Abstract
Nanoparticles (NPs) may behave like atoms or molecules in the self-assembly into artificial solids with stimuli-responsive properties. However, the functionality engineering of nanoparticle-assembled solids is still far behind the aesthetic approaches for molecules, with a major problem arising from the lack of atomic-precision in the NPs, which leads to incoherence in superlattices. Here we exploit coherent superlattices (or supercrystals) that are assembled from atomically precise Au103S2(SR)41 NPs (core dia. = 1.6 nm, SR = thiolate) for controlling the charge transport properties with atomic-level structural insights. The resolved interparticle ligand packing in Au103S2(SR)41-assembled solids reveals the mechanism behind the thermally-induced sharp transition in charge transport through the macroscopic crystal. Specifically, the response to temperature induces the conformational change to the R groups of surface ligands, as revealed by variable temperature X-ray crystallography with atomic resolution. Overall, this approach leads to an atomic-level correlation between the interparticle structure and a bi-stability functionality of self-assembled supercrystals, and the strategy may enable control over such materials with other novel functionalities.
Supercrystals assembled from atomically precise gold nanoclusters exhibit a thermally responsive transition in charge transport.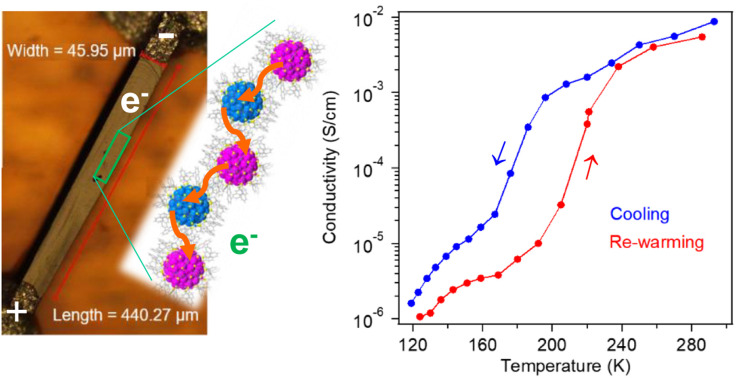
Introduction
Stimuli-responsive materials of molecules or organometallics can show dramatically improved functionalities owing to the tailoring strategy by molecular chemistry.1 In contrast, the functionality engineering of self-assembled colloidal nanoparticles (NPs)2 still lags behind the aesthetic approaches for molecules, although research on the NP systems has made tremendous progress in controlling the packing structures2–4 as well as surface functionalization for versatile nanomaterials.5–7 The major problem hindering the realization of molecular-precision assembly of NPs arises from the lack of atomic-precision of those NPs in both the core and the surface shell of ligands. While much work on charge transport in nanoparticle assemblies has been reported, the inherent size dispersity of conventional NPs (e.g. standard deviation of ∼5%) and their elusive surface structure preclude the attainment of atomically coherent superlattices (i.e. supercrystals), because the coherence from the crystalline cores of NPs to the nanometer periodicity of superlattices is destroyed by the rotation of NPs and random packing of interparticle ligands in the NP assemblies.8 Therefore, no atomic-level insight could be achieved.8,9
It has long been a major dream to obtain atomically precise NPs and assemble them into atomically coherent superlattices for achieving versatile functionalities.5,6,10 Recent advances in nanochemistry11–17 have offered access to gold NPs with atomically precise cores and definitive numbers of surface ligands (e.g., thiolate –SR or phosphine PPh3),10,16,17 which are represented by exact formulae (e.g., Aun(SR)m for thiolate-protected NPs), akin to molecules.18–21 Such ultrasmall NPs (1–3 nm core diameter) are often called nanoclusters (NCs). More importantly, crystallization of such NCs can lead to long-range coherence from the angstrom level to the macroscopic length scale (e.g., millimeter) in the assemblies,22–24 which offers new opportunities in transport measurements, including photoconductivity25 and electrical conductivity.26–29 The structural order is very important in charge transport.27 Yuan et al.28 discovered an anisotropic effect (in plane vs. out of plane) in supercrystals assembled from nanoclusters. In recent work, Zhu et al.29 demonstrated an electronic spin-polarized transport (i.e. a spin valve effect) in assembled nanoclusters by observing a magnetoresistance of 1.6% even at room temperature, which was attributed to the spin–orbit coupling (SOC) effect of NCs.
Here we report a thermally-induced sharp transition (i.e. a bi-stability effect) in electron transport through supercrystals self-assembled from atomically-precise gold NCs, including Au103S2(SR)41, Au133(SR)52, and Au144(SR)60. As for Au103S2(SR)41, the interparticle ligand packing structure is rationalized to be responsible for the thermally responsive transition of charge transport through the atomically coherent crystals. X-ray crystallography with atomic resolution (0.8–1.1 Å) reveals that the response to external temperature induces conformational changes to the naphthalene groups of surface ligands. The obtained structural insights reveal a correlation between the surface structure and functionality of self-assembled supercrystals with atomically-precise gold NPs. The strategy described here will help material scientists to improve the tailoring method for atomic-level control over nanostructures and solid materials for novel functionalities.
