37.28) | Z = −1.126, p = 0.260 |
| PLT, ×109/L | 173(131, 216) | 169.5 (127, 196.5) | Z = −0560, p = 0.575 |
| K, mmol/L | 4.55 (3.96, 5.12) | 4.67 (3.97, 5.09) | Z = −0.616, p = 0.538 |
| CL, mmol/L | 100.7 (98.33, 104) | 100.7 (97.73, 102.73) | Z = −0.649, p = 0.516 |
| Ca, mmol/L | 2.13 (2, 2.26) | 2.13 (1.98, 2.26) | Z = −0.315, p = 0.753 |
| Mg, mmol/L | 0.99 (0.90, 1.07) | 0.99 (0.90, 1.05) | Z = −0.420, p = 0.674 |
| P, mmol/L | 1.56 (1.25, 1.85) | 1.56 (1.22, 1.65) | Z = −0.775, p = 0.438 |
| HDL-C, mmol/L | 1.13 (0.99,1.27) | 1.13 (0.88, 1.28) | Z = −0.606, p = 0.544 |
| LDL-C, mmol/L | 2.31 (1.91, 2.81) | 2.30 (1.77, 2.75) | Z = −0.715, p = 0.474 |
| hs-CRP, mg/L | 25.95 (11.62, 36.10) | 26.25 (14.63, 33.40) | Z = −1.105, p = 0.269 |
| PTH, pg/mL | 267.4 (134.3, 311.3) | 318.95 (177.4,429.14) | Z = −3.623, p = 0.001 |
| ARD, mm | 30 (28.75, 31) | 30 (28, 31) | Z = −0.257, p = 0.797 |
| LVDD, mm | 54 (50, 58) | 54 (50.58.5) | Z = −0.876, p = 0.381 |
| RAD, mm | 42 (40, 42) | 42 (41, 42.75) | Z = −0.970, p = 0.332 |
| LVPWD, mm | 10 (10, 11) | 10 (9, 11) | Z = −0.770, p = 0.441 |
| LVEF, % | 57 (49, 62) | 50 (44, 57) | Z = −4.388, p = 0.001 |
| TRV, m/s | 3.62 (3.17, 3.66) | 3.66 (3.44, 3.96) | Z = −4.076, p = 0.001 |
BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease; HGB, hemoglobin; PLT, platelet; ALB, albumin; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; K, potassium; Ca, calcium; Cl, chlorine; P, serum phosphorus; Mg, magnesium; PTH, parathyroid hormone; hs-CRP, high-sensitivity C-reactive protein; ARD, aortic root diameter; RAD, right atrium diameter; LVDD, left ventricular end-diastolic diameter; LVPWD, left ventricular posterior wall diameter; LVEF, left ventricular ejection fraction; TRV, tricuspid regurgitation velocity.
Cox Regression with Univariate and Multivariate Variables to Predict All-Cause Mortality
Univariate and multivariate Cox regression analyzes were used to identify the risk variables for death from all causes (Table 2). Age, smoking, body mass index, HTN, DM, CVD, PTH, HGB, TRV, and LVEF were all connected to a higher risk of total mortality, according to the univariate Cox regression models (all p < 0.05). In the multivariate Cox regression analysis, the independent risk factors for all-cause mortality were age (adjusted odds ratio [aOR]: 2.222; 95% CI: 1.196–4.126), CVD (aOR: 1.963; 95% CI: 1.136–3.390), DM (aOR: 1.806; 95% CI: 1.054–3.095), HGB (aOR: 0.981; 95% CI: 0.966–0.997), LVEF (aOR: 0.963; 95% CI: 0.935–0.991), and TRV (aOR: 2.707, 95% CI: 1.278–5.735).
Table 2.
