Abstract
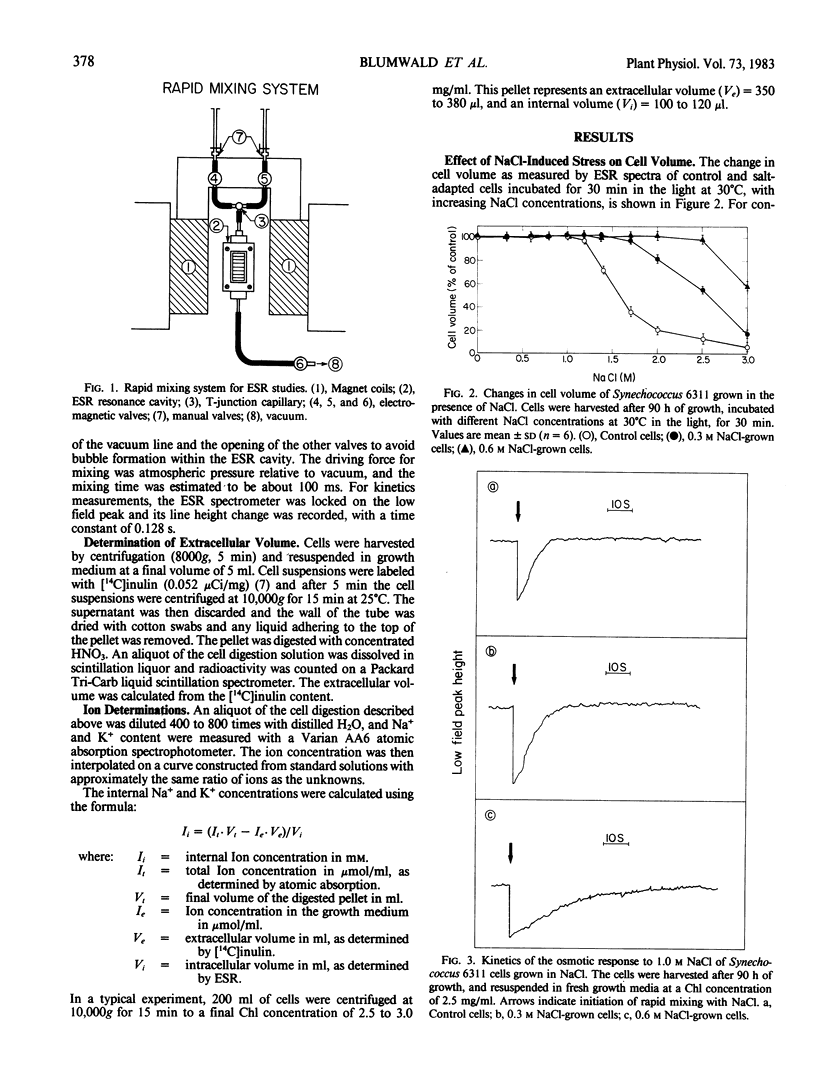
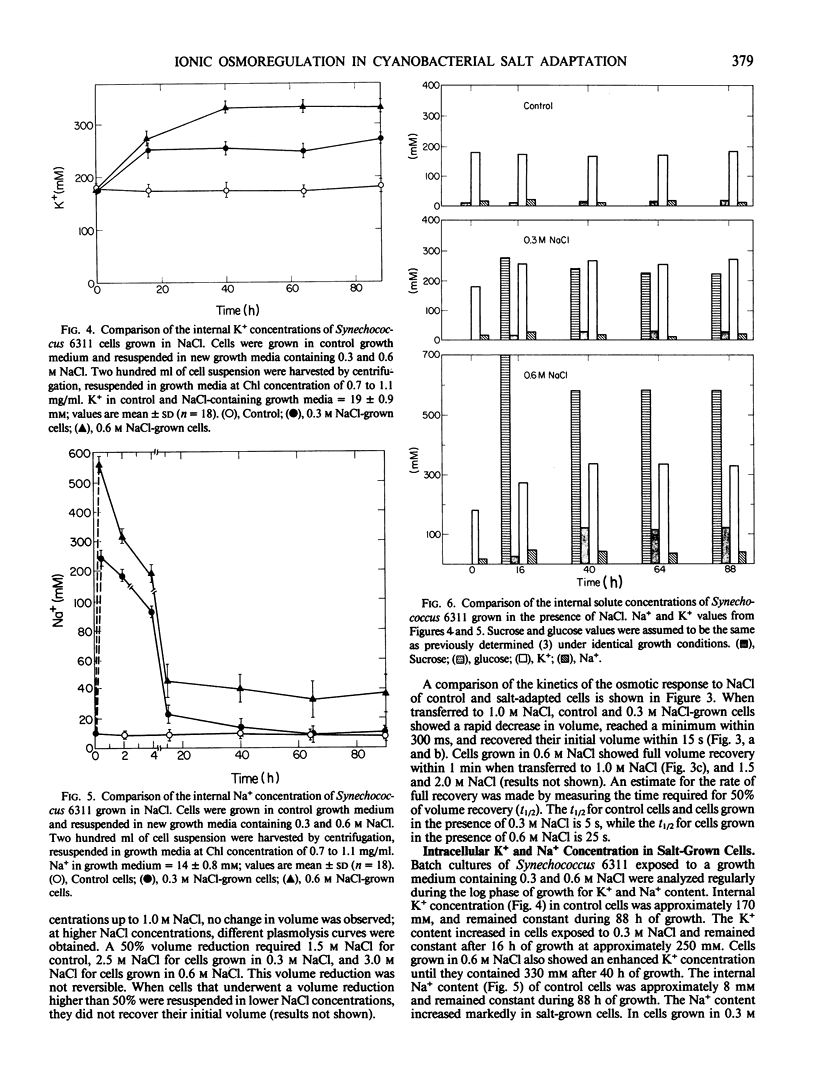
The mechanisms of salt adaptation were studied in the cyanobacterium Synechococcus 6311. Intracellular volumes and ion concentrations were measured before and after abrupt increases of external NaCl concentrations up to 0.6 molar NaCl. Equilibrium volumes, measured with a rapid and accurate electron spin resonance spin probe method, showed that at low NaCl concentrations the cells did not shrink as expected for an impermeable solute. However, when the NaCl concentration exceeded a critical value, volume losses occurred. These losses were not fully reversed by hypoosmotic treatment, suggesting membrane damage. The critical value of irreversible volume loss paralleled the increase in salinity during cell growth. Rapid mixing experiments showed that exposure of Synechococcus 6311 to non-damaging NaCl concentrations caused water extrusion from the cells; the volume decreases were time resolved to about 200 milliseconds. Subsequently, volumes increased rapidly as NaCl moved into the cells. Controls recovered their volumes within 15 seconds, while salt-adapted cells grown at 0.6 molar NaCl required 1 minute for volume equilibration. This decrease in the rate of cell volume recovery indicates that salt adaptation is accompanied by changes in cell membrane properties. Subsequent to these initial rapid volume changes, a more gradual sequence of ion movement and sugar accumulation was observed. Under conditions for photoautotrophic growth, significant Na+ extrusion was observed 30 min after salt shock. Sucrose accumulation reached a maximum value after 16 hours and K+ accumulation reached equilibrium after 40 hours. The final concentrations of K+ and Na+ and sucrose and glucose inside the 0.6 molar NaCl-grown cells indicate that the inorganic ions and organic `compatible' solutes are the major osmotic species which account for the adaptation of Synechococcus 6311 to salt.