Results and discussion
The atomically precise Au103S2(SR)41, Au133(SR)52, and Au144(SR)60, where the R groups are 2-naphthalene, 4-tert-butyl benzene, and benzyl group, respectively, were synthesized by “size-focusing” and/or “ligand-exchange” methods,30–32 followed by supercrystal growth. The as-prepared crystals were then used for current–voltage (I–V) measurements at variable temperatures (from 300 K down to 120 K) to study thermal-responsive conductivity of the NC self-assembled crystal. The stimuli-responsive conductivity is further correlated with the structural changes by performing X-ray crystallography at different temperatures.
Charge transport through the Au103 supercrystal
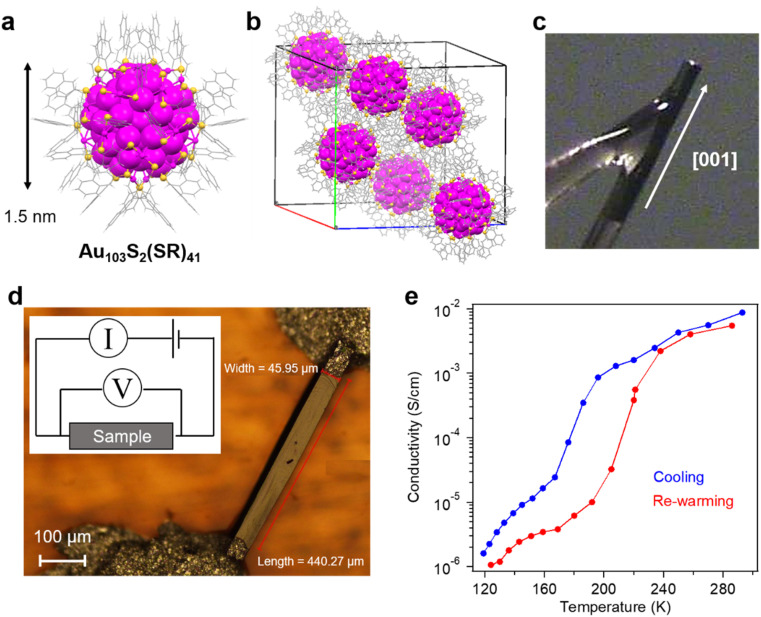
We first discuss the charge transport properties of the Au103S2(SR)41-assembled crystals (Au103 for short). The synthesis of atomically-precise Au103 was performed by a ligand-exchange reaction from Au99(SPh)42 with 2-naphthalenethiol.30 A vapor diffusion of methanol into 1,2,4-trichlorobenzene solution of Au103 yielded needle-shaped crystals. Each Au103 NC shows an atomically-precise structure made up of a Au79 core protected by oligomeric –S–(AuI–S)n– motifs which can be called “staples” (Fig. 1a), and the crystal exhibits a rod-like morphology (Fig. 1b and c). The crystal is in a monoclinic crystal system with C2/c space group. Crystallographic face indexing determined the longitudinal axis of the crystals as [001], thus, the charge transport measurements were performed along the [001] axis of single crystals (Fig. 1b and c). Both ends of the crystal were covered by silver paste in order to form good contacts (Fig. 1d). At room temperature, the conductivity (σ) of a typical Au103 crystal was ∼10−2 S cm−1. Interestingly, it drastically decreased to ∼10−6 S cm−1 at 120 K with a sharp drop (∼two orders of magnitude) over 196–167 K (Fig. 1e).
Fig. 1. Self-assembled crystal of atomically-precise Au103 NCs for charge transport measurement. (a) Crystal structure of Au103S2(SR)41. (b) Unit cell of a single crystal of Au103. (c) Face indexing of a Au103 crystal to determine the long axis. (d) Charge transport study on a Au103 assembled crystal along the [001] direction. (e) Conductivity of a Au103 assembled crystal at variable temperatures.
Upon re-warming, the conductivity gradually increases with increasing temperature to 192 K, followed by a sharp increase of conductivity over 192 to 238 K. The hysteresis loop shows a two-orders-of-magnitude difference at ∼180 K (mid-point) for cooling and at ∼210 K for re-warming (Fig. 1e). Such a hysteresis is reversible, indicating no thermally caused damage to particle arrangements in the crystal or other irreversible processes.
