Abstract
Purpose:
To provide 4-year data on the efficacy and safety of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma.
Design:
Retrospective case series.
Participants:
Eyes of patients > 18 years of age who underwent GATT by a single surgeon at Wills Eye Hospital with at least 36 months follow-up.
Methods:
Postoperative changes in outcome measures including intraocular pressure (IOP), medication use and visual acuity were recorded. Failure was defined as IOP > 21 mmHg or less than 20% reduction below baseline at any postoperative visit after 3 months or need for further glaucoma surgery.
Main Outcome Measures:
Main outcome measures were failure rate, IOP, number of glaucoma medications, and visual acuity at 4 years.
Results:
Fifty-nine patients (74 eyes), age 57.1 ± 18.5 years (37.8% female) underwent the GATT procedure. Average follow-up was 47.0 ± 6.7 months (range 35.6–76.5 months). Mean IOP was 27.0 ± 10.0 mmHg preoperatively and 14.8 ± 6.5 mmHg at 4 years (45% IOP decrease; P < 0.01). Mean number of medications decreased from 3.2 ± 1.0 preoperatively to 2.3 ± 1.0 at 4 years (P < 0.01). The cumulative failure rate at 4 years was 53.9%, and the cumulative reoperation rate was 42.0%. No significant differences between patients with primary open-angle glaucoma and other types of glaucoma were found.
Conclusions:
Gonioscopy-assisted transluminal trabeculotomy can be a safe and effective conjunctival-sparing surgery for treating various forms of open-angle glaucoma at 4 years.
Financial Disclosure(s):
Proprietary or commercial disclosure may be found after the references.
Keywords: GATT, Glaucoma surgery, Gonioscopy-assisted, Minimally invasive, Trabeculotomy
Gonioscopy-assisted transluminal trabeculotomy (GATT) is a circumferential ab interno trabeculotomy that lowers the intraocular pressure (IOP) by improving aqueous outflow through Schlemm’s canal and adjacent collector channels without bleb formation or device implantation.1,2 Gonioscopy-assisted transluminal trabeculotomy alone or combined with cataract surgery has shown success in lowering IOP and the number of glaucoma medications in primary, juvenile and secondary open-angle glaucomas, including uveitic, steroid-induced, traumatic, pigmentary, and pseudoexfoliation.3–11 Even in patients who had undergone previous incisional glaucoma surgery, GATT can be safe and effective.12
Several studies have provided short- and mid-term (up to 24 months) safety and efficacy data for GATT in open-angle glaucoma.3–5,11 However, longer term data is still lacking. This study seeks to provide 4-year follow-up for this surgery and report on efficacy and safety.
Methods
A retrospective chart review was performed for all patients who underwent a GATT procedure by a single surgeon (M.R.M.) between July 2014 and May 2018 with the goal of evaluating at least a 3-year follow-up. Inclusion criteria were patients > 18 years old with uncontrolled IOP despite maximum medical therapy or who were intolerant to glaucoma medications, consequently requiring surgical intervention at the discretion of the surgeon. Only patients with > 36 months of follow-up were included. This study cohort includes some patients that were included in our initial report.11 Patients with chronic angle closure glaucoma, inability to stop anticoagulant medications, bleeding disorders, unidentifiable angle structures, severe corneal endothelial decompensation, or an unstable intraocular lens were considered as unsuitable candidates for GATT. All patients had a preoperative gonioscopic examination that revealed an open-angle with visible trabecular meshwork. Eyes with neovascular glaucoma were included if the angles were open and the eyes were quiescent with no active neovascularization of the angle or iris at the time of surgery. Both eyes were included for patients who underwent sequential, bilateral GATT. For these eyes, the between-eye correlation in their 4-year survival was found to be low (intraclass correlation coefficient 0.2). Wills Eye Hospital Institutional Review Board approved the study, which was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
Surgical Procedure
The GATT procedure was performed as described previously.2,11 In brief, a nasal trabecular meshwork goniotomy was made with a 25-gauge microvitreoretinal blade. The microcatheter (iTRACK, Ellex iScience Inc) was passed through Schlemm’s canal 360 degrees while an assistant viscodilated the canal approximately 1 turn every clock hour. The catheter was pulled out of the paracentesis to bisect the trabecular meshwork. The anterior chamber was filled with a balanced salt solution until the IOP went to about 30 mmHg and the episcleral perilimbal vessels were studied. An episcleral venous fluid wave was observed when the blood vessels blanched and the limbus turned white. The intraoperative wave was graded on a scale of 1 to 4 based on the number of quadrants that blanched.
For the patients who underwent concurrent cataract surgery, GATT was performed first, followed by cataract extraction with phacoemulsification and intraocular lens implantation. At the end of the surgery, antibiotic and steroid drops were administered.
Postoperative Assessment
Postoperative data was collected from 1 day, 1 week, 1 month, 3 months, 6 months, and every 6 to 12 months thereafter, if available, to assess results of the GATT surgery. Atthe2-, 3-, and 4-year visit time points, visits within 6 months of that time point were included. Postoperative IOP, number of glaucoma medications, best-corrected visual acuity as well as any surgical complications were recorded.
