Abstract
Background
In patients with spinal cord injuries (SCIs), infections continue to be a leading cause of morbidity, mortality and hospital admission.
Objectives
This study evaluated the long-term impact of a weekly, multidisciplinary Spinal/Antimicrobial Stewardship (AMS) meeting for acute-care SCI inpatients, on antimicrobial prescribing over 3 years.
Methods
A retrospective, longitudinal, pre-post comparison of antimicrobial prescribing was conducted at our tertiary hospital in Melbourne. Antimicrobial prescribing was audited in 6 month blocks pre- (25 April 2017 to 24 October 2017), immediately post- (27 March 2018 to 25 September 2018) and 3 years post-implementation (2 March 2021 to 31 August 2021). Antimicrobial orders for patients admitted under the spinal unit at the meeting time were included.
Results
The number of SCI patients prescribed an antimicrobial at the time of the weekly meeting decreased by 40% at 3 years post-implementation [incidence rate ratio (IRR) 0.63; 95% CI 0.51–0.79; P ≤ 0.001]. The overall number of antimicrobial orders decreased by over 22% at 3 years post-implementation (IRR 0.78; 95% CI 0.61–1.00; P = 0.052). A shorter antimicrobial order duration in the 3 year post-implementation period was observed (−28%; 95% CI −39% to −15%; P ≤ 0.001). This was most noticeable in IV orders at 3 years (−36%; 95% CI −51% to −16%; P = 0.001), and was also observed for oral orders at 3 years (−25%; 95% CI −38% to −10%; P = 0.003). Antimicrobial course duration (days) decreased for multiple indications: skin and soft tissue infections (−43%; 95% CI −67% to −1%; P = 0.045), pulmonary infections (−45%; 95% CI −67% to −9%; P = 0.022) and urinary infections (−31%; 95% CI −47% to −9%; P = 0.009). Ninety-day mortality rates were not impacted.
Conclusions
This study showed that consistent, collaborative meetings between the Spinal and AMS teams can reduce antimicrobial exposure for acute-care SCI patients without adversely impacting 90 day mortality.
Introduction
In the spinal cord injury (SCI) population, infections continue to be a leading cause of hospital admission, morbidity and mortality.1–3 In particular, the high frequency of urinary tract infections, pneumonia and primary bacteraemia results in repeated antimicrobial exposure, and the emergence of multidrug resistant organisms (MDROs) and Clostridioides difficile infection (CDI).4–7 Patients with SCI have additional risk factors for colonization with MDROs, such as regular contact with the healthcare system, and the need for invasive devices such as urinary catheters.8
Despite a high burden of infection and antimicrobial consumption, published antimicrobial stewardship (AMS) studies in the SCI population are limited.8 This study aimed to examine the impact of a weekly, multidisciplinary team (MDT) Spinal/AMS meeting introduced for SCI acute-care inpatients on antimicrobial prescribing patterns. Primary outcome measure was median duration of antimicrobial orders. Secondary outcome measures were the number of antimicrobial orders, median duration (days) of therapy stratified by indication (courses), and 90-day mortality.
Materials and methods
Design
A retrospective, longitudinal, pre-post comparison of antimicrobial prescribing was conducted at Austin Health (Melbourne, Australia). Austin Health provides state-wide, tertiary referral spinal care services in a 26-bed acute SCI ward. Despite a well-embedded, hospital-wide AMS policy (Figure S1, available as Supplementary data at JAC-AMR Online), local quality assurance audits highlighted the need for improved assistance and guidance when prescribing for complex, acute SCI patients at our institution. The meeting was first introduced in March 2018. The intervention included a weekly MDT meeting attended by the Spinal consultants and junior medical staff from the Spinal team, an Infectious Diseases (ID) physician, acute SCI pharmacist and AMS pharmacist. The core interventional team of Spinal consultants, ID physician and AMS pharmacist have remained the same over the 3 year period. Preparation for the meeting approximated 1.5 h, whereas the meeting itself was approximately 30 min each week. Post-meeting documentation and implementation took up to 30 min, performed jointly by junior medical staff and the AMS pharmacist. Further details of the meeting structure are outlined in Figure S2. Antimicrobial usage was audited in 6 month blocks, pre- (25 April 2017 to 24 October 2017), immediately post- (27 March 2018 to 25 September 2018) and 3 years post-implementation (2 March 2021 to 31 August 2021).
