Abstract
PURPOSE
Mozambique has one of the highest burdens of cervical cancer globally. Treatment options are few as most women present with advanced disease, and there are limited trained health professionals and health care resources. The objective of this study was to describe the outcomes of women diagnosed with invasive cancer as part of the Mozambican women undergoing cervical cancer screening with human papillomavirus (HPV) testing in conjunction with family planning services (MULHER) study.
MATERIALS AND METHODS
Women age 30-49 years were prospectively enrolled in the MULHER study and offered screening with primary HPV testing followed by treatment of screen-positive women with thermal ablation or excision as appropriate. Women with cervical examination findings suspicious for cancer were referred to one of the three gynecologic oncologists in the country.
RESULTS
Between January 2020 and January 2023, 9,014 women underwent cervical cancer screening and 30 women were diagnosed with cervical cancer. In this cohort, four patients (13.3%) had early-stage disease, 18 (60.0%) had locally advanced disease, one (3.3%) had distant metastatic disease, and seven (23.3%) did not have staging information available. Five patients (16.6%) died without receiving oncologic treatment, and seven patients (23.3%) are still awaiting treatment. Of the remaining 18 patients, three (17.6%) underwent surgery and four (23.5%) received radiotherapy. Eleven (36.7%) patients received only chemotherapy.
CONCLUSION
As cervical screening programs are implemented in low-resource settings, there will likely be an increase in the number of women diagnosed with invasive cervical cancer. Our results in Mozambique demonstrate the need to increase access to advanced surgery, radiation, and palliative care services.
Barriers to cervical cancer treatment must be addressed as we scale-up cervical cancer screening programs in LMICs.
INTRODUCTION
Mozambique, a Portuguese-speaking nation in sub-Saharan Africa, has one of the highest cervical cancer burdens in the world. Among the population of 16.5 million women, cervical cancer remains the cancer with the highest incidence and the leading cause of cancer-related mortality, despite being a preventable illness.1,2 In 2020, cervical cancer incidence and mortality were the highest of any cancer site, accounting for 20.9% of new cancer cases in the country across all sexes and ages and 21.4% of cancer-related deaths.2 When restricting these data to females only, cervical cancer incidence accounted for 34.6% of new cancer cases in 2020.2 The high prevalence (11.5% in 2020) of HIV in Mozambique, a known risk factor for the development of cervical cancer,3 significantly contributes to its high burden of cervical cancer.
CONTEXT
Key Objective
As cervical cancer screening programs are increased in low- and middle-income countries (LMICs), what are some potential barriers to diagnosing and treating cervical cancer?
Knowledge Generated
Long wait times for limited resources, such as access to radiation therapy, often lead to deviations from standard-of-care therapy.
Lack of a robust medical record system contributes to treatment delays as workups may need to be started anew or patients may receive delays in follow-up.
Relevance
Our results demonstrate the need to simultaneously increase access to advanced surgery, radiation, and palliative care while increasing screening and prevention programs in LMICs.
Cervical cancer prevention efforts in Mozambique have been hindered by a lack of coordinated screening programs, few providers trained in treatment of preinvasive disease, and limited coverage of human papillomavirus (HPV) vaccination.4 It has been estimated that <5% of women have undergone screening and many screen-positive women are lost to follow-up—in turn, most women with cervical cancer in Mozambique present with advanced-stage disease and the majority die from their disease.4
Challenges with building local capacity for the treatment of invasive cancer also contribute to the high burden and mortality of cervical cancer. While the WHO minimum standard for health professionals per 100,000 people is set at 20 physicians and 100 nurses, in 2011, Mozambique had just 2.6 physicians and 20 nurses per 100,000 people.5 There are particularly shortages in specialty care, and in 2020, Mozambique had only 11 pathologists, three medical oncologists, and no surgical oncologists serving the entire population at the time of 31 million people.1,6 Radiation therapy has been available since August 2019 although there is only one linear accelerator that is located in the capital city of Maputo, and there are very long wait times.6 Fortunately, there are now three gynecologic oncologists in the country, who graduated in 2020 from the International Gynecologic Cancer Society (IGCS) global fellowship.7
Despite these improvements in capacity for cervical cancer treatment, many barriers persist and survival outcomes after a diagnosis of cervical cancer in Mozambique are poor. A 2019 study across 13 sub-Saharan African cancer registries, including Maputo, Mozambique, found an average age at diagnosis of 48.2 years and 1-year and 3-year survival rates in Maputo of 66.2% and 55.2%, respectively.8 Five-year survival data are unavailable. By contrast, the 1-year survival for cervical cancer across all stages in the United Kingdom is >80%, and the 5-year survival is >60%.9 In this report, we seek to describe the outcomes among women diagnosed with cervical cancer as part of the Mozambican women undergoing cervical cancer screening with HPV testing in conjunction with family planning services (MULHER) cervical cancer screening research study in Mozambique.