Full text
PDF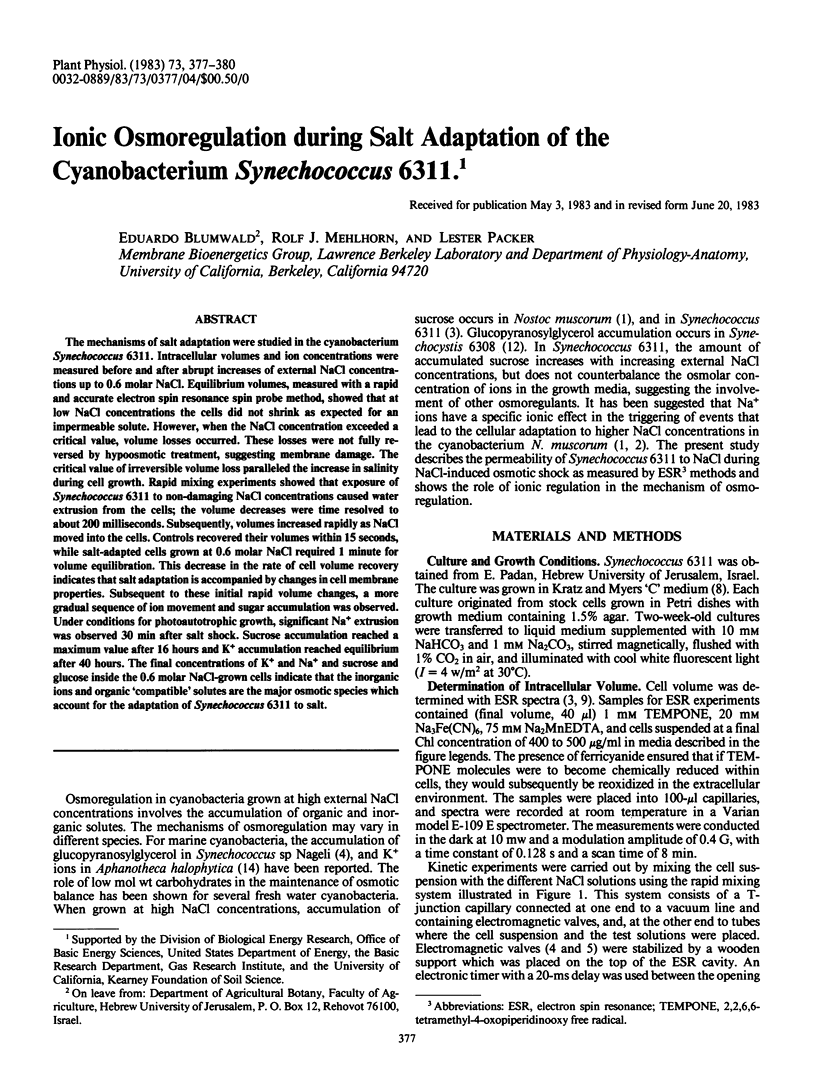

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumwald E., Mehlhorn R. J., Packer L. Studies of osmoregulation in salt adaptation of cyanobacteria with ESR spin-probe techniques. Proc Natl Acad Sci U S A. 1983 May;80(9):2599–2602. doi: 10.1073/pnas.80.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitzka L. J., Demmerle S., Mackay M. A., Norton R. S. Carbon-13 nuclear magnetic resonance study of osmoregulation in a blue-green alga. Science. 1980 Nov 7;210(4470):650–651. doi: 10.1126/science.210.4470.650. [DOI] [PubMed] [Google Scholar]
- Brown A. D., Simpson J. R. Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J Gen Microbiol. 1972 Oct;72(3):589–591. doi: 10.1099/00221287-72-3-589. [DOI] [PubMed] [Google Scholar]
- Paschinger H. DCCD induced sodium uptake by Anacystis nidulans. Arch Microbiol. 1977 Jun 20;113(3):285–291. doi: 10.1007/BF00492037. [DOI] [PubMed] [Google Scholar]
- Reed R. H., Rowell P., Stewart W. D. Characterization of the transport of potassium ions in the cyanobacterium Anabaena variabilis Kütz. Eur J Biochem. 1981 May 15;116(2):323–330. doi: 10.1111/j.1432-1033.1981.tb05337.x. [DOI] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]