The conductance in the Au103-assembled crystal increased with temperature, indicating that thermal activation (kBT) is required to overcome the energy barriers between NCs.33,34 This is typical of the charge hopping mechanism,35,36 and the activation energy is expressed by the equation:
| σ(d, T) = σ0e−βde−EA/kBT | 1 |
where, d is the interparticle distance (Å), T is the temperature (K), β is the electron-tunneling coefficient, and EA is the activation energy (eV). A system with weak electronic coupling is known for this type of conducting behavior (such as the highly disordered organics or NPs' film).37–40 Therefore, our observation indicates that the charge carriers are localized in each NP, resulting in smaller interparticle electronic coupling (β ≪ kBT). The plot from eqn (1) shows that the activation barriers in the Au103 crystal varied in different temperature ranges (see Fig. S1†), with the activation energies determined to be I: 0.12 eV (293–196 K), II: 0.35 eV (196–167 K), and III: 0.093 eV (167–119 K). These values are comparable to EA values in weakly coupled organic crystals.41
Sizes and energy-gaps of nanoclusters
To investigate the potential NC size and bandgap (Eg) effects, we compared the charge transport properties of Au103 crystals with other crystals assembled from Au133(SR′)52 or Au144(SR′′)60 (Fig. S2 and S3†). All these are thiolate-protected (with >100 Au atoms) and show clearly resolved carbon tail structures by X-ray crystallography (Fig. S2 and S3†). Under the same measurement conditions, the crystals exhibited a trend of decreasing conductivity with cooling, and the room temperature conductivity is similar (∼10−2 S cm−1) among the crystals of 3 sizes of NCs, indicating negligible effects of energy-gaps of each type of NCs (note: the Eg varies from 0.5 eV of Au103 to 0.1 eV of Au144); thus, the core sizes are less critical, rather the ligands should play a more important role. Among the three systems, the naphthalenethiolate-protected Au103 crystals showed the most distinctive thermal responsivity (width of the hysteresis: ∼40 K, Fig. 1e), while the Au144(SR′′)60 crystal (where R′′ = benzyl group) showed a similar two-orders of magnitude transition but a much less prominent hysteresis due to less extensive C–H⋯π interaction of the smaller benzene ring (Fig. S3b†) than the naphthalene groups on Au103 (Fig. S4†), and the case of Au133(SR′)52 (R′ = 4-tert-butyl benzene) exhibited neither transition nor hysteresis due to the almost destroyed C–H⋯π interaction by the tert-butyl group on the benzene ring (Fig. S2b†). Overall, the observed trend implies the important role of the ligand type and the interparticle ligand packing in the crystal.
Variable temperature single crystal XRD analysis
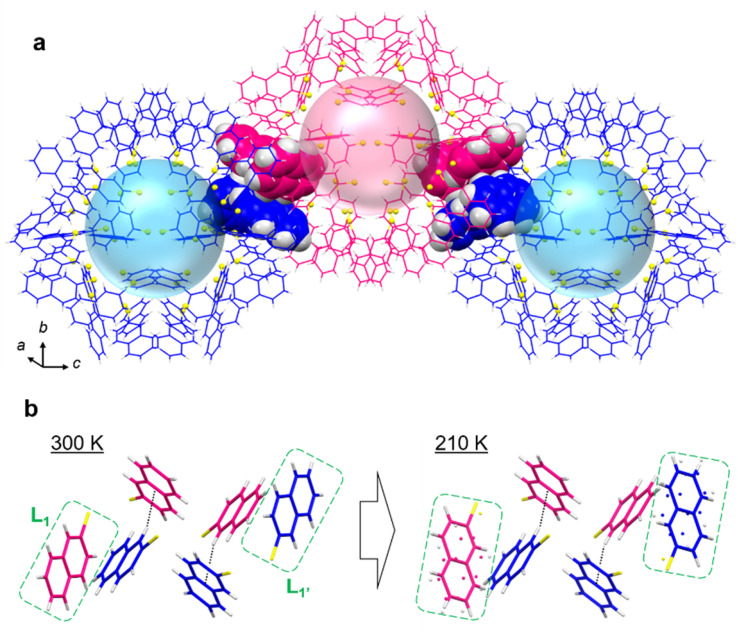
In order to obtain further structural insight into the thermally-induced sharp transition and hysteresis in the Au103 crystal, variable temperature single crystal XRD (SCXRD) was performed (Fig. 2). We find that Au103 NCs are linearly assembled along the [001] direction of the crystal, which is the same direction as the charge transport (Fig. 2a). The metal core structures do not show any change during the cooling/rewarming processes. Fig. 2b summarizes temperature-dependent unit cell lengths normalized by cell volume. Upon re-warming from 120 K, the crystal experiences an elongation along the [001] direction, which continues with the temperature rise to 240 K. Above 240 K, the unit cell starts to shrink along the [001] direction until reaching room temperature. The unit cell length along the b-axis shows opposite changes: a gradual decrease between 120 K and 240 K but an increase between 240 and 300 K. The schematic illustration of NC arrangement is shown in Fig. 2c. It is interesting to observe that the unit cell parameters for b- and c-axis show volcano-like behavior with the transition temperature at ∼240 K upon re-warming (Fig. 2b). This temperature is indeed comparable to the temperature of sharp conductivity changes during the re-warming process. Thus, the correlation between the observed temperature-induced changes for unit cell parameters and conductivity suggests that the sharp conductivity transition should be induced by the interparticle conformational change in response to external heat. Previous work on charge transport in NP assemblies42,43 could not observe any sharp transition possibly because of the polydispersity of NPs and lack of atomic-level coherence. In our system, we observed a two-orders-of-magnitude variation in conductivity over the sharp transition and distinct hysteresis behavior in the atomically coherent assembly of precise NPs. The underlying mechanism is revealed by resolving interparticle ligands' conformation.