MATERIALS AND METHODS
The MULHER study was a prospective cohort study in which women age 30-49 years were prospectively enrolled and offered cervical cancer screening in conjunction with voluntary family planning services in Maputo and Gaza provinces. Institutional Review Board approval was obtained, and all patients provided informed consent. Patients underwent primary HPV testing and were given the choice of provider collection versus patient self-collection. The overall results of the MULHER screening study will be reported separately. Women with a positive HPV test result underwent visual assessment for treatment (VAT) with visual inspection with acetic acid—a process by which dilute acetic acid is applied to the cervix—by study nurses, followed by immediate treatment with thermal ablation if eligible per the WHO guidelines.10,11 Women with lesions not eligible for ablation (ie, lesion size too large, lesion extending into the cervical canal, squamocolumnar junction not visualized, glandular disease, or suspicion for cancer) were referred to a gynecologist for excision with a loop electrosurgical excision procedure (LEEP) or cold knife cone biopsy. Study personnel worked to ensure that the LEEP specimens were processed and interpreted by the pathology department and that the patients and providers received timely follow-up of their results.
Women with a cervical lesion suspicious for cancer were referred to a study gynecologist for a gynecologic examination with a cervical biopsy and preliminary staging. If there was pathologic confirmation of invasive carcinoma, the patient was then referred to the abovementioned gynecologic oncologists for evaluation and treatment planning. The gynecologic oncologists performed any indicated surgeries and referred patients to the radiation and medical oncologists for radiation therapy and/or chemotherapy as appropriate. Data were collected for all women who were diagnosed with invasive cancer to determine patterns in their treatment modalities and outcomes. Whenever possible, these data were collected from available hospital records. When medical record data were not available, this information was collected by speaking to the patients, family members, or other medical providers. Data collected included age, HIV status, patient region, treatment type, date of diagnosis, pathology results, time from biopsy to release of pathology report, and time between biopsy and first treatment although approximation through use of patient or provider recollection for these dates was often necessary because of lack of hospital records. These data were then stored in Research Electronic Data Capture (REDCap),12,13 a secure web-based application. Descriptive statistics were used to summarize the demographic and clinical characteristics of patients. Age was summarized with a median value, whereas categorical variables were summarized with counts and frequencies. The chi-square test was used to compare groups with respect to treatment type and patient province using Stata v.17.0 (StataCorp, LLC, College Station, TX), and an alpha of .05 was considered significant. No patients were missing data on the type of treatment received or patient province. Patients with an unknown time to first treatment (n = 18) were not included in the analysis.
RESULTS
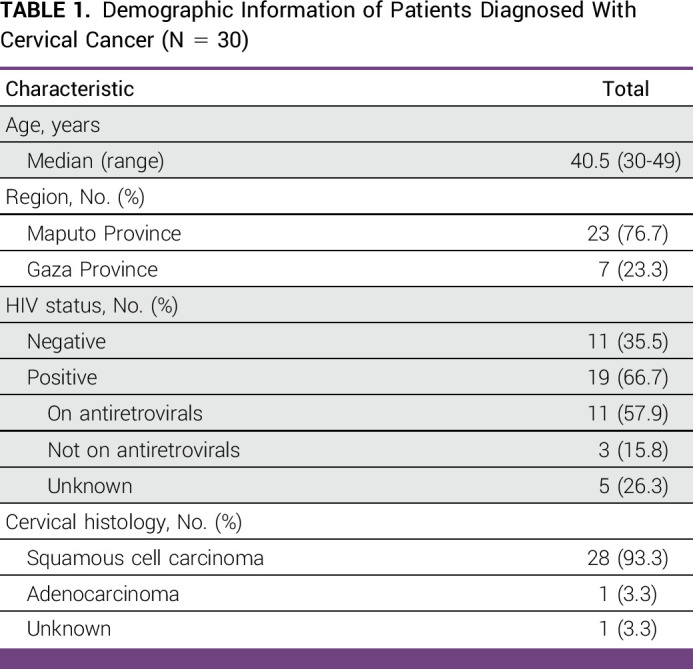
Between January 2020 and January 2023, 9,014 women underwent cervical cancer screening on the MULHER study. A total of 2,805 (31.1%) women tested positive for HPV and were scheduled for VAT by a study nurse. Of these, 30 women were diagnosed with invasive cervical cancer. As shown in Table 1, the median age at diagnosis was 40.5 years (range, 30-49 years) and 19 (63.3%) were living with HIV. Four patients (13.3%) had early-stage disease, 18 (60.0%) had locally advanced disease, one (3.3%) had distant metastatic disease, and seven (23.3%) did not have staging information available. In terms of histology, 28 patients had squamous cell carcinoma (93.3%), one patient had adenocarcinoma (3.3%), and histology was not available for one patient (3.3%). Of note, medical records were only available for 14 patients (46.7%) and the cancer diagnosis and treatment information were obtained by speaking with the patients, family members, and health care providers as described above.