Collation of microbiological results, antimicrobial selection, dosing, route and duration review was prepared by the AMS pharmacist prior to the meeting and shared with attending ID physicians. The meeting transitioned from in-person attendance to an online model during the COVID global pandemic (May 2020). When electronic patient medical records (EMRs) were introduced in May 2021, an antimicrobial plan, summarizing relevant clinical actions, was sent by the AMS pharmacist, post-meeting, to all attendees, and documented in the patient’s EMR. This study was approved by the Austin Human Research Ethics Committee (LNR17/Austin/312).
Inclusion and exclusion criteria
Antimicrobial orders were included in the study if they were prescribed for patients admitted under the Spinal unit bed-card, on the Acute SCI ward, at 10.00 am on a Tuesday. The entire course durations of IV, oral (PO) and topical orders were included (even if commenced in the emergency department or the ICU).
Antimicrobial orders were excluded if they were prescribed for long-term prophylaxis (i.e. methenamine hippurate, antimicrobials prescribed for chronic osteomyelitis suppression), or commenced prior to the study periods. Patients transferred to another hospital, where antimicrobial durations were unknown, were also excluded.
Occasionally, a patient with a severe infection such as infective endocarditis or Staphylococcus aureus bacteraemia would present, requiring more frequent review in the early phase of admission (Days 0–7). This was performed by ID advanced trainees in conjunction with the ID physicians attached to the spinal AMS round, and thus was included in analysis.
Prescribing data
Antimicrobial consumption was quantified as days of therapy (DOT) per 1000 occupied bed days (OBD). DOT data were obtained from an automated report generated by the electronic prescribing system.
Total antimicrobial duration information was obtained via reconciliation of electronic prescriptions and hospital dispensing records. A single antimicrobial order was prescribed via a single route for consecutive day or days, at a regular frequency. An antimicrobial course was any combination of antimicrobial orders prescribed for a single indication (see Additional information in Supplementary data).
AMS interventions were classified according to previously published ‘Five Moments of AMS’—escalation, de-escalation, discontinuation, switch to oral therapy and optimization.9
Statistical analysis
Immediately post- and 3 years post-implementation periods were compared with the pre-implementation period. Fisher’s exact and rank sum tests were used for comparison of participant characteristics. Count data (number of patients, orders, courses) were compared using negative binomial regression (total number of SCI patients considered). Antimicrobial course duration was compared using linear regression, adjusting for indication (outcome transformed, standard errors adjusted for clustering within participants). Results are expressed as incidence rate ratios (IRRs) and exponentiated coefficients, respectively, both with 95% CIs. Stata 17.0 was used for analysis.
Results
Across the study periods (pre, 68; immediately post, 66; 3 years post, 51) 185 patients were reviewed. Tables 1 and S1 summarize the complete results.
Table 1.