Fig. 2. Variable temperature SCXRD analysis of a Au103-assembled crystal. (a) Packing of Au103 NCs along [001] direction (the c axis) in the crystal. Blue and magenta illustrate Au atoms in different Au103 enantiomers. (b) Unit cell parameters at 120–300 K normalized by cell volume. (c) Schematic illustration of rearrangement of Au103 NCs while re-warming.
The conformation-induced transition/hysteresis mechanism is also supported by the much less distinct behavior in the cases of Au133 or Au144 crystals that do not show a linearly-assembled wire-like structure with interlocked aromatic carbon tails of naphthalene groups; note that the potential interlocking of tert-butylbenzene groups in the Au133 sample is destroyed by tert-butyl, whereas the benzyl groups in the Au144 sample can partially interlock but much less than the naphthalene case. Therefore, the distinct transition/hysteresis in Au103 crystals should originate from the linearly assembled Au103 NCs along the [001] direction, which is the direction of the measured electrical conductance, and the conformational changes to interparticle naphthalene groups along the [001] induces the sharp transition due to the phase transition between the less conductive conformation (yet more stable) at low-T and the more conductive conformation at high-T. While molecular packing is involved in the charge transport properties of organic crystals,44–46 the systems of thiolate-protected NCs are much more complex due to the inorganic/organic hybrid and spherical topology (as opposed to planar organic molecules, which involves relatively simple π–π stacking interactions). In randomly assembled films of atomically precise NCs25,26 or coherent crystals without interlocked aromatic groups,27,28 no coherent effect of ligands was observed. In order to resolve the changes with atomic resolution, we further performed full-structural analysis by X-ray crystallography at 210 and 300 K, respectively, and the obtained insights are discussed below.
First of all, ample C–H⋯π interactions are found in the crystal of Au103 NCs. The intra-particle C–H⋯π distance is 2.58 ± 0.08 Å, which is considerably shorter than 2.73 ± 0.13 Å for typical C–H⋯π distance in organic crystals.47 In addition, the Au103 NCs are linearly assembled via C–H⋯π interaction between the surface-protecting 2-naphthalenethiolate ligands (Fig. 3a). Of note, the interparticle distance (core center-to-center) is 23.74 Å along the c-axis (Fig. S4†), while for other directions the nearest distances (27.41 Å and 27.48 Å) are significantly longer. Thus, the charge hopping should largely proceed along the c-axis or [001] direction. Charge carriers are considered to travel through the interlocked naphthalene groups as highlighted in Fig. 3a during the charge transport along the [001] axis. At 300 K, the interparticle ligands' C–H⋯π interactions show a distance of 2.920 Å, which increases to 2.941 Å at 210 K, hence, the conductivity drops at low temperatures. Further insights into the conformational changes are obtained for adjacent ligands (noted as L1 and L1′) near interlocked ligands (Fig. 3b). Interestingly, at 210 K, conformational disorders are observed for these neighboring ligands, which are not observed at 300 K. This observation suggests that the decrease in conductivity is induced by the conformational changes of interparticle ligands' interactions, rather than the interparticle distance (invariant at 210 K and 300 K). Although the observed structure difference does not seem large, more dramatic changes are expected to be observed at much lower temperatures considering the significantly lower conductivity (see above Fig. 1e).
Fig. 3. Carbon tail structures between self-assembled Au103 NCs. (a) Packing of Au103 along the [001] direction with highlights in interlocked naphthalene carbon tails between adjacent NCs. (b) Temperature-dependent conformation changes in interparticle C–H⋯π interactions for interlocked naphthalene carbon tails.
We note that the non-linear assembly in the cases of Au133 and Au144 crystals and their lack of strong inter-locking pattern of ligands lead to significantly less distinct transition and hysteresis in the crystals of Au133 and Au144 compared to the naphthalenethiolate-protected Au103 NCs.