Outcome Measures
The primary outcome measure was failure rate. Secondary outcome measures included change in IOP, change in best-corrected visual acuity, and number of medications used. Participants were divided into 2 groups: the primary open-angle glaucoma (POAG) group and the secondary open-angle glaucoma (SOAG) group. Failure was defined as IOP > 21 mmHg or less than 20% reduction below baseline on any postoperative visit after 3 months, or need for further glaucoma surgery.11,12 Medication use was not a criterion for failure. This definition was used to generate Kaplan–Meier survival curves based on the cumulative rate of surgical failure. Data on IOP and numbers of glaucoma medications were censored once a patient underwent a reoperation for glaucoma. Visual acuities were not censored after reoperation.
Statistical Analysis
Statistical analysis was performed using SPSS software. Continuous variables were expressed as mean ± standard deviation or range. Proportions (%) were used to describe categorical variables. Paired sample t tests were used to compare continuous variables within the same group. Between-group comparisons for continuous and categorical variables were performed using 2-sided t tests and chi-square test (Fisher exact test), respectively. Visual acuities were converted from Snellen to approximate logMAR [−1×log (Snellen fraction)] for analysis. Risk factors for failure were evaluated with Cox proportional hazards regression with forward stepwise elimination. Covariates with P > 0.2 in the univariable model were included in the multivariable model.
Results
Baseline Characteristics
Table 1 summarizes demographic characteristics of the study patients. Fifty-nine patients and 74 eyes with mean age 56.8 ± 18.3 years (range 20–91 years) underwent the GATT procedure, with 37.8% females. The racial distribution was 56.8% White, 32.4% Black, 8.1% Hispanic, and 2.7% Asian. The average follow-up period was 47.0 ± 6.7 months (range 35.6–76.5 months). In total, 78.4% of eyes were diagnosed with POAG. The remaining 21.6% were diagnosed with SOAG including pseu-doexfoliation, pigmentary, uveitic, neovascular, or neovascular glaucoma. In addition, 16.2% eyes were pseudophakic and 12.1% underwent combined GATT and cataract extraction.
Table 1.
Demographics by Study Group
| POAG | SOAG | Total | P | |
|---|---|---|---|---|
|
| ||||
| No. patients | 44 | 15 | 59 | |
| No. eyes | 58 | 16 | 74 | |
| Mean age, yr, ± SD | 56.8 ± 18.3 | 58.4 ± 19.7 | 57.1 ± 18.5 | 0.76 |
| Range | 22–84 | 30–91 | ||
| Female | 22 | 6 | 28 (37.8%) | 0.98 |
| GATT alone in phakic eyes | 43 | 10 | 53 (71.6%) | 0.59 |
| GATT alone in pseudophakic eyes | 9 | 3 | 12 (16.2%) | |
| GATT combined with CE | 6 | 3 | 9 (12.1%) | |
| Ethnicity | ||||
| White | 28 | 14 | 42 (56.8%) | <0.001 |
| Black | 24 | 0 | 24 (32.4%) | |
| Hispanic | 6 | 0 | 6 (8.1%) | |
| Asian | 0 | 2 | 2 (2.7%) | |
| Diagnosis | ||||
| POAG | 58 | 0 | 58 | |
| PXF | 0 | 5 | 5 | |
| Pigmentary | 0 | 3 | 3 | |
| Uveitic | 0 | 3 | 3 | |
| NVG | 0 | 2 | 2 | |
| Other | 0 | 3 | 3 | |
| VF MD, mean ± SD | 8.2 ± 5.2 | 8.2 ± 6.3 | 8.2 ± 5.4 | 1.0 |
Data are presented as mean ± SD or n (%). Bold indicates statistical significance.
CE = cataract extraction; GATT = gonioscopy-assisted transluminal trabeculotomy; MD = mean deviation; NVG= neovascular glaucoma; POAG = primary open-angle glaucoma; PXF = pseudoexfoliation glaucoma; SD = standard deviation; SOAG = secondary open-angle glaucoma; VF= visual field.
IOP Reduction and Medical Therapy
Table 2 shows detailed surgical efficacy data, including mean IOP and mean number of medications for each follow-up visit. Patients who underwent additional glaucoma surgery were censored from analysis after reoperation. By the 4-year visit, there were 28 eyes that were censored because of glaucoma reoperation. Among the 31 eyes that did not undergo subsequent glaucoma surgery, GATT produced a significant reduction in IOP at all postoperative time points (P < 0.01). Mean IOP was 27.0 ± 10.0 mmHg preoperatively and 14.8 ± 6.5 mmHg at 4 years (45% IOP decrease; P < 0.01). There was a significant and sustained reduction in the use of medical therapy. Mean number of medications decreased from 3.2 ± 1.0 preoperatively to 2.3 ± 1.0 at 4 years (P < 0.01).
Table 2.