Baseline demographics, AMS round details and antimicrobial prescribing for studied cohorts
| Pre-implementation | Immediately post-implementation | 3 years post-implementation | P valuea | P valueb | |
|---|---|---|---|---|---|
| Stewardship round | |||||
| Number of rounds | 27 (potential) | 27 | 27 | ||
| Number of all patients on ward per round, median (IQR) | 25 (25–27) | 23 (21–26) | 26 (23–27) | ||
| Number of SCI patients on ward per round, median (IQR) | 20 (19–21) | 18 (15–20) | 19 (15–21) | 0.52 | 0.24 |
| Number of SCI patients prescribed antimicrobials on ward per round, median (IQR) | 8 (7–9) | 6 (5–8) | 4 (3–6) | 0.86 | <0.001 |
| Antimicrobial orders reviewed, n | 148 | 141 | 108 | 0.66 | 0.05 |
| Antimicrobial courses reviewed, n | 104 | 103 | 82 | 0.51 | 0.25 |
| Average AMS moments per round, n | NA | 5 | 5 | NA | NA |
| AMS acceptance rate, % | NA | 92 | 95 | NA | NA |
| Patient demographics (patients included in review) | |||||
| N | 68 | 66 | 51 | ||
| LOS, days, median (IQR) | 15 (7–49) | 25 (9–65) | 20 (9–49) | 0.14 | 0.50 |
| Male, n (%) | 50 (74) | 50 (76) | 43 (84) | 0.84 | 0.18 |
| Age, years, median (IQR) | 55 (44–67) | 54 (39–64) | 59 (38–71) | 0.36 | 0.59 |
| CCI, median (IQR) | 2 (0–4) | 2 (0–4) | 2 (1–4) | 0.74 | 0.95 |
| 90 day mortality, n (%) | 2 (3) | 3 (5) | 3 (6) | 0.67 | 0.65 |
| Antimicrobial (Abx) order duration, days, median (IQR) | |||||
| N | 148 | 141 | 108 | ||
| Total Abx duration | 7 (5–11) | 6 (4–11) | 5 (3–7) | <0.001 | <0.001 |
| IV Abx duration | 7 (4–8) | 6 (4–14) | 3 (2–7) | 0.78 | <0.001 |
| PO Abx duration | 8 (6–14) | 6 (4–10) | 6 (4–10) | <0.001 | 0.003 |
| Topical antimicrobial duration | 20 (8–31) | 14 (4–26) | 42 (13–42) | 0.35 | 0.53 |
| Antimicrobial course duration by indication, days, median (IQR) | |||||
| Overall course duration | 11 (8–19) (n = 104) | 10 (6–15) (n = 103) | 7 (5–11) (n = 82) | 0.001 | <0.001 |
| Skin/soft tissue infection | 12 (7–14) (n = 17) | 13 (10–15) (n= 16) | 5 (4–12) (n = 7) | 0.62 | 0.045 |
| Bacteraemia | 15 (14–15) (n = 5) | 14 (12–14) (n = 6) | 21 (12–30) (n = 4) | 0.16 | 0.64 |
| Pulmonary infection | 10 (7–12) (n= 23) | 6 (5–7) (n = 17) | 5 (5–8) (n = 11) | 0.001 | 0.022 |
| Osteomyelitis/septic arthritis | 42 (15–72) (n= 8) | 46 (41–50) (n = 10) | 42 (38–46) (n = 3) | 0.78 | 0.97 |
| Gastrointestinal infection | 12 (9–17) (n = 4) | 11 (10–20) (n = 6) | 10 (10–13) (n = 8) | 0.72 | 0.85 |
| Urogenital infection | 9 (6–12) (n= 30) | 7 (5–10) (n= 27) | 7 (5–8) (n = 39) | 0.073 | 0.009 |
| Prophylactic antimicrobials | 10 (9–10) (n = 2) | 3 (1–5) (n = 4) | 3 (3–4) (n = 2) | 0.042 | 0.010 |
| Superficial fungal infection | 20 (24–52) (n = 12) | 15 (12–24) (n = 14) | 30 (19–41) (n = 6) | 0.019 | 0.49 |
| Other (deep source) | 19 (13–20) (n = 3) | 40 (38–54) (n = 3) | 15 (1–23) (n = 2) | 0.033 | 0.59 |
AMS, antimicrobial stewardship; CCI, Charlson Comorbidity Index; LOS, length of stay; NA, not applicable; PO, per os (orally); SCI, spinal cord injury.
aImmediately post- versus pre-implementation.
bThree years versus pre-implementation; non-parametric tests.
