Abstract
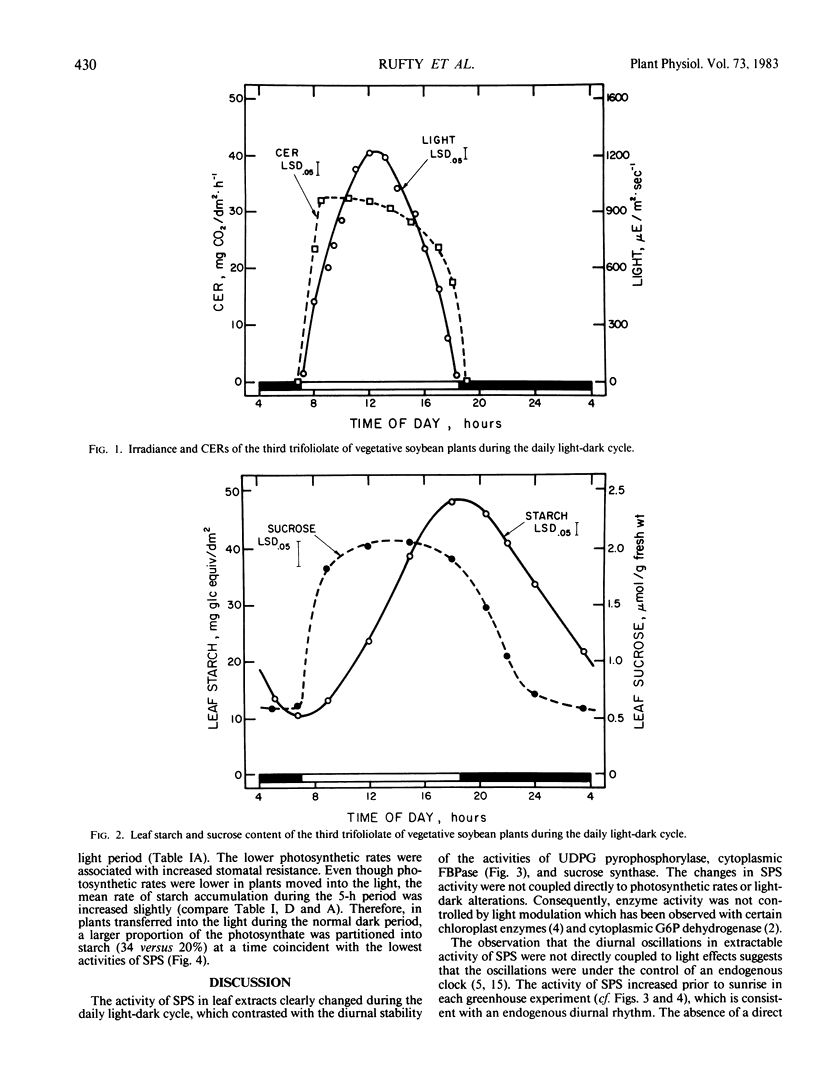
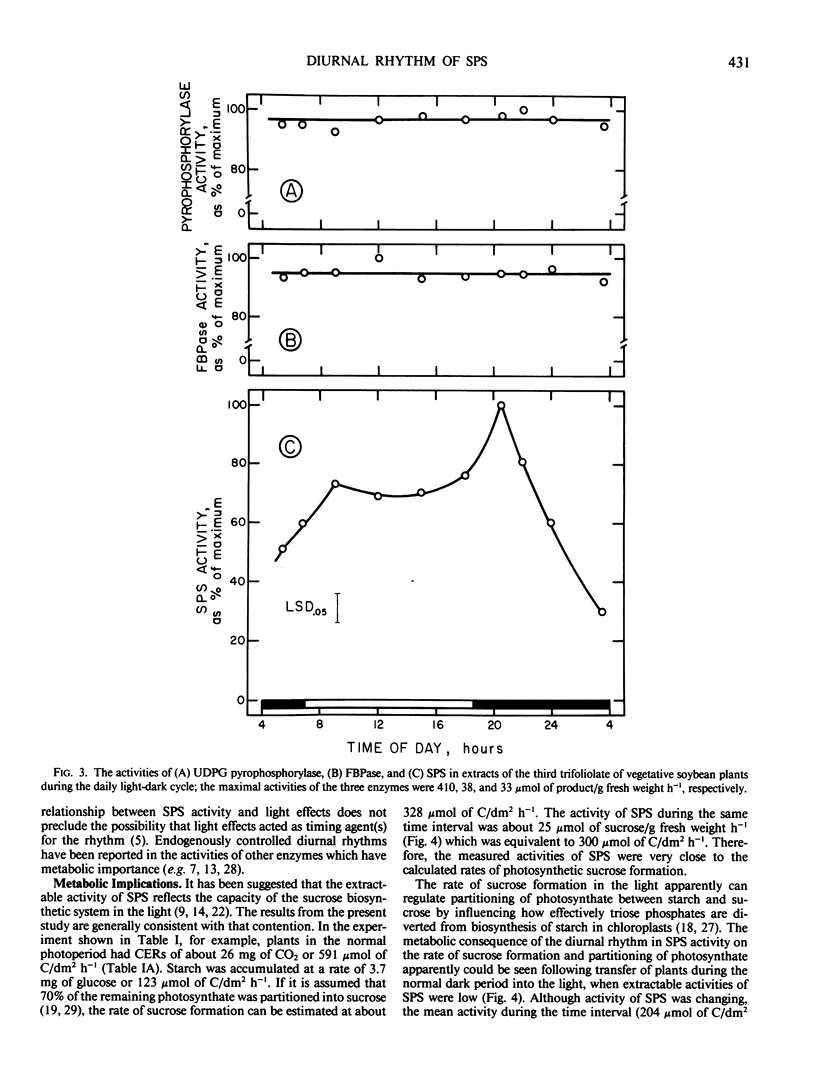
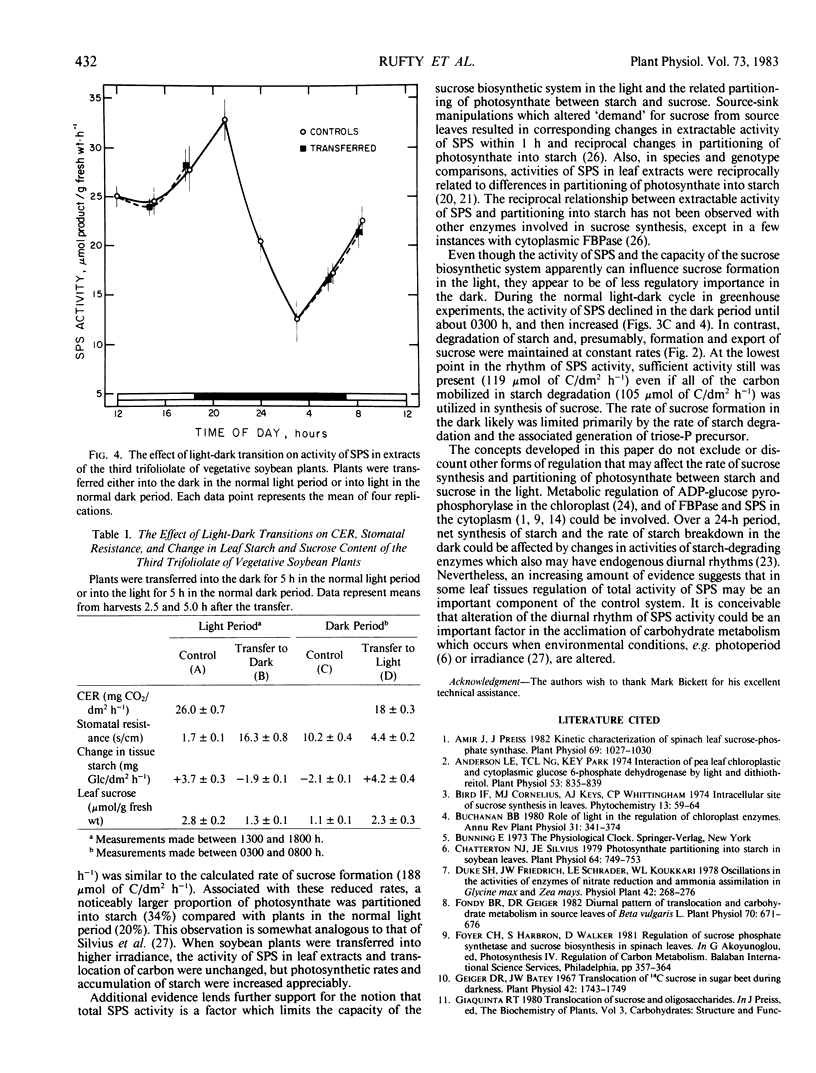
Experiments were conducted with vegetative soybean plants (Glycine max [L.] Merr., `Ransom') to determine whether the activities in leaf extracts of key enzymes in sucrose metabolism changed during the daily light/dark cycle. The activity of sucrose-phosphate synthase (SPS) exhibited a distinct diurnal rhythm, whereas the activities of UDP-glucose pyrophosphorylase, cytoplasmic fructose-1,6-bisphosphatase, and sucrose synthase did not. The changes in extractable SPS activity were not related directly to photosynthetic rates or light/dark changes. Hence, it was postulated that the oscillations were under the control of an endogenous clock. During the light period, the activity of SPS was similar to the estimated rate of sucrose formation. In the dark, however, SPS activity declined sharply and then increased even though degradation of starch was linear. The activity of SPS always exceeded the estimated maximum rate of sucrose formation in the dark. Transfer of plants into light during the normal dark period (when SPS activity was low) resulted in increased partitioning of photosynthate into starch compared to partitioning observed during the normal light period. These results were consistent with the hypothesis that SPS activity in situ was a factor regulating the rate of sucrose synthesis and partitioning of fixed carbon between starch and sucrose in the light.