Efficacy Data for Study Population, Separated by Study Group
| POAG | SOAG | Total | P | |
|---|---|---|---|---|
|
| ||||
| Months followed, mean ± SD | 47.4 ± 7.0 | 45.7 ± 5.5 | 47.0 ± 6.7 | 0.31 |
| Cumulative eyes censored because of reoperation at each follow-up visit (n) | ||||
| Baseline | 58 | 16 | 74 | |
| 6 mos | 15 | 2 | 17 | |
| 1 yr | 15 | 4 | 19 | |
| 2 yrs | 18 | 4 | 22 | |
| 3 yrs | 19 | 5 | 24 | |
| 4 yrs | 22 | 6 | 28 | |
| Eyes lost to follow-up between 3 and 4 years (n) | 11 | 4 | 15 | |
| IOP in mmHg, mean ± SD | ||||
| Baseline | 26.8 ± 10.5 | 27.8 ± 8.3 | 27.0 ± 10.0 | 0.73 |
| 6 mos | 12.9 ± 3.1 | 14.3 ± 4.3 | 13.2 ± 3.4 | 0.33 |
| 1 yr | 13.5 ± 3.7 | 16.1 ± 5.6 | 14.1 ± 4.2 | 0.15 |
| 2 yrs | 14.6 ± 5.1 | 17.3 ± 9.2 | 15.1 ± 5.9 | 0.31 |
| 3 yrs | 13.8 ± 5.9 | 15.4 ± 4.0 | 14.2 ± 5.5 | 0.43 |
| 4 yrs | 14.6 ± 7.1 | 15.7 ± 3.2 | 14.8 ± 6.5 | 0.72 |
| Medications, mean ± SD | ||||
| Baseline | 3.2 ± 0.9 | 3.3 ± 1.2 | 3.2 ± 1.0 | 0.78 |
| 6 mos | 2.0 ± 0.4 | 2.4 ± 0.7 | 2.1 ± 0.5 | 0.17 |
| 1 yr | 2.0 ± 0.4 | 2.3 ± 0.5 | 2.1 ± 0.4 | 0.09 |
| 2 yrs | 2.2 ± 0.9 | 2.3 ± 0.5 | 2.2 ± 0.8 | 0.66 |
| 3 yrs | 2.4 ± 0.7 | 1.9 ± 1.3 | 2.3 ± 0.9 | 0.28 |
| 4 yrs | 2.2 ± 0.8 | 2.5 ± 1.6 | 2.3 ± 1.0 | 0.68 |
Data are presented as mean ± SD.
IOP = intraocular pressure; POAG = primary open-angle glaucoma; SD = standard deviation; SOAG = secondary open-angle glaucoma.
There were 58 eyes with POAG. At 4 years, this group had an average decrease in IOP of 12.2 ± 12.8 mmHg (46%; P < 0.01). The average number of medications decreased from baseline by 1.0 ± 1.2 (P < 0.01). There were 16 eyes in the SOAG group. At 4 years, this group had an average decrease in IOP of 12.1 ± 8.9 mmHg (44%; P < 0.01). There was no significant difference in the average number of medications decreased from baseline in this group (P = 0.34). There were no statistical differences between the POAG and SOAG groups with regard to mean IOP or the number of medications in use.
We performed additional analysis to include the 28 eyes that underwent additional glaucoma surgery. Among the 59 eyes that completed the 4-year visit, the IOP was 14.0 ± 4.9 mmHg and the mean number of medications was 2.0 ± 1.2, which were significantly reduced from baseline (P < 0.01).
Postoperative Complications
Table 3 shows the intraoperative findings and postoperative complications. The total amount of canal opened at the time of surgery was 360 degrees in 70 eyes (94.6%). The remaining eyes had 180 to 270 degrees of canal opening. A > 50% intraoperative episcleral venous fluid wave was found in 27 (36.5%) eyes. Twenty-seven (36.5%) eyes had a hyphema at postoperative week 1, which was reduced to 8 (10.8%) at month 1 and 1 (1.4%) at month 3. One eye had a significant hyphema with IOP spike requiring anterior chamber washout and trabeculectomy at month 1. There were 16 eyes (21.6%) with IOP spike > 30 mmHg within 1 month. Six eyes (8.1%) had an IOP spike at postoperative day 1, 8 eyes (10.8%) at week 1, and 5 eyes (6.8%) at month 1. Four of these eyes also had a hyphema. Six eyes with IOP spikes underwent reoperation by month 3. There were no eyes with persistent inflammation at month 1. No eyes experienced post-operative hypotony (IOP < 5 mmHg), suprachoroidal hemorrhage, or other vision-threatening complications.
Table 3.
Intraoperative and Postoperative Findings
| POAG | SOAG | Total | P | |
|---|---|---|---|---|
|
| ||||
| 360 degree canal opening | 56 (96.6) | 14 (87.5) | 70 (94.6) | 0.20 |
| Intraoperative fluid wave (> 50%) (n, %) | 19 (32.8) | 6 (37.5) | 27 (36.5) | 0.77 |
| Hyphema (n, %) | ||||
| Week 1 | 19 (32.8) | 8 (50.0) | 27 (36.5) | 0.23 |
| Month 1 | 7 (12.1) | 1 (12.5) | 8 (10.8) | 0.80 |
| Month 3 | 1 (1.7) | 0 | 1 (1.4) | 0.50 |
| IOP spike > 30 mmHg within 1 mo (n, %) | 12 (20.7) | 4 (25.0) | 16 (21.6) | 0.74 |
IOP = intraocular pressure; POAG = primary open-angle glaucoma; SOAG = secondary open-angle glaucoma.