Thermal analysis on the transition
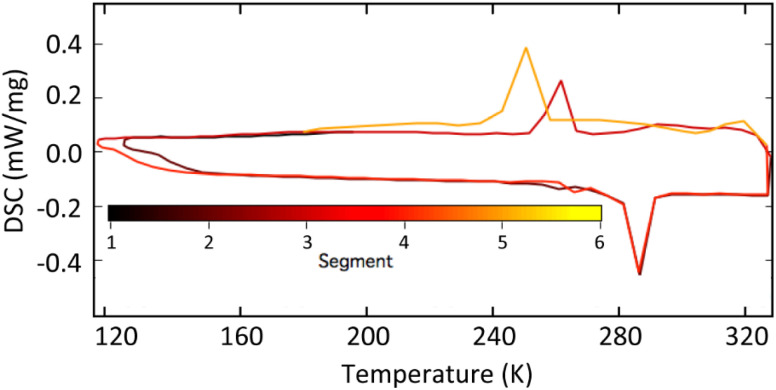
We further carried out differential scanning calorimetry (DSC) analysis between −150 and 50 °C (123−323 K, Fig. 4). Two reversible transformations were observed in the crystal assembled from Au103 NCs, and the transition observed in DSC occurs near the temperature of the transition in the conductivity measurements, indicating that the electron transport change was induced by the phase transition, consistent with the X-ray crystallographic analysis.
Fig. 4. DSC analysis of the phase transition in the supercrystal assembled from Au103 NCs.
Conclusions
In summary, this work presents the discovery of thermally induced sharp transition and hysteresis of conductivity in crystals assembled from atomically precise Au103 NCs and reveals atomic-level insight into the structural correlation with charge transport properties. The crystals exhibit charge hopping transport with decrease in conductivity upon cooling, due to the weak interparticle electronic coupling, and abrupt changes in activation energy are revealed by Arrhenius-like plots. Further crystallographic analysis rules out the interparticle distance change and unravels the conformational changes to the interparticle ligands, the latter explains the temperature dependent conductivity as well as the thermal hysteresis. This work elucidates the critical role of interparticle ligands' conformation on the charge transport of assembled NPs with atomic-level coherence. The bi-stability behavior of coherent crystal materials of nanoclusters via ligand tailoring may hold potential in future exploration for memory, sensing, and actuation applications.
Data availability
All the data are included in the ESI; Crystallographic structure files (cif deposition numbers: 2 153 700 for Au103–300 K, 2 153 701 for Au103–210 K) can be retrieved at CCDC, https://www.ccdc.cam.ac.uk.
Author contributions
T. H. and J. C. R. contributed equally to this work. R. J. and X. R. conceived the project. T. H. performed all the synthesis and single crystal growth. J. C. R. conducted the variable temperature charge transport measurements. D. W. P. collected and analyzed X-ray diffraction data at different temperatures. T. H., J. C. R., X. R. and R. J. wrote the manuscript with the contributions from all the other authors.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank Dr Chong Liu, Dr Tian-Yi Luo, and Prof. Nathaniel L. Rosi for face-indexing analysis of Au103 single crystal. R. J. acknowledges the financial support from the AFOSR (work of the synthesis of NCs). X. R. acknowledges the AFOSR funding for the electrical transport measurements under AFOSR Award no. FA9550-18-1-0020. J. C. R. is supported by the Department of Defense through the National Defense Science and Engineering Graduate (NDSEG) Fellowship.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc02753h
References
- Sato O. Dynamic Molecular Crystals with Switchable Physical Properties. Nat. Chem. 2016;8:644–656. doi: 10.1038/nchem.2547. [DOI] [PubMed] [Google Scholar]
- Boles M. A. Engel M. Talapin D. V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016;116:11220–11289. doi: 10.1021/acs.chemrev.6b00196. [DOI] [PubMed] [Google Scholar]
- Lin Q.-Y. Mason J. A. Li Z. Zhou W. O’Brien M. N. Brown K. A. Jones M. R. Button S. Lee B. Dravid V. P. Aydin K. Mirkin C. A. Building Superlattices from Individual Nanoparticles via Template-Confined DNA-Mediated Assembly. Science. 2018;359:669–672. doi: 10.1126/science.aaq0591. [DOI] [PubMed] [Google Scholar]
- Huang X. Zhu J. Ge B. Deng K. Wu X. Xiao T. Jiang T. Quan Z. Cao Y. C. Wang Z. Understanding Fe3O4 Nanocube Assembly with Reconstruction of a Consistent Superlattice Phase Diagram. J. Am. Chem. Soc. 2019;141:3198–3206. doi: 10.1021/jacs.8b13082. [DOI] [PubMed] [Google Scholar]