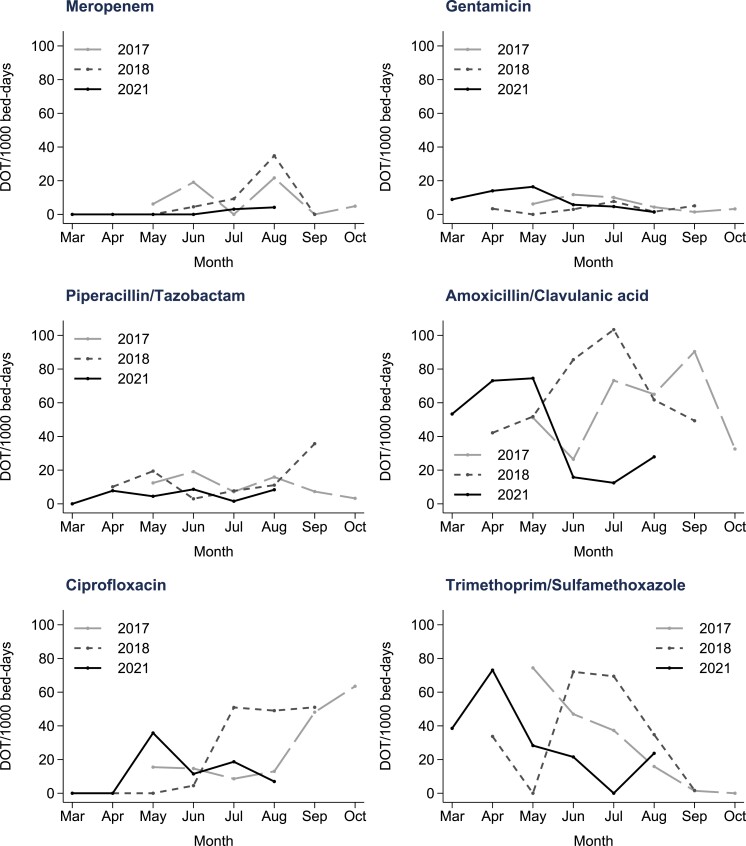
The number of SCI inpatients prescribed an antimicrobial at the time of the weekly meeting decreased by 40% over the 3 year period (IRR 0.63; 95% CI 0.51–0.79; P ≤ 0.001). The median number of SCI patients admitted to the ward did not change significantly over the same time (Table 1). The overall number of antimicrobial orders decreased by 22% over the 3 year period (IRR 0.78; 95% CI 0.61–1.00; P = 0.052), whereas the number of antimicrobial courses showed a smaller reduction of 15% (IRR 0.85; 95% CI 0.63–1.13; P = 0.25). Figure 1 shows specific antimicrobial use patterns over the 3 year period (DOT/1000 OBD). Narrower spectrum antimicrobials were used preferentially where possible (Tables S2 and S3). Figure S3 highlights the downward trend in total systemic antimicrobial DOT/1000 OBD, particularly at 3 years.
Figure 1.
Days of therapy (DOT) per 1000 patient bed days for broad spectrum antimicrobials and narrower spectrum alternatives.
After adjusting for indication, shorter antimicrobial order durations were observed at both subsequent time periods, when compared with the pre-implementation period (24% shorter immediately post; 95% CI −34% to −12%; P < 0.001; and 28% shorter 3 years post; 95% CI −39% to −15%; P ≤ 0.001). This was most noticeable in IV orders at 3 years (36% shorter; 95% CI −51% to −16%; P = 0.001). A significant reduction in PO order duration was also observed at 3 years (25% shorter; 95% CI −38% to −10%; P = 0.003). No significant changes were observed in the frequency or duration of topical antimicrobial orders or courses.