Univariate and multivariate Cox regression analysis of variables predicting mortality
| Variables | Univariate analysis, OR (95% CI) | p value | Multivariate analysis, aOR (95% CI) | p value |
|---|---|---|---|---|
| Age (≥60 years) | 2.057 (1.234, 3.429) | 0.006 | 2.222 (1.196, 4.126) | 0.011 |
| BMI (kg/m2) | 1.144 (1.068, 1.226) | 0.000 | 1.055 (0.968, 1.148) | 0.221 |
| Smoking | 1.704 (1.012, 2.868) | 0.045 | 1.313 (0.735, 2.344) | 0.358 |
| DM | 1.875 (1.129, 3.116) | 0.015 | 1.806 (1.054,3.095) | 0.032 |
| HTN | 2.085 (1.057, 4.113) | 0.034 | 0.978 (0.475, 2.013) | 0.951 |
| CVD | 2.808 (1.691, 4.663) | 0.000 | 1.963 (1.136, 3.390) | 0.016 |
| HGB (g/L) | 0.983 (0.969, 0.997) | 0.022 | 0.981 (0.966, 0.997) | 0.017 |
| PTH (pg/mL) | 1.002 (1.001, 1.003) | 0.002 | 1.001 (1.000, 1.002) | 0.082 |
| LVEF (%) | 0.959 (0.935, 0.985) | 0.002 | 0.963 (0.935, 0.991) | 0.009 |
| TRV (m/s) | 3.463 (1.781, 6.733) | 0.000 | 2.707 (1.278, 5.735) | 0.009 |
OR, odds ratio; aOR, adjusted odds ratio; CI, confidence interval; BMI, body mass index; HTN, hypertension; DM, diabetes mellitus; CVD, cardiovascular disease; HGB, hemoglobin; PTH, parathyroid hormone; LVEF, left ventricular ejection fraction; TRV, tricuspid regurgitation velocity.
Creating and Testing a Nomogram to Predict All-Cause Mortality
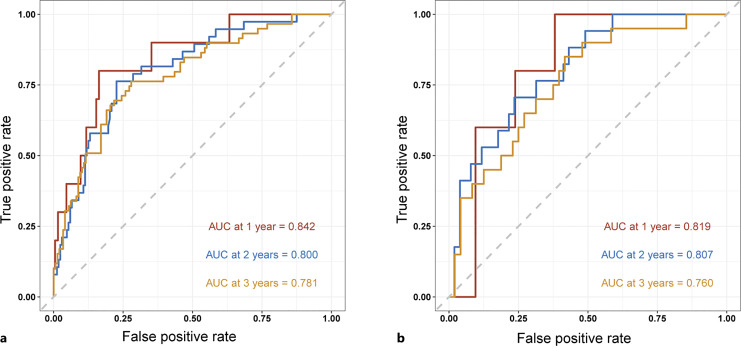
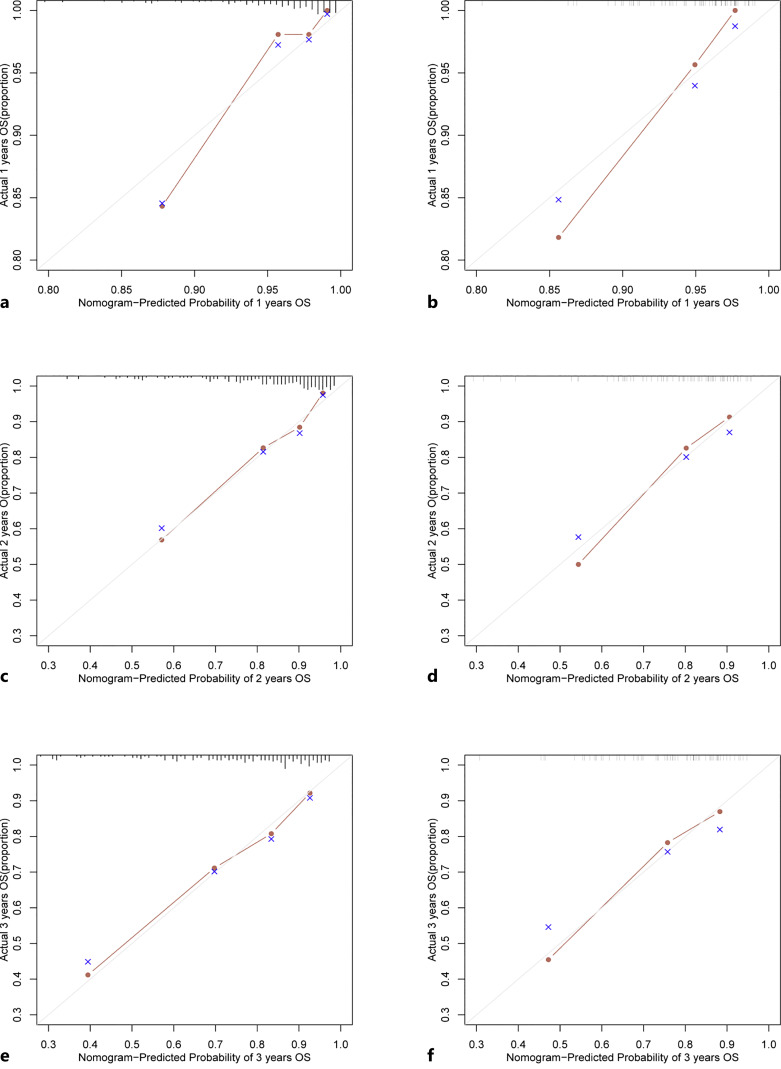
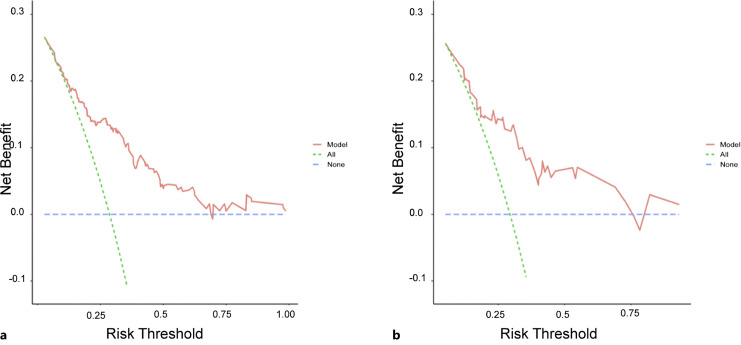