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amir J., Preiss J. Kinetic characterization of spinach leaf sucrose-phosphate synthase. Plant Physiol. 1982 May;69(5):1027–1030. doi: 10.1104/pp.69.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton N. J., Silvius J. E. Photosynthate Partitioning into Starch in Soybean Leaves: I. Effects of Photoperiod versus Photosynthetic Period Duration. Plant Physiol. 1979 Nov;64(5):749–753. doi: 10.1104/pp.64.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Diurnal Pattern of Translocation and Carbohydrate Metabolism in Source Leaves of Beta vulgaris L. Plant Physiol. 1982 Sep;70(3):671–676. doi: 10.1104/pp.70.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Batey J. W. Translocation of C Sucrose in Sugar Beet during Darkness. Plant Physiol. 1967 Dec;42(12):1743–1749. doi: 10.1104/pp.42.12.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbron S., Foyer C., Walker D. The purification and properties of sucrose-phosphate synthetase from spinach leaves: the involvement of this enzyme and fructose bisphosphatase in the regulation of sucrose biosynthesis. Arch Biochem Biophys. 1981 Nov;212(1):237–246. doi: 10.1016/0003-9861(81)90363-5. [DOI] [PubMed] [Google Scholar]
- Hartt C. E., Kortschak H. P. Translocation of C in the sugarcane plant during the day and night. Plant Physiol. 1967 Jan;42(1):89–94. doi: 10.1104/pp.42.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley T. L., Schrader L. E., Miller M., Setter T. L. Partitioning of C-photosynthate, and long distance translocation of amino acids in preflowering and flowering, nodulated and nonnodulated soybeans. Plant Physiol. 1979 Jul;64(1):94–98. doi: 10.1104/pp.64.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongratz P., Beck E. Diurnal oscillation of amylolytic activity in spinach chloroplasts. Plant Physiol. 1978 Nov;62(5):687–689. doi: 10.1104/pp.62.5.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. P., Walker D. A. The site of sucrose synthesis in isolated leaf protoplasts. FEBS Lett. 1979 Nov 15;107(2):295–299. doi: 10.1016/0014-5793(79)80394-4. [DOI] [PubMed] [Google Scholar]
- Rufty T. W., Huber S. C. Changes in Starch Formation and Activities of Sucrose Phosphate Synthase and Cytoplasmic Fructose-1,6-bisphosphatase in Response to Source-Sink Alterations. Plant Physiol. 1983 Jun;72(2):474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Chatterton N. J., Kremer D. F. Photosynthate partitioning in soybean leaves at two irradiance levels: comparative responses of acclimated and unacclimated leaves. Plant Physiol. 1979 Nov;64(5):872–875. doi: 10.1104/pp.64.5.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer B. T. Control of Diurnal Variations in Photosynthetic Products: II. Nitrate Reductase Activity. Plant Physiol. 1974 Nov;54(5):762–765. doi: 10.1104/pp.54.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]