After adjusting for indication, a significant reduction in overall course duration was also observed at both time periods (28% shorter immediately post; 95% CI −40% to −13%; P = 0.001; and 31% shorter 3 years post; 95% CI −44% to −17%; P < 0.001). When stratified for indication, this reduction was observed for skin and soft tissue infections (SSTIs) (43% shorter; 95% CI −67% to −1%]; P = 0.045), pulmonary infections (45% shorter; 95% CI −67% to −9%; P = 0.022) and urinary infections (31% shorter; 95% CI −47% to −9%; P = 0.009) at 3 years. Other non-significant reductions in course durations were noted for bone and joint infections, and gastrointestinal infections (P > 0.05) (Tables 1 and S4). Ninety-day mortality rates were not impacted.
At least one AMS moment was recommended for 79% (immediately post) and 84% (3 years post) of antimicrobial orders. These were accepted on 95% of occasions in the 3 year post-implementation period (Table S5).
Discussion
We demonstrated that a sustained, weekly MDT Spinal-AMS meeting on the acute SCI ward can significantly reduce the frequency of antimicrobial prescribing and reduce antimicrobial order duration with high compliance. This is consistent with two other similar interventional pre/post studies in SCI patients that demonstrated a reduction in days of antimicrobial therapy per 100 patient days.8,10 Our results uniquely demonstrated a reduction in both IV and oral antimicrobial prescribing in the SCI population. Reduced IV prescribing has been shown in other populations to reduce nursing care time, line-associated infections and hospital length of stay.11,12 This is in addition to the established benefits of reducing overall duration on the emergence of CDI and MDRO infections.5 These outcomes require validation in the SCI population.
The sustained reduction in antimicrobial course duration, stratified for indication, is a unique feature of our study. Clarke et al.8 delineate the initial indication for antimicrobials, with genitourinary being the most common, followed by SSTIs and pulmonary infections. Rigazzi et al.13 show the high variability in antimicrobial course duration for osteomyelitis in a single specialized SCI centre where no AMS services are provided. We observed similar prevalences of pulmonary infections, SSTIs and urinary infections to Clarke et al.; however, we were also able to demonstrate a sustained reduction in median course duration for all three indication groups. Shorter durations have been shown to be as effective as longer courses for a range of clinical indications and often result in fewer adverse events and/or reduced prevalence of drug-resistant infections.14,15 For indications where a reduction was not observed, short durations were already being prescribed (e.g. bone and joint infection).
Other AMS interventions in the SCI population have demonstrated a significant impact on broad-spectrum antimicrobial prescribing.16,17 Tedeschi et al.10 reduced carbapenem use by 97% from 13 to 0.4 defined daily dose (DDD)/100 patient days over 3 years, whereas fluoroquinolones decreased by 92% from 11.8 to 0.99 DDD/100 patient days. Due to a well-embedded, institution-wide AMS policy (Figure S1), our hospital had comparatively low starting rates of broad-spectrum antimicrobial use (Figure 1).9
Our study demonstrates that when broad-spectrum antimicrobials are not frequently prescribed, opportunities remain within the SCI population to deploy narrower antimicrobials and decrease overall antimicrobial exposure with reduced days of therapy. The narrower antibiotic spectrum may be attributable, in part, to combined effects of the AMS meeting and secular prescribing habits within a hospital with a strong AMS presence. However, the reduction in antibiotic treatment duration, contrasted against a highly vulnerable population with complex infections, is thought to be a specific achievement of the AMS meeting. With enhanced control of antibiotic spectrum and duration, future studies may move towards stewardship of topical therapies, which are common in this population due to complex wound care, but were not directly targeted or impacted by our intervention.