Based on the findings of the univariable and multivariable Cox regression analyses, six factors were chosen to construct the nomogram (shown in Fig. 1). To determine how confident the model was in its outcome prediction, we used the ROC curve. The AUC-ROCs for the 1-, 2-, and 3-year development sets were 0.842 (95% CI: 0.722–0.961), 0.800 (95% CI: 0.725–0.875), and 0.781 (95% CI: 0.711–0.851), while those for the validation set were 0.819 (95% CI: 0.693–0.945), 0.807 (95% CI: 0.698–0.917), and 0.760 (95% CI: 0.639–88.16) (shown in Fig. 2). The calibration charts for the development and internal validation datasets showed a strong fit to the ideal standard line (shown in Fig. 3), indicating that the predicted results of our prediction model match the actual results with a high accuracy. Finally, the DCA curve was used to evaluate the degree of patient benefit, as it introduces threshold probability that triggers a medical intervention at the same threshold probability. When the threshold probabilities ranged from 11.1% to 68.6%, the results of DCA in the training set suggested that using the nomogram to predict results would produce a greater net benefit than the “treat all” or “treat none” methods (shown in Fig. 4). For example, if a 19% risk threshold was used, patients would receive some treatment measures if the likelihood of 3-year survival rate was greater than 19%, and net benefit was approximately 0.16.