- Kagan C. R. Lifshitz E. Sargent E. H. Talapin D. V. Building Devices from Colloidal Quantum Dots. Science. 2016;353:aac5523. doi: 10.1126/science.aac5523. [DOI] [PubMed] [Google Scholar]
- Zhang M. Magagnosc D. J. Liberal I. Yu Y. Yun H. Yang H. Wu Y. Guo J. Chen W. Shin Y. J. Stein A. Kikkawa J. M. Engheta N. Gianola D. S. Murray C. B. Kagan C. R. High-Strength Magnetically Switchable Plasmonic Nanorods Assembled from a Binary Nanocrystal Mixture. Nat. Nanotechnol. 2017;12:228–232. doi: 10.1038/nnano.2016.235. [DOI] [PubMed] [Google Scholar]
- Klajn R. Stoddart J. F. Grzybowski B. A. Nanoparticles Functionalised with Reversible Molecular and Supramolecular Switches. Chem. Soc. Rev. 2010;39:2203–2237. doi: 10.1039/B920377J. [DOI] [PubMed] [Google Scholar]
- Nagaoka Y. Tan R. Li R. Zhu H. Eggert D. Wu Y. A. Liu Y. Wang Z. Chen O. Superstructures Generated from Truncated Tetrahedral Quantum Dots. Nature. 2018;561:378–382. doi: 10.1038/s41586-018-0512-5. [DOI] [PubMed] [Google Scholar]
- Wu L. Willis J. J. McKay I. S. Diroll B. T. Qin J. Cargnello M. Tassone C. J. High-Temperature Crystallization of Nanocrystals into Three-Dimensional Superlattices. Nature. 2018;548:197–201. doi: 10.1038/nature23308. [DOI] [PubMed] [Google Scholar]
- Zhou M. Higaki T. Hu G. Sfeir M. Y. Chen Y. Jiang D. Jin R. Three-Orders-of-Magnitude Variation of Carrier Lifetimes with Crystal Phase of Gold Nanoclusters. Science. 2019;364:279–282. doi: 10.1126/science.aaw8007. [DOI] [PubMed] [Google Scholar]
- Shi W.-Q. Guan Z.-J. Li J.-J. Han X.-S. Wang Q.-M. Site-Specific Doping of Silver Atoms into a Au25 Nanocluster as Directed by Ligand Binding Preferences. Chem. Sci. 2022;13:5148–5154. doi: 10.1039/D2SC00012A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. Cao Y. Yao Q. Xie J. Revealing the Composition-Dependent Structural Evolution Fundamentals of Bimetallic Nanoparticles through an Inter-Particle Alloying Reaction. Chem. Sci. 2022;13:4598–4607. doi: 10.1039/D1SC06296D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S. Masuda S. Takano S. Harano K. Kikkawa J. Tsukuda T. Synergistically Activated Pd Atom in Polymer-Stabilized Au23Pd1 Cluster. ACS Nano. 2022;16:16932–16940. doi: 10.1021/acsnano.2c06996. [DOI] [PubMed] [Google Scholar]
- Wei X. Xu C. Li H. Kang X. Zhu M. Fabrication of a Family of Atomically Precise Silver Nanoclusters via Dual-Level Kinetic Control. Chem. Sci. 2022;13:5531–5538. doi: 10.1039/D2SC01016J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X.-M. Huang S. Luo P. Ma K. Wang Z.-Y. Dong X.-Y. Zang S.-Q. Snapshots of Key Intermediates Unveiling the Growth from Silver Ions to Ag70 Nanoclusters. Chem. Sci. 2022;13:11110–11118. doi: 10.1039/D2SC04204E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenzler S. Schnepf A. Metalloid Gold Clusters - Past, Current and Future Aspects. Chem. Sci. 2021;12:3116–3129. doi: 10.1039/D0SC05797E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touchton A. J. Wu G. Hayton T. W. [Ni30S16(PEt3)11]: an Open-Shell Nickel Sulfide Nanocluster with a “Metal-Like” Core. Chem. Sci. 2022;13:5171–5175. doi: 10.1039/D2SC00960A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Guo J. Qiu X. Zhao S. Tang Z. Optical Activity of Chiral Metal Nanoclusters. Acc. Mater. Res. 2020;2:21–35. doi: 10.1021/accountsmr.0c00057. [DOI] [Google Scholar]
- Zeng C. Chen Y. Kirschbaum K. Lambright K. J. Jin R. Emergence of Hierarchical Structural Complexities in Nanoparticles and Their Assembly. Science. 2016;354:1580–1584. doi: 10.1126/science.aak9750. [DOI] [PubMed] [Google Scholar]