The number of antimicrobial orders initiated by the Spinal team for SCI patients reduced significantly over the study period, and the predominant AMS intervention changed from discontinuation of antimicrobials to optimization (Table S5), suggesting a prescribing practice change within the spinal unit over 3 years. Although this requires qualitative analysis and validation, we postulate that a collaborative and co-operative learning culture, with consistent senior representation from both the Spinal and ID teams, resulted in more appropriate prescribing and enhanced confidence to use antimicrobials less frequently. The meeting also created anticipation of regular prescribing review and thereby triggered self-evaluation by the Spinal team themselves. The effects of consistent and continued AMS education on improved prescribing in SCI patients have been discussed elsewhere.5,17,18
Limitations of this study include the retrospective cohort design, single centre examination, patients/antimicrobial courses missed between rounds, and the unknown impact on antimicrobial resistance. Further studies examining the incidence of hospital readmission, ICU admission and prevalence of CDI/MDRO-related infections in the SCI population at our hospital are warranted. Future examinations of outpatient prescribing and subacute admissions are also required.
This study has shown that a sustained, consistent and collaborative meeting between the Spinal unit and AMS team can result in reduced antimicrobial initiation and duration of both IV and PO orders, and reduced overall course duration without adversely impacting 90 day mortality of reviewed patients. Weekly meetings represent a time-effective approach that can be replicated for other speciality units frequently prescribing antimicrobials and whose patients are at risk of developing increasingly difficult-to-treat infections.
Supplementary Material
Acknowledgements
We would like to acknowledge the Acute SCI ward pharmacists for their ongoing support in implementing AMS recommendations and commitment to attending the AMS meetings. We would also like to acknowledge previous AMS pharmacists for assisting with the preliminary stages of introducing the Spinal-AMS meeting.
Contributor Information
D Perera, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Pharmacy, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
S Vogrin, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Medicine, St Vincent's Health, The University of Melbourne, 29 Regent Street, Fitzroy 3065, Victoria, Australia.
S Khumra, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Pharmacy, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
S Motaganahalli, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
A Batrouney, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Pharmacy, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
K Urbancic, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Pharmacy, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
M Devchand, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Pharmacy, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
E Mitri, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Pharmacy, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Infectious Diseases, Doherty Institute, University of Melbourne, 792 Elizabeth St, Melbourne 3000, Victoria, Australia.
R Clements, Victorian Spinal Cord Service, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
A Nunn, Victorian Spinal Cord Service, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia.
G Reynolds, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, 305 Grattan Street, Melbourne 3000, Victoria, Australia; Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne 3000, Victoria, Australia.
J A Trubiano, Department of Infectious Diseases, Austin Health, 145 Studley Road, Heidelberg 3084, Victoria, Australia; Department of Infectious Diseases, Doherty Institute, University of Melbourne, 792 Elizabeth St, Melbourne 3000, Victoria, Australia.
Funding
No funding sources were available for this study.
Transparency declarations
G.R. is supported by an National Health and Medical Research Council (NHMRC) post-graduate PhD Scholarship (#2013970) - unrelated to this work. All other authors: none to declare
Supplementary data
Figures S1 to S3, Tables S1 to S5 and Additional information are available as Supplementary data at JAC-AMR Online.