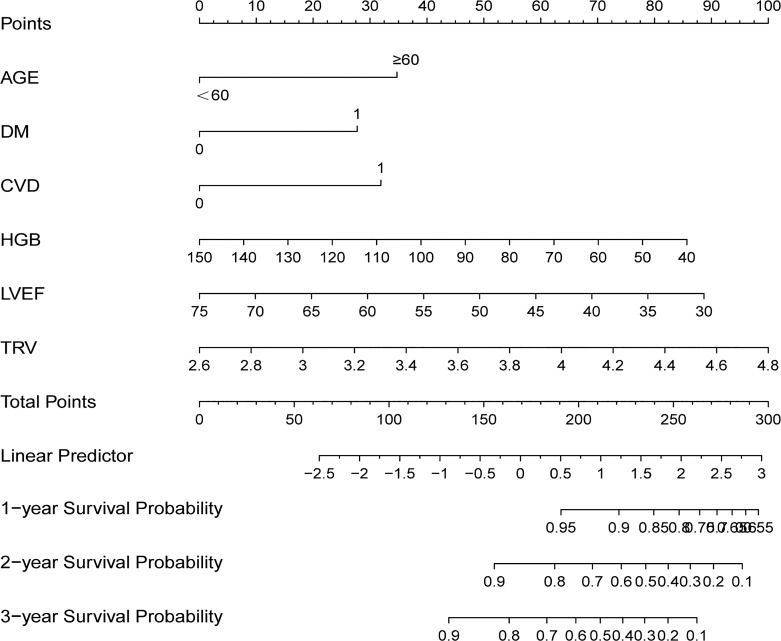
Fig. 1.
Nomogram for assessing HD patients with PH’s risk of all-cause mortality. The top line points are the values for each subgroup of the variables. The sum of the corresponding points for each subgroup of the variables is the total points. From this total point, a vertical line is drawn, and the expected proportion of survivors is found where it intersects the 1-, 3-, and 5-year survival probability lines. DM, diabetes mellitus; CVD, cardiovascular disease; HGB, hemoglobin; TRV, tricuspid regurgitant velocity; LVEF, left ventricular ejection fraction.
Fig. 2.
Based on the development set (a) and validation set (b), the ROC curves of the clinical prediction model are presented. AUC, area under the receiver-operating characteristic; ROC, receiver-operating characteristic.
Fig. 3.
Nomogram calibration curve using data from the development set (a, c, e) and validation set (b, d, f). The diagonal line depicts the ideal forecast made by a perfect model, while the y-axis represents the actual death rate and the x-axis represents the expected mortality rate.
Fig. 4.
Data from the development set (a) and the validation set (b) were used to assess the clinical benefit of the predictive model. The threshold probability and the net benefit were plotted. Both “all” and “none” lines represent the net benefits of treating all patients, respectively.
Discussion
This study aimed to provide a tool for predicting all-cause mortality based on clinical parameters and risk factors in HD patients with PH. Through univariate and multivariate analyses, our findings first revealed that HD patients who combined PH with advanced age, a history of DM and CVD, lower baseline serum levels of HGB and LVEF, and a higher TRV were more likely to present with death within 3 years. All these variables were integrated into our study to set up a nomogram to predict all-cause mortality risk for HD patients with PH. AUC-ROC values for 1, 2, and 3 years were 0.842, 0.800, and 0.781, respectively, and additionally calibrated. The DCA demonstrated satisfactory net benefits.
In the present study, mortality is higher in ESRD patients with PH than in ESRD patients without PH; these findings are in line with a systematic review and meta-analysis by Schoenberg et al. [22] and Tang et al. [9]. However, compared to their published forest maps for predicting the risk of mortality [23], our model is a simple, convenient, accurate, and personalized visualization tool to predict the probability of death per patient by calculating the weighted contributions of independent risk factors.
As they aged, HD patients had a higher probability of developing PH [24, 25]. Older patients have poor organ function, poor nutrition, and more comorbidities. Reque et al. showed that elderly patients with HD combined with PH had a poor prognosis [8], and older patients with PH showed more inferior right ventricular function and worse pulmonary artery compliance [26, 27]. DM, however, is the most common cause of ESRD and has the worst outcomes [28, 29]. Whitaker et al. found an association between DM and right ventricular dysfunction and that DM may affect right ventricular afterload and remodeling in patients with PH, thereby reducing survival in PH patients [30]. The most prevalent and significant risk factor for HD patients with PH was CVD, which was present in 45.65% of HD patients in China [31]. The primary cause of mortality in ESRD patients is CVD [32–34], which is also associated with PH [9]. Even the best dialysis modality cannot reverse cardiovascular damage [35].