- Xia N. Xing J. Peng D. Ji S. Zha J. Yan N. Su Y. Jiang X. Zeng Z. Zhao J. Wu Z. Assembly-Induced Spin Transfer and Distance-Dependent Spin Coupling in Atomically Precise AgCu Nanoclusters. Nat. Commun. 2022;13:5934. doi: 10.1038/s41467-022-33651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. Kong J. Li Y. Kuang Z. Wang H. Zhou M. Coherent Vibrational Dynamics of Au144(SR)60 Nanoclusters. Chem. Sci. 2022;13:8124–8130. doi: 10.1039/D2SC02246J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rival J. V. Mymoona P. Lakshmi K. M. Nonappa Pradeep T. Shibu E. S. Self-Assembly of Precision Noble Metal Nanoclusters: Hierarchical Structural Complexity, Colloidal Superstructures, and Applications. Small. 2021;17:2005718. doi: 10.1002/smll.202005718. [DOI] [PubMed] [Google Scholar]
- Hossain S. Imai Y. Motohashi Y. Chen Z. Suzuki D. Suzuki T. Kataoka Y. Hirata M. Ono T. Kurashige W. Kawawaki T. Yamamoto T. Negishi Y. Understanding and Designing One-Dimensional Assemblies of Ligand-Protected Metal Nanoclusters. Mater. Horiz. 2020;7:796–803. doi: 10.1039/C9MH01691K. [DOI] [Google Scholar]
- Li Y. Zhou M. Song Y. Higaki T. Wang H. Jin R. Double-Helical Assembly of Heterodimeric Nanoclusters into Supercrystals. Nature. 2021;594:380–384. doi: 10.1038/s41586-021-03564-6. [DOI] [PubMed] [Google Scholar]
- Galchenko M. Black A. Heymann L. Klinke C. Field Effect and Photoconduction in Au25 Nanoclusters Films. Adv. Mater. 2019;31:1900684. doi: 10.1002/adma.201900684. [DOI] [PubMed] [Google Scholar]
- Reato M. Dainese T. Antonello S. Maran F. Electron Transfer in Films of Atomically Precise Gold Nanoclusters. Chem. Mater. 2021;33:4177–4187. doi: 10.1021/acs.chemmater.1c00936. [DOI] [Google Scholar]
- (a) Fetzer F. Maier A. Hodas M. Geladari O. Braun K. Meixner A. J. Schreiber F. Schnepf A. Scheele M. Structural Order Enhances Charge Carrier Transport in Self-Assembled Au-Nanoclusters. Nat. Commun. 2020;11:6188. doi: 10.1038/s41467-020-19461-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hagel J. Kelemen M. T. Fischer G. Pilawa B. Wosnitza J. Dormann E. Löhneysen H. v. Schnepf A. Schnöckel H. Neisel U. Beck J. Superconductivity of a Crystalline Ga84-Cluster Compound. J. Low Temp. Phys. 2002;129:133–142. doi: 10.1023/A:1020892022453. [DOI] [Google Scholar]; (c) Bono D. Schnepf A. Hartig J. Schnöckel H. Nieuwenhuys G. J. Amato A. de Jongh L. J. Muon Spin Relaxation Studies of Superconductivity in a Crystalline Array of Weakly Coupled Metal Nanoparticles. Phys. Rev. Lett. 2006;97:077601. doi: 10.1103/PhysRevLett.97.077601. [DOI] [PubMed] [Google Scholar]; (d) Bakharev O. N. Bono D. Brom H. B. Schnepf A. Schnöckel H. de Jongh L. J. Superconductivity in a Molecular Metal Cluster Compound. Phys. Rev. Lett. 2006;96:117002. doi: 10.1103/PhysRevLett.96.117002. [DOI] [PubMed] [Google Scholar]
- Yuan P. Zhang R. Selenius E. Ruan P. Yao Y. Zhou Y. Malola S. Häkkinen H. Teo B. K. Cao Y. Zheng N. Solvent-Mediated Assembly of Atom-Precise Gold–Silver Nanoclusters to Semiconducting One-Dimensional Materials. Nat. Commun. 2020;11:2229. doi: 10.1038/s41467-020-16062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. Guo L. Guo J. Zhao L. Li C. Qiu X. Qin Y. Gu X. Sun X. Tang Z. Room-Temperature Spin Transport in Metal Nanocluster-Based Spin Valves. Angew. Chem., Int. Ed. 2023;62:e202213208. doi: 10.1002/anie.202213208. [DOI] [PubMed] [Google Scholar]
- Higaki T. Liu C. Luo T.-Y. Rosi N. R. Jin R. Tailoring the Structure of 58-Electron Gold Nanoclusters: Au103S2(S-Nap)41 and Its Implications. J. Am. Chem. Soc. 2017;139:9994–10001. doi: 10.1021/jacs.7b04678. [DOI] [PubMed] [Google Scholar]