Treatment Outcomes
Table 4 compares the outcomes and reasons of failure of study patients at year 4. Overall, 38 eyes failed at 4 years. Of the failures, 78.9% were due to reoperation for glaucoma, and 21.1% were due to inadequate IOP control without need for additional surgery. The reasons for failure were similar across study groups.
Table 4.
Reasons for Treatment Failure at 4 Years
| POAG | SOAG | Total | P | |
|---|---|---|---|---|
|
| ||||
| Failures (n) | 29 | 9 | 38 | 0.78 |
| Reasons for Failure (n, %) | ||||
| Inadequate IOP reduction without additional glaucoma surgery | 6 (20.7) | 2 (22.2) | 8 (21.1) | 1.0 |
| Reoperation for glaucoma | 23 (79.3) | 7 (77.8) | 30 (78.9) | |
| Types of reoperations (n, %) | ||||
| Trabeculectomy | 13 (56.5) | 3 (42.9) | 16 (53.3) | |
| Tube shunts (Baerveldt/Ahmed) | 3 (13.0) | 4 (57.1) | 7 (23.3) | |
| Subconjunctival minimally invasive glaucoma surgery (ab externo Xen, Microshunt) | 7 (30.4) | 0 | 7 (23.3) | |
IOP = intraocular pressure; POAG = primary open-angle glaucoma; SOAG = secondary open-angle glaucoma.
Kaplan–Meier survival analysis was used to compare failure rates between the 2 study groups (Fig 1). The cumulative proportion of failure at 4 years was 51.8% for the POAG group and 62.5% for the SOAG group (P = 0.53, log-rank test), with an overall failure rate of 53.9%. The majority of failures occurred in the first year. The cumulative proportion of failure at 1 year, 2 years, and 3 years were 27.0%, 31.1%, and 45.9%, respectively.
Figure 1.
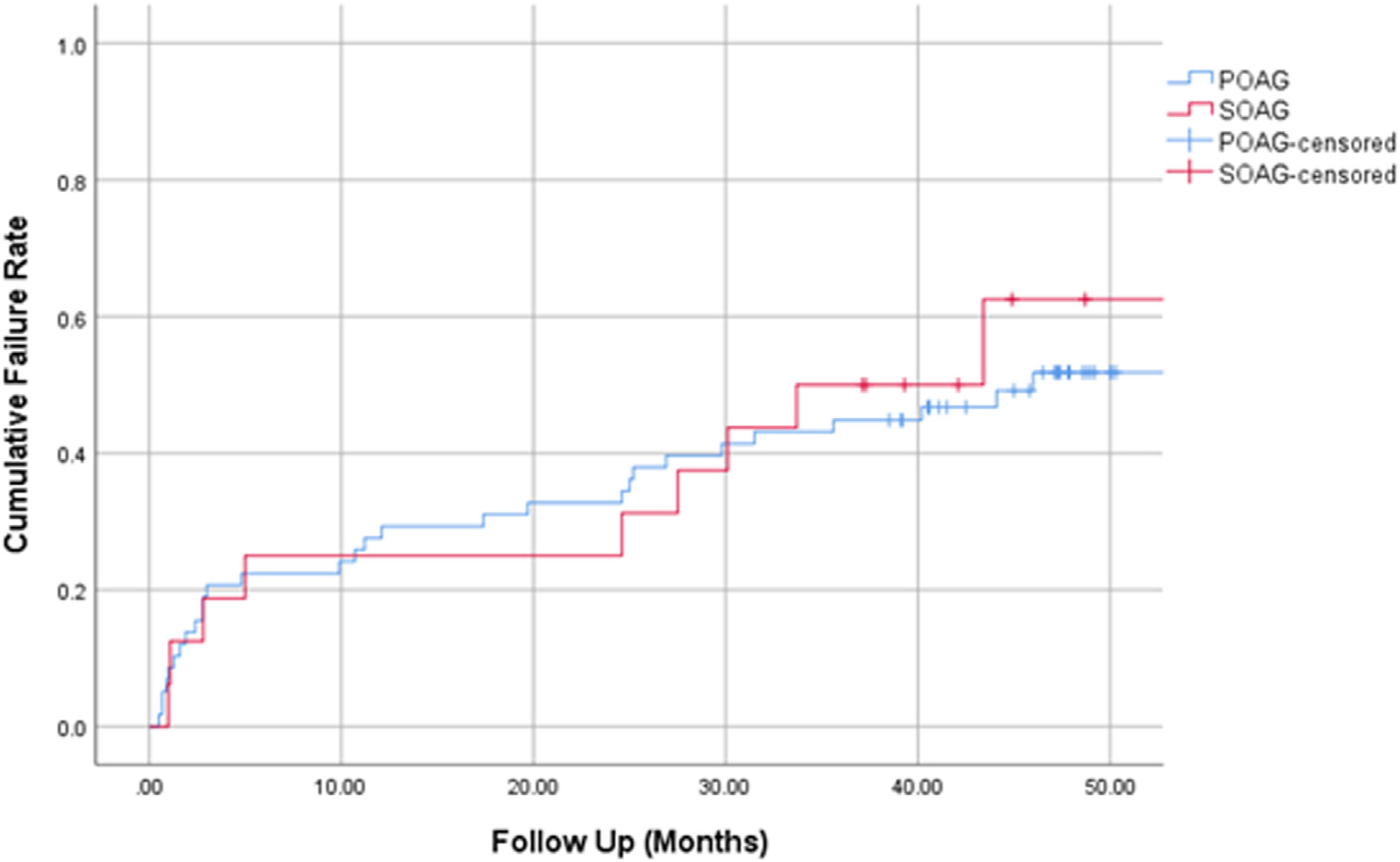
Kaplan–Meier curve demonstrating the cumulative proportion of failure over time separated by study group. POAG = primary open-angle glaucoma; SOAG = secondary open-angle glaucoma.