Anemia in CKD is a multifactorial process related to the relative deficiency of erythropoietin and dialysis adequacy [36, 37]. HGB concentration had prognostic significance in dialysis patients, and low levels of HGB impair the ability to transport oxygen, which results in hypoxemia, which triggers pulmonary vasoconstriction and ultimately results in PH [38]. Additionally, lower HGB concentrations were associated with reduced quality of life, increased burden on the heart, and increased patient morbidity and mortality [39, 40].
LVEF is the index of systolic function [41] and does not improve after HD despite the significant reduction in the left atrial volume [42]. In comparison to individuals with LVEF >45%, HD patients with poor LVEF had a 1.48-fold higher risk of death [43], which could classify the risk of death from heart disease and other causes [44]. In addition, after successful renal transplantation, patients with pretransplant PH have reduced LVEF, which increases the risk of mortality and graft loss [45]. In those with CKD and those receiving HD, PH is associated with a significant burden of morbidity and death [7–9, 24, 46]. Higher TRV values are independent risk factors for mortality and cardiovascular events, as well as the risk of heart failure [24]. In addition, Ramasubbu et al. [46] found that long-term dialysis patients with PH died with more severe TRV, larger right atria and right ventricular sizes, and worse right ventricular function.
Our study had some limitations. First, this was a monocentric, observational, retrospective study with no external validation. Therefore, the results need to be confirmed by multicenter studies. Second, as our data were obtained from a hospital in-patient database, we had no data on the nutritional status, volume status, or weight gain between dialysis sessions. In the future, we can assess their connection to mortality in HD patients with PH. Third, given the invasive nature of the RHC, we only used echocardiography to estimate the risk of PH. Further studies should use RHC to confirm our findings.
Conclusion
Age, DM, CVD, HGB, LVEF, and TRV values were independent predictors of all-cause mortality. To accurately predict the mortality of HD patients with PH, a simple-to-use nomogram must be created and internally validated, which may help clinicians develop individualized treatment regimens and improve the prognosis. For this paradigm to be universal, external validation is required.
Acknowledgments
This study was funded by the Department of Cardiology and Nephrology of the Affiliated Hospital of Xuzhou Medical University. We thank all the staff for their contribution in data collection.
Statement of Ethics
This study was approved by the Research Ethics Committee of the Affiliated Hospital of Xuzhou Medical University (Approval number XYFY2022-KL093). The study was a single-center retrospective study, and the requirement for informed consent was therefore waived by the Ethics Committee.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be interpreted as a potential conflict of interest.
Funding Sources
This work was supported by grants from the Applied Basic Research Project of XUZHOU to Defeng Pan (Grant number: KC20097).
Author Contributions
Huimin Wu developed the analysis plan and wrote the paper. Chunyan Huanand Yue Hu undertook the data analysis. Shengjue Xiao, Xiaotong Wang, Tao Xu, Minjia Guo, Ailin Liu, Jiayi Sun, Chunqing Wang, and Jia Wang collected the dataset and provided advice on its analysis. Defeng Pan guided the analysis and made substantial improvements to the paper. Hong Zhu supervised the study and contributed to the data analysis plan. Huimin Wu, Chunyan Huan, and Yue Hu contributed equally to this work.
Funding Statement
This work was supported by grants from the Applied Basic Research Project of XUZHOU to Defeng Pan (Grant number: KC20097).
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.