- Zeng C. Chen Y. Kirschbaum K. Appavoo K. Sfeir M. Y. Jin R. Structural Patterns at All Scales in a Nonmetallic Chiral Au133(SR)52 Nanoparticle. Sci. Adv. 2015;1:e1500045. doi: 10.1126/sciadv.1500045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N. Xia N. Liao L. Zhu M. Jin F. Jin R. Wu Z. Unraveling the Long-Pursued Au144 Structure by X-Ray Crystallography. Sci. Adv. 2018;4:eaat7259. doi: 10.1126/sciadv.aat7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels J. M. Nothofer H.-G. Ford W. E. von Wrochem F. Scholz F. Vossmeyer T. Schroedter A. Weller H. Yasuda A. Optical and Electrical Properties of Three-Dimensional Interlinked Gold Nanoparticle Assemblies. J. Am. Chem. Soc. 2004;126:3349–3356. doi: 10.1021/ja0377605. [DOI] [PubMed] [Google Scholar]
- Wang Y. Duan C. Peng L. Liao J. Dimensionality-Dependent Charge Transport in Close-Packed Nanoparticle Arrays: from 2D to 3D. Sci. Rep. 2014;4:7565. doi: 10.1038/srep07565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brédas J. L. Beljonne D. Coropceanu V. Cornil J. Charge-Transfer and Energy-Transfer Processes in π-Conjugated Oligomers and Polymers: a Molecular Picture. Chem. Rev. 2004;104:4971–5003. doi: 10.1021/cr040084k. [DOI] [PubMed] [Google Scholar]
- Oberhofer H. Reuter K. Blumberger J. Charge Transport in Molecular Materials: an Assessment of Computational Methods. Chem. Rev. 2017;117:10319–10357. doi: 10.1021/acs.chemrev.7b00086. [DOI] [PubMed] [Google Scholar]
- Wuelfing W. P. Murray R. W. Electron Hopping through Films of Arenethiolate Monolayer-Protected Gold Clusters. J. Phys. Chem. B. 2002;106:3139–3145. doi: 10.1021/jp013987f. [DOI] [Google Scholar]
- Zabet-Khosousi A. Dhirani A.-A. Charge Transport in Nanoparticle Assemblies. Chem. Rev. 2008;108:4072–4124. doi: 10.1021/cr0680134. [DOI] [PubMed] [Google Scholar]
- Li Q. Russell J. C. Luo T.-Y. Roy X. Rosi N. L. Zhu Y. Jin R. Modulating the Hierarchical Fibrous Assembly of Au Nanoparticles with Atomic Precision. Nat. Commun. 2018;9:3871. doi: 10.1038/s41467-018-06395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuelfing W. P. Green S. J. Pietron J. J. Cliffel D. E. Murray R. W. Electronic Conductivity of Solid-State, Mixed-Valent, Monolayer-Protected Au Clusters. J. Am. Chem. Soc. 2000;122:11465–11472. doi: 10.1021/ja002367+. [DOI] [Google Scholar]
- Ueda A. Yamada S. Isono T. Kamo H. Nakao A. Kumai R. Nakao H. Murakami Y. Yamamoto K. Nishio Y. Mori H. Hydrogen-Bond-Dynamics-Based Switching of Conductivity and Magnetism: a Phase Transition Caused by Deuterium and Electron Transfer in a Hydrogen-Bonded Purely Organic Conductor Crystal. J. Am. Chem. Soc. 2014;136:12184–12192. doi: 10.1021/ja507132m. [DOI] [PubMed] [Google Scholar]
- Zabet-Khosousi A. Dhirani A.-A. Charge Transport in Nanoparticle Assemblies. Chem. Rev. 2008;108:4072–4124. doi: 10.1021/cr0680134. [DOI] [PubMed] [Google Scholar]
- Kim J.-Y. Kotov N. A. Charge Transport Dilemma of Solution-Processed Nanomaterials. Chem. Mater. 2014;26:134–152. doi: 10.1021/cm402675k. [DOI] [Google Scholar]
- Sancho-García J. C. Pérez-Jiménez A. J. Olivier Y. Cornil J. Molecular Packing and Charge Transport Parameters in Crystalline Organic Semiconductors from First-Principles Calculations. Phys. Chem. Chem. Phys. 2010;12:9381–9388. doi: 10.1039/B925652K. [DOI] [PubMed] [Google Scholar]
- Datta A. Mohakud S. Pati S. K. Comparing the Electron and Hole Mobilities in the α and β Phases of Perylene: Role of π-stacking. J. Mater. Chem. 2007;17:1933–1938. doi: 10.1039/B700625J. [DOI] [Google Scholar]
- Ostroverkhova O. Organic Optoelectronic Materials: Mechanisms and Applications. Chem. Rev. 2016;116:13279–13412. doi: 10.1021/acs.chemrev.6b00127. [DOI] [PubMed] [Google Scholar]
- Nishio M. The CH/π Hydrogen Bond in Chemistry. Conformation, Supramolecules, Optical Resolution and Interactions Involving Carbohydrates. Phys. Chem. Chem. Phys. 2011;13:13873–13900. doi: 10.1039/C1CP20404A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are included in the ESI; Crystallographic structure files (cif deposition numbers: 2 153 700 for Au103–300 K, 2 153 701 for Au103–210 K) can be retrieved at CCDC, https://www.ccdc.cam.ac.uk.