Table 5 evaluates the risk factors for failure at 4 years. Postoperative IOP spike >30 mmHg was the only significant factor associated with failure in univariable analyses, with unadjusted hazard ratio (HR) 2.61 (95% confidence interval [CI], 1.30–5.24; P = 0.01). In multivariable analyses that included sex, race, number of baseline medications, baseline IOP, and postoperative IOP spike as covariates, the adjusted HR for postoperative IOP spike was 3.24 (95% CI, 1.57–6.69; P = 0.002). Black race was a significant risk factor for failure (adjusted HR, 1.95; 95% CI, 1.02–3.73; P = 0.045). Baseline IOP was also associated with a mildly increased risk of failure (adjusted HR, 1.04; 95% CI, 1.00–1.07; P = 0.023). Factors that were not associated with failure in both univariable and multivariable analyses included sex, age, glaucoma type, number of baseline medications, baseline visual field mean deviation, GATT with concurrent cataract surgery, lens status, and intraoperative vessel blanching (P > 0.5).
Table 5.
Risk Factors for Treatment Failure at 4 Years
| Univariable | Multivariable* | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Unadjusted HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| Sex (male vs female) | 0.07 | 0.288 | ||||
| Age | 0.54 | |||||
| Race (Black vs non-Black) | 0.06 | 1.95 | 1.02–3.73 | 0.045 | ||
| Glaucoma type (POAG vs SOAG) | 0.70 | |||||
| Number of baseline medications | 0.14 | 0.117 | ||||
| Baseline IOP | 0.08 | 1.04 | 1.00–1.07 | 0.023 | ||
| Baseline VF MD | 0.60 | |||||
| GATT combined with CE (vs GATT alone) | 0.29 | |||||
| Lens status | 0.32 | |||||
| Intraoperative fluid wave > 50% | 0.26 | |||||
| Postoperative IOP spike > 30 mmHg | 2.61 | 1.30–5.24 | 0.01 | 3.24 | 1.57–6.69 | 0.002 |
Bold indicates statistical significance.
CE = cataract extraction; CI = confidence interval, given for significant variables; GATT = gonioscopy-assisted transluminal trabeculotomy; HR = hazard ratio, given for significant variables; IOP = intraocular pressure; MD = mean deviation; POAG = primary open-angle glaucoma; SOAG = secondary open-angle glaucoma; VF= visual field.
Multivariable analysis with sex, race, number of baseline medications, baseline IOP, and postoperative IOP spike as covariates.
Reoperation for Glaucoma
The cumulative proportion of patients undergoing reoperation for glaucoma at 4 years was 40.6% for the POAG group and 47.9% for the SOAG group (P = 0.63, log-rank test), with an overall reoperation rate of 42.0% (Fig 2). The cumulative proportion of reoperations at 1 year, 2 years, and 3 years were 25.7%, 31.1%, and 37.8%, respectively. Table 4 shows the specific glaucoma reoperations for each group. Thirty eyes required reoperation for uncontrolled glaucoma at a mean of 12.5 ± 14.4 months after GATT. Glaucoma procedures included trabeculectomy (53.3%), tube shunts (23.3%), and subconjunctival minimally invasive glaucoma surgeries (23.3%). An additional 11 eyes (9 in the POAG group, 2 in the SOAG group) underwent cataract surgery during the follow-up period.
Figure 2.