Supplementary Material
References
- 1. Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. 2022 Apr;12(1):7–11. 10.1016/j.kisu.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jager KJ, Kovesdy C, Langham R, Rosenberg M, Jha V, Zoccali C. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Kidney Int. 2019 Nov 1;96(5):1048–50. 10.1016/j.kint.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 3. GBD Chronic Kidney Disease Collaboration, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385(9981):1975–82. 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 5. Al-Qadi M, LeVarge B, Ford HJ. Epidemiology, pathogenesis, and clinical approach in group 5 pulmonary hypertension. Front Med. 2020;7:616720. 10.3389/fmed.2020.616720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walther CP, Nambi V, Hanania NA, Navaneethan SD. Diagnosis and management of pulmonary hypertension in patients with CKD. Am J Kidney Dis. 2020 Jun;75(6):935–45. 10.1053/j.ajkd.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Agarwal R. Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant. 2012 Oct;27(10):3908–14. 10.1093/ndt/gfr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reque J, Quiroga B, Ruiz C, Villaverde MT, Vega A, Abad S, et al. Pulmonary hypertension is an independent predictor of cardiovascular events and mortality in haemodialysis patients. Nephrology. 2016 Apr;21(4):321–6. 10.1111/nep.12595. [DOI] [PubMed] [Google Scholar]
- 9. Tang M, Batty JA, Lin C, Fan X, Chan KE, Kalim S. Pulmonary hypertension, mortality, and cardiovascular disease in CKD and ESRD patients: a systematic review and meta-analysis. Am J Kidney Dis. 2018 Jul;72(1):75–83. 10.1053/j.ajkd.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 10. Devasahayam J, Oliver T, Joseph V, Nambiar S, Gunasekaran K. Pulmonary hypertension in end-stage renal disease. Respir Med. 2020 Apr;164:105905. 10.1016/j.rmed.2020.105905. [DOI] [PubMed] [Google Scholar]
- 11. Tudoran M, Ciocarlie T, Mates A, Pescariu SA, AbuAwwad A, Tudoran C. Pulmonary hypertension in patients with end stage renal disease undergoing hemodialysis. Niger J Clin Pract. 2020 Feb;23(2):198–204. 10.4103/njcp.njcp_278_19. [DOI] [PubMed] [Google Scholar]
- 12. Goyal VK, Solanki SL, Baj B. Pulmonary hypertension and post-operative outcome in renal transplant: a retrospective analysis of 170 patients. Indian J Anaesth. 2018 Feb;62(2):131–5. 10.4103/ija.IJA_529_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022 Oct 11;43(38):3618–731. 10.1093/eurheartj/ehac237. [DOI] [PubMed] [Google Scholar]
- 14. Lau EM, Humbert M, Celermajer DS. Early detection of pulmonary arterial hypertension. Nat Rev Cardiol. 2015 Mar;12(3):143–55. 10.1038/nrcardio.2014.191. [DOI] [PubMed] [Google Scholar]
- 15. Augustine DX, Coates-Bradshaw LD, Willis J, Harkness A, Ring L, Grapsa J, et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Res Pract. 2018 Sep;5(3):G11–24. 10.1530/ERP-17-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Douglas PS, Carabello BA, Lang RM, Lopez L, Pellikka PA, Picard MH, et al. 2019 ACC/AHA/ASE key data elements and definitions for transthoracic echocardiography: a report of the American College of Cardiology/American Heart Association task force on clinical data standards (writing committee to develop cardiovascular endpoints data standards) and the American Society of Echocardiography. J Am Coll Cardiol. 2019 Jul 23;74(3):403–69. 10.1016/j.jacc.2019.02.027. [DOI] [PubMed] [Google Scholar]
- 17. Rahaghi FF, Kolaitis NA, Adegunsoye A, de Andrade JA, Flaherty KR, Lancaster LH, et al. Screening strategies for pulmonary hypertension in patients with interstitial lung disease: a multidisciplinary delphi study. Chest. 2022 Jul;162(1):145–55. 10.1016/j.chest.2022.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D’Alto M, Di Maio M, Romeo E, Argiento P, Blasi E, Di Vilio A, et al. Echocardiographic probability of pulmonary hypertension: a validation study. Eur Respir J. 2022 Aug;60(2):2102548. 10.1183/13993003.02548-2021. [DOI] [PubMed] [Google Scholar]