References
- 1. National Spinal Cord Injury Statistical Center . Spinal cord injury facts and figures at a glance. University of Alabama at Birmingham, 2020. https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202020.pdf [Google Scholar]
- 2. DiPiro ND, Murday D, Corley EH et al. The primary and secondary causes of hospitalizations during the first five years after spinal cord injury. Spinal Cord 2022; 60: 574–9. 10.1038/s41393-022-00750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Access Economics Pty Ltd . The economic cost of spinal cord injury and traumatic brain injury in Australia. The Victoria Neurotrauma Initiative, 2009. https://scia.org.au/wp-content/uploads/2022/02/Access_Economics_Economic_Cost_of_sci_and_tbi_in_Australia_June_2009.pdf [Google Scholar]
- 4. Liu B, Reid J, Silverman M et al. High risk of Clostridium difficile infection among spinal cord injured patients after the use of antibiotics commonly used to treat urinary tract infections. Neurourol Urodyn 2020; 39: 2401–8. 10.1002/nau.24502 [DOI] [PubMed] [Google Scholar]
- 5. Skelton F, Suda K, Evans C et al. Effective antibiotic stewardship in spinal cord injury: challenges and a way forward. J Spin Cord Med 2019; 42: 251–4. 10.1080/10790268.2017.1396183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitzpatrick MA, Suda KJ, Safdar N et al. Changes in bacterial epidemiology and antibiotic resistance among veterans with spinal cord injury/disorder over the past 9 years. J Spinal Cord Med 2018; 41: 199–207. 10.1080/10790268.2017.1281373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Balbale SN, Johnson S, Burns SP et al. Community-associated Clostridium difficile infection among veterans with spinal cord injury and disorder. Infect Control Hosp Epidemiol 2014; 35: 577–80. 10.1086/675830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clarke D, Nguyen D, Overton K. Antimicrobial stewardship in spinal cord injury: a multidisciplinary approach. J Spin Cord Med 2021; 44: 770–4. 10.1080/10790268.2020.1731225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Devchand M, Stewardson AJ, Urbancic KF et al. Outcomes of an electronic medical record (EMR)-driven intensive care unit (ICU)-antimicrobial stewardship (AMS) ward round: assessing the “five moments of antimicrobial prescribing”. Infect Control Hosp Epidemiol 2019; 40: 1170–5. 10.1017/ice.2019.218 [DOI] [PubMed] [Google Scholar]
- 10. Tedeschi S, Trapani F, Giannella M et al. An antimicrobial stewardship program based on systematic infectious disease consultation in a rehabilitation facility. Infect Control Hosp Epidemiol 2017; 38: 76–82. 10.1017/ice.2016.233 [DOI] [PubMed] [Google Scholar]
- 11. Mouwen AMA, Dijkstra JA, Jong E et al. Early switching of antibiotic therapy from intravenous to oral using a combination of education, pocket-sized cards and switch advice: a practical intervention resulting in reduced length of hospital stay. Int J Antimicrob Agents 2020; 55: 105769. 10.1016/j.ijantimicag.2019.07.020 [DOI] [PubMed] [Google Scholar]
- 12. Mertz D, Koller M, Haller P et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother 2009; 64: 188–99. 10.1093/jac/dkp131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rigazzi J, Fahndrich C, Osinga R et al. Osteomyelitis and antibiotic treatment in patients with grade IV pressure injury and spinal cord lesion—a retrospective cohort study. Spinal Cord 2022; 60: 540–7. 10.1038/s41393-022-00758-1 [DOI] [PubMed] [Google Scholar]
- 14. Wald-Dickler N, Spellberg B. Short course antibiotic therapy–replacing constantine units with “shorter is better”. Clin Infect Dis 2019; 69: 1476–9. 10.1093/cid/ciy1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Royer S, DeMerle KM, Dickson RP et al. Shorter versus longer courses of antibiotics for infection in hospitalized patients: a systematic review and meta-analysis. J Hosp Med 2018; 13: 336–42. 10.12788/jhm.2905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kitigawa K, Shigemura K, Nomi M et al. Use of third generation cephalosporins and quinolones and occurrence of antibiotic-resistant strains in the neurogenic bladder (NB) outpatient setting: a retrospective chart audit. Spinal Cord 2020; 58: 705–10. 10.1038/s41393-020-0416-8 [DOI] [PubMed] [Google Scholar]
- 17. Skelton F, May SMS, Grigoryan L et al. Spinal cord injury provider knowledge and attitudes toward bacteriuria management and antibiotic stewardship. PM R 2020; 12: 1187–94. 10.1002/pmrj.12384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skelton F, Salemi JL, Akpati L et al. Genitourinary complications are a leading and expensive cause of emergency department and inpatient encounters for persons with spinal cord injury. Arch Phys Med Rehabil 2019; 100: 1614–21. 10.1016/j.apmr.2019.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.