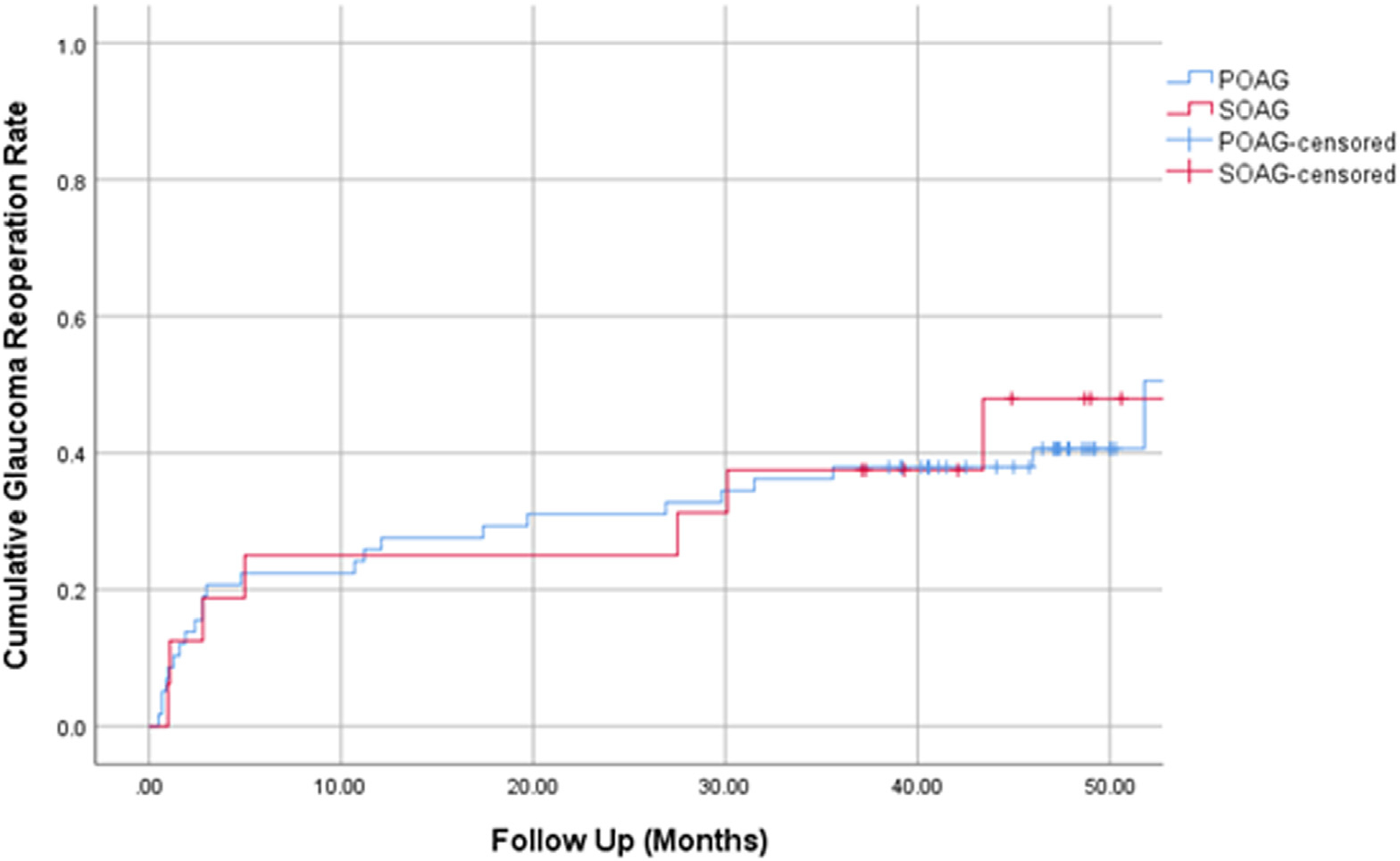
Kaplan–Meier curve demonstrating the cumulative proportion of glaucoma reoperation over time separated by study group. POAG = primary open-angle glaucoma; SOAG = secondary open-angle glaucoma.
Visual Acuity
Visual acuity results are shown in Table 6. There was no significant difference in best-corrected visual acuity over the 4-year study period compared with baseline (P > 0.05). Eleven patients lost 3 lines or more of Snellen acuity at 4 years, and 8 of those patients (72.7%) underwent reoperation for glaucoma.
Table 6.
Preoperative and Postoperative BCVA after GATT in All Patients after 4 Years
| BCVA (LogMAR) | Mean LogMAR (SD) | P |
|---|---|---|
|
| ||
| Baseline | 0.25 ± 0.46 | |
| 6 mos | 0.22 ± 0.36 | 0.34 |
| 1 yr | 0.23 ± 0.36 | 0.40 |
| 2 yrs | 0.22 ± 0.43 | 0.37 |
| 3 yrs | 0.18 ± 0.36 | 0.16 |
| 4 yrs | 0.38 ± 0.59 | 0.09 |
Data are presented as mean ± standard deviation (SD).
BCVA = best-corrected visual acuity; GATT = gonioscopy-assisted transluminal trabeculotomy.
Discussion
In this retrospective comparative study, we provide 4-year data on the safety and efficacy of GATT in open-angle glaucoma in 74 eyes with at least 36 months follow-up. At 4 years, the cumulative failure rate was 53.9% and the reoperation rate was 42.0%. There were no significant differences in the failure and reoperation rates between the POAG and SOAG groups. Among the 31 eyes at the 4-year follow-up who did not undergo subsequent glaucoma surgery, GATT produced significant reductions in IOP, from baseline of 27.0 mmHg to 14.8 mmHg at 4 years, with an average decrease of 0.9 medications. The IOP reduction represents a 45% decrease from baseline.