- 19. van de Veerdonk MC, Vonk-Noordegraaf A, Vachiery JL. Unbowed, unbent, unbroken: predicting pulmonary hypertension using echocardiography. Eur Respir J. 2022 Aug;60(2):2200481. 10.1183/13993003.00481-2022. [DOI] [PubMed] [Google Scholar]
- 20. Zhu J, Tang C, Ouyang H, Shen H, You T, Hu J. Prediction of all-cause mortality using an echocardiography-based risk score in hemodialysis patients. Cardiorenal Med. 2021;11(1):33–43. 10.1159/000507727. [DOI] [PubMed] [Google Scholar]
- 21. You X, Huang YY, Wang Y, Yu MX, Li XY, Xu L, et al. Prediction model for cardiovascular disease risk in hemodialysis patients. Int Urol Nephrol. 2022 May;54(5):1127–34. 10.1007/s11255-021-02984-7. [DOI] [PubMed] [Google Scholar]
- 22. Schoenberg NC, Argula RG, Klings ES, Wilson KC, Farber HW. Prevalence and mortality of pulmonary hypertension in ESRD: a systematic review and meta-analysis. Lung. 2020 Jun;198(3):535–45. 10.1007/s00408-020-00355-0. [DOI] [PubMed] [Google Scholar]
- 23. Jaroszyński A, Schlegel TT, Zaborowski T, Zapolski T, Załuska W, Janion-Sadowska A, et al. The value of ventricular gradient for predicting pulmonary hypertension and mortality in hemodialysis patients. Sci Rep. 2022 Jan 10;12(1):456. 10.1038/s41598-021-04186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navaneethan SD, Roy J, Tao K, Brecklin CS, Chen J, Deo R, et al. Prevalence, predictors, and outcomes of pulmonary hypertension in CKD. J Am Soc Nephrol. 2016 Mar;27(3):877–86. 10.1681/ASN.2014111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zeng Y, Yang DD, Feng S, Shen HY, Wang Z, Jiang S, et al. Risk factors for pulmonary hypertension in patients receiving maintenance peritoneal dialysis. Braz J Med Biol Res. 2016 Mar;49(3):e4733. 10.1590/1414-431X20154733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nishimura M, Tokoro T, Yamazaki S, Hashimoto T, Kobayashi H, Ono T. Idiopathic pre-capillary pulmonary hypertension in patients with end-stage kidney disease: effect of endothelin receptor antagonists. Clin Exp Nephrol. 2017 Dec;21(6):1088–96. 10.1007/s10157-016-1344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hjalmarsson C, Radegran G, Kylhammar D, Rundqvist B, Multing J, Nisell MD, et al. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J. 2018 May;51(5):1702310. 10.1183/13993003.02310-2017. [DOI] [PubMed] [Google Scholar]
- 28. Zhang L, Long J, Jiang W, Shi Y, He X, Zhou Z, et al. Trends in chronic kidney disease in China. N Engl J Med. 2016 Sep 1;375(9):905–6. 10.1056/nejmc1602469. [DOI] [PubMed] [Google Scholar]
- 29. Giorda CB, Carna P, Salomone M, Picariello R, Costa G, Tartaglino B, et al. Ten-year comparative analysis of incidence, prognosis, and associated factors for dialysis and renal transplantation in type 1 and type 2 diabetes versus non-diabetes. Acta Diabetol. 2018 Jul;55(7):733–40. 10.1007/s00592-018-1142-y. [DOI] [PubMed] [Google Scholar]
- 30. Whitaker ME, Nair V, Sinari S, Dherange PA, Natarajan B, Trutter L, et al. Diabetes mellitus associates with increased right ventricular afterload and remodeling in pulmonary arterial hypertension. Am J Med. 2018 Jun;131(6):702 e7–702702.e13. 10.1016/j.amjmed.2017.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang C, Gao B, Zhao X, Su Z, Sun X, Wang HY, et al. Executive summary for China kidney disease network (CK-NET) 2016 annual data report. Kidney Int. 2020 Dec;98(6):1419–23. 10.1016/j.kint.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 32. Tong J, Liu M, Li H, Luo Z, Zhong X, Huang J, et al. Mortality and associated risk factors in dialysis patients with cardiovascular disease. Kidney Blood Press Res. 2016;41(4):479–87. 10.1159/000443449. [DOI] [PubMed] [Google Scholar]