Compared with other retrospective studies with shorter follow-up, our cumulative failure rates were similar. Rahmatnejad et al11 reported in a study of 66 patients with open-angle glaucoma that the cumulative failure rate was 37% at 12 months. Grover et al4 reported in a study of 198 patients with open-angle glaucoma that the cumulative failure rate at 24 months ranged from 18% to 48%, depending on the type of glaucoma and lens status. Compared with these short- and mid-term studies that employed similar success/failure criteria, our failure rates were comparable at those time points, with a cumulative failure rate of 27.0% at 12 months and 31.1% at 24 months.
Thirty eyes required reoperation for glaucoma by 4 years after GATT, representing a cumulative reoperation rate of 42.0%. Patients who underwent glaucoma reoperation after GATT most commonly underwent a trabeculectomy or tube shunt. Large-scale prospective studies reporting outcomes of tubes and trabeculectomies showed failure rates ranging from 30% to 53% at 5 years, with reoperation rates ranging from 9% to 29%.13–16 The failure and reoperation rates for GATT in this study were, in general, higher than those reported for tubes and trabeculectomies. This may not be surprising, as the decision for additional glaucoma surgery was left to the surgeon’s discretion, and there may be a lower threshold to reoperate after GATT than after traditional glaucoma surgeries. In some patients, there may be an intention to first perform a conjunctival-sparing surgery such as GATT to delay the need for traditional glaucoma surgery. Interestingly, in eyes that did not require further glaucoma surgery after GATT, the percentage of IOP lowering was comparable to that reported for tube shunts and trabeculectomies.13–16
Risk factors associated with failure at 4 years include postoperative IOP spike, Black race, and preoperative IOP. Postoperative IOP spike and Black race were also previously reported as risk factors associated with surgical failure at 12 months.11 In this study, the majority of reoperations occurred in the first 12 months, and reoperation after postoperative IOP spikes account for some of these early failures. Black race has been associated with surgical failures after other glaucoma procedures including trabeculectomy, Ex-PRESS shunt, tube shunt, and canaloplasty.17 Other studies have also reported that Blacks have a higher risk of inflammation after routine cataract surgery and endoscopic cyclophotocoagulation.18,19 It is possible that increased intraocular inflammation in Blacks after GATT may promote closure of the canal opening or distal collector channels, although none of the patients in this study had any clinical signs of inflammation by postoperative month 1. There was also a slightly higher risk of failure in patients with higher preoperative IOP, suggesting that GATT may not provide sufficient IOP reduction in some of these patients. The episcleral venous fluid wave has been suggested as an intraoperative gauge of trabecular outflow patency during angle procedures and correlates with outcomes after Trabectome at 12 months.20 We did not find this wave to be associated with surgical success at 4 years. It is possible that venous blanching has a stronger correlation with short-term outcomes after angle surgery, which would be an interesting topic for future investigation.
We did not find concurrent or previous cataract surgery to affect the outcome of GATT at 4 years, considering that these eyes represent a relatively small subset of our cohort. Previous studies did not find a significant difference in IOP reduction after GATT for those who underwent concurrent or previous cataract surgery at 12 months or 24 months,4,11 although pseudophakic POAG patients have been suggested to have a higher cumulative proportion of failure after GATT at 24 months.4
GATT showed a strong safety profile at 4 years, and we did not detect any late-onset complications attributed to the procedure. As an angle-based procedure that does not involve an implant or a bleb, GATT avoids many delayed complications reported for tubes and trabeculectomies, such as hypotony, bleb leak, bleb-related endophthalmitis, and tube erosion.21 Consistent with previous reports, transient hyphema was the most common complication.4,11 Almost all of these resolved without surgical intervention. Postoperative IOP spike was also a common occurrence. Most of these occurred in the absence of a hyphema and may be an indicator for early surgical failure.
Best-correct visual acuity remained stable throughout the 4-year follow-up period. A small number of eyes (11) lost 3 lines or more of Snellen acuity, and 8 of these eyes (72.7%) underwent reoperation for glaucoma. This is likely due to the advanced nature of disease in these eyes and not directly attributable to GATT.
The limitations of the study include its retrospective nature. There may be potential for selection bias as the decision to perform GATT was purely determined by the discretion of the surgeon. Further, postoperative drop management and the decision to reoperate were left to the surgeon’s discretion. As all cases were done by a single surgeon, there is less variation in terms of surgical technique and postoperative management. The study is also limited by size and follow-up. With the goal of evaluating 4-year outcomes, we only included patients with > 36 months of follow-up.
In conclusion, our results suggest that GATT is an effective minimally invasive surgery that produces significant IOP reduction at 4 years. This is a promising approach that can spare the conjunctiva for future glaucoma surgeries.
Acknowledgments
The authors made the following disclosures: W.W.L.: Financial support – Stanford Mentored Career Development Award (KL2TR003143), American Glaucoma Society Young Clinician Scientist Award, Research to Prevent Blindness Career Development Award, NIH NEI P30 Vision Research Core (EY026877, Stanford Ophthalmology), and Research to Prevent Blindness (Stanford Ophthalmology).
M.R.M.: Financial Support – AbbVie, Aerie Pharmaceuticals, Alcon, Allergan, Bausch and Lomb, Glaukos, InnFocus, New World Medical, Nicox, Novartis, Santen; Consultant/Advisor – AbbVie, Aerie Pharmaceuticals, Alcon, Allergan, Bausch and Lomb, Glaukos, Gore, InnFocus, MedEdicus, New World Medical, Novartis, Santen, QURA; Lecture Fees/Honoraria – Abbvie, Aerie Pharmaceuticals, Alcon, Allergan, Bausch and Lomb, Glaukos, Gore, InnFocus, Novartis, MedEdicus, New World Medical, Santen; Equity – QURA.
Abbreviations and Acronyms:
- CI
confidence interval
- GATT
gonioscopy-assisted transluminal trabeculotomy
- HR
hazard ratio
- IOP
intraocular pressure
- POAG
primary open-angle glaucoma
- SOAG
secondary open-angle glaucoma
Footnotes
Disclosures:
All authors have completed and submitted the ICMJE disclosures forms.
HUMAN SUBJECTS: Human subjects were included in this study. Wills Eye Hospital Institutional Review Board approved the study, which was conducted in accordance with the Declaration of Helsinki. The requirement for informed consent was waived because of the retrospective nature of the study.
No animal subjects were included in this study.
Presented as a poster at the American Glaucoma Society Annual Meeting, March 3–6, 2022, Nashville, Tennessee.
References
- 1.Grover DS, Fellman RL. Gonioscopy-assisted transluminal trabeculotomy (GATT): thermal suture modification with a dye-stained rounded tip. J Glaucoma. 2016;25:501–504. [DOI] [PubMed] [Google Scholar]
- 2.Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121:855–861. [DOI] [PubMed] [Google Scholar]
- 3.Aktas Z, Ucgul AY, Bektas C, Sahin Karamert S. Surgical outcomes of prolene gonioscopy-assisted transluminal trabeculotomy in patients with moderate to advanced open-angle glaucoma. J Glaucoma. 2019;28:884–888. [DOI] [PubMed] [Google Scholar]
- 4.Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma. 2018;27:393–401. [DOI] [PubMed] [Google Scholar]
- 5.Sharkawi E, Lindegger DJ, Artes PH, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy in pseudoexfoliative glaucoma: 24-month follow-up. Br J Ophthalmol. 2021;105:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boese EA, Shah M. Gonioscopy-assisted transluminal trabeculotomy (GATT) is an effective procedure for steroid-induced glaucoma. J Glaucoma. 2019;28:803–807. [DOI] [PubMed] [Google Scholar]
- 7.Grover DS, Smith O, Fellman RL, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy for the treatment of primary congenital glaucoma and juvenile open angle glaucoma. Br J Ophthalmol. 2015;99:1092–1096. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann-Clarke L, Sadeghi Y, Guarnieri A, Sharkawi E. Gonioscopy-assisted transluminal trabeculotomy using an illuminated catheter for infantile primary congenital glaucoma. Case series. Am J Ophthalmol Case Rep. 2020;19, 100733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Wang H, Han Y, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy in juvenile-onset primary open-angle glaucoma. Eye (Lond). 2021;35:2848–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan AV, Yannuzzi NA, Chen J, et al. Gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with secondary open-angle glaucoma following vitreoretinal surgery. J Glaucoma. 2020;29:e23–e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahmatnejad K, Pruzan NL, Amanullah S, et al. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma. 2017;26:1137–1143. [DOI] [PubMed] [Google Scholar]
- 12.Grover DS, Godfrey DG, Smith O, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma. 2017;26:41–45. [DOI] [PubMed] [Google Scholar]
- 13.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153: 789–803.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budenz DL, Barton K, Gedde SJ, et al. Five-year treatment outcomes in the Ahmed Baerveldt comparison study. Ophthalmology. 2015;122:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christakis PG, Kalenak JW, Tsai JC, et al. The Ahmed versus Baerveldt study: five-year treatment outcomes. Ophthalmology. 2016;123:2093–2102. [DOI] [PubMed] [Google Scholar]
- 16.Gedde SJ, Feuer WJ, Lim KS, et al. Treatment outcomes in the primary Tube Versus Trabeculectomy study after 5 years of follow-up. Ophthalmology. 2022;129: 1344–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taubenslag KJ, Kammer JA. Outcomes disparities between Black and White populations in the surgical management of glaucoma. Semin Ophthalmol. 2016;31:385–393. [DOI] [PubMed] [Google Scholar]
- 18.Edmiston AM, SooHoo JR, Seibold LK, et al. Postoperative inflammation after endoscopic cyclophotocoagulation: racial distribution and effect on outcomes. J Glaucoma. 2018;27: 266–268. [DOI] [PubMed] [Google Scholar]
- 19.Reddy AK, Patnaik JL, Miller DC, et al. Risk factors associated with persistent anterior uveitis after cataract surgery. Am J Ophthalmol. 2019;206:82–86. [DOI] [PubMed] [Google Scholar]
- 20.Fellman RL, Feuer WJ, Grover DS. Episcleral venous fluid wave correlates with trabectome outcomes: intraoperative evaluation of the trabecular outflow pathway. Ophthalmology. 2015;122:2385–2391.e1. [DOI] [PubMed] [Google Scholar]
- 21.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–814.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]