- 33. Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet. 2016;388(10041):276–84. 10.1016/S0140-6736(16)30508-6. [DOI] [PubMed] [Google Scholar]
- 34. Johansen KL, Chertow GM, Gilbertson DT, Herzog CA, Ishani A, Israni AK, et al. US renal data system 2021 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2022 Apr;79(4 Suppl 1):A8–12. 10.1053/j.ajkd.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lameire N, Van Biesen W, Vanholder R. Did 20 years of technological innovations in hemodialysis contribute to better patient outcomes? Clin J Am Soc Nephrol. 2009 Dec;4(Suppl 1):S30–40. 10.2215/CJN.04000609. [DOI] [PubMed] [Google Scholar]
- 36. Gaweda AE, Goldsmith LJ, Brier ME, Aronoff GR. Iron, inflammation, dialysis adequacy, nutritional status, and hyperparathyroidism modify erythropoietic response. Clin J Am Soc Nephrol. 2010 Apr;5(4):576–81. 10.2215/CJN.04710709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol. 2012 Oct;23(10):1631–4. 10.1681/ASN.2011111078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, et al. Pulmonary hypertension in CKD. Am J Kidney Dis. 2013 Apr;61(4):612–22. 10.1053/j.ajkd.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 39. Locatelli F, Pisoni RL, Akizawa T, Cruz JM, DeOreo PB, Lameire NH, et al. Anemia management for hemodialysis patients: kidney disease outcomes quality initiative (K/DOQI) guidelines and dialysis outcomes and practice patterns study (DOPPS) findings. Am J Kidney Dis. 2004 Nov;44(5 Suppl 2):27–33. 10.1053/j.ajkd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 40. Locham S, Mathlouthi A, Dakour-Aridi H, Nejim B, Malas MB. Association between severe anemia and outcomes of hemodialysis vascular access. Ann Vasc Surg. 2020 Jan;62:295–303. 10.1016/j.avsg.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 41. Mele D, Andrade A, Bettencourt P, Moura B, Pestelli G, Ferrari R. From left ventricular ejection fraction to cardiac hemodynamics: role of echocardiography in evaluating patients with heart failure. Heart Fail Rev. 2020 Mar;25(2):217–30. 10.1007/s10741-019-09826-w. [DOI] [PubMed] [Google Scholar]
- 42. Malik J, Lachmanova J, Kudlicka J, Rocinova K, Valerianova A, Bartkova M, et al. Left atrial dysfunction in end-stage renal disease patients treated by hemodialysis. Nephron. 2016;133(3):169–74. 10.1159/000447500. [DOI] [PubMed] [Google Scholar]
- 43. Hickson LJ, Negrotto SM, Onuigbo M, Scott CG, Rule AD, Norby SM, et al. Echocardiography criteria for structural heart disease in patients with end-stage renal disease initiating hemodialysis. J Am Coll Cardiol. 2016 Mar 15;67(10):1173–82. 10.1016/j.jacc.2015.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamada S, Ishii H, Takahashi H, Aoyama T, Morita Y, Kasuga H, et al. Prognostic value of reduced left ventricular ejection fraction at start of hemodialysis therapy on cardiovascular and all-cause mortality in end-stage renal disease patients. Clin J Am Soc Nephrol. 2010 Oct;5(10):1793–8. 10.2215/CJN.00050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Obi C, Frost AE, Graviss EA, Nguyen DT, Gaber AO, Suki WN. The association of pretransplant pulmonary hypertension with patient and graft survival after kidney transplantation: a retrospective cohort study. Transplant Proc. 2020 Dec;52(10):3023–32. 10.1016/j.transproceed.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 46. Ramasubbu K, Deswal A, Herdejurgen C, Aguilar D, Frost AE. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: prevalence and clinical significance. Int J Gen Med. 2010 Oct 5;3:279–86. 10.2147/IJGM.S12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material files. Further inquiries can be directed to the corresponding author.