TABLE 1.
Demographic Information of Patients Diagnosed With Cervical Cancer (N = 30)
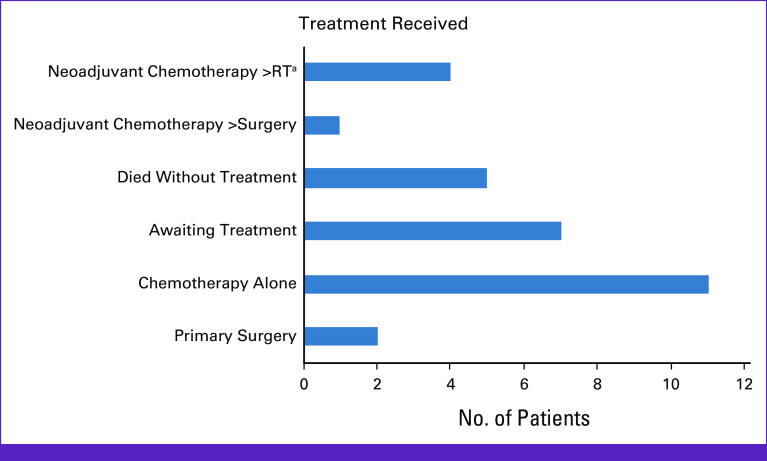
The treatments provided are shown in Figure 1. Three patients (10.0%) underwent surgery (two radical hysterectomy, one LEEP); both patients who underwent radical hysterectomy met the criteria to receive adjuvant radiotherapy (RT) and/or chemotherapy. One was found to have a 5.5-cm tumor with a 6-mm depth of invasion and lymphovascular space invasion (LVSI) present. The other had a 4-cm tumor with a 1.4-mm depth of invasion and LVSI. None of the patients received primary chemoradiation because of limited capacity and long wait times for RT. Seven patients (23.3%) underwent neoadjuvant chemotherapy, which was followed by surgery in one patient and RT in four patients; two patients are still awaiting RT. Eleven patients received chemotherapy only. Seven patients are still awaiting workup/treatment at the time of manuscript writing. Ten patients (32.3%) have died, five of whom (50.0%) died without having received any oncologic treatment.
FIG 1.
Oncologic treatment for 30 patients diagnosed with invasive cervical cancer. aSix patients had a treatment plan of neoadjuvant chemotherapy followed by RT; however, two are still awaiting RT. RT, radiotherapy.
Treatment Time
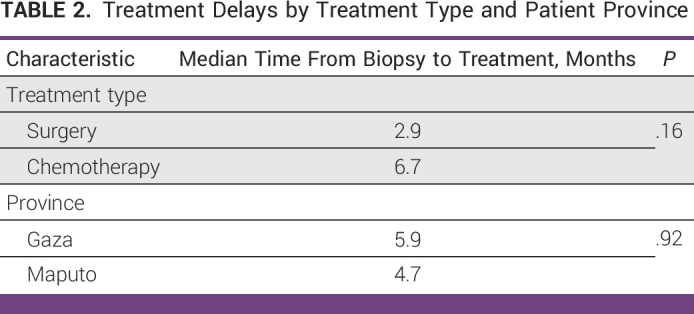
The median time from biopsy to pathology report was 1.1 months (range, 0.5-8.5 months). In Mozambique, patients are asked to carry their own tissue specimen to pathology for processing and are told a window of time to return for the pathology report. Reports are not directly sent from the pathology department to the gynecologist who performed the biopsy. In cases in which the delay was over 2 months, we noted that there was often an error in the spelling of the patient’s name or a mismatch in hospital record numbers that led to misfiling and an inability to locate the appropriate pathology report. The median time from biopsy to first treatment was 5.5 months (range, 2.2-16.6 months). As shown in Table 2, the median time from biopsy to treatment was 2.9 months for surgery and 6.7 months (range, 2.2-16.6 months) for chemotherapy (P = .16). The median time from biopsy to treatment was 6.3 months (range, 2.2-16.6 months) for patients in Maputo compared with 5.9 months (range, 4.6-7.1) for patients in Gaza (P = .92).
TABLE 2.
Treatment Delays by Treatment Type and Patient Province
Time to Radiation Therapy
In our cohort, no patients were treated with primary radiation therapy, the current standard of care for locally advanced cervical cancer. To better understand this finding, we were able to examine the treatment records of four randomly selected patients with cervical cancer not enrolled in our study to better understand where delays in treatment may be occurring. The median age of this subset was 48.5 years (range, 42.0-68.0 years), and all patients had stage IIIB disease. The average time from the date of biopsy to RT simulation in this cohort was 385.8 days (range, 158-720 days). Once simulated, the average time to begin radiation treatment was an additional 19.9 days (range, 7-43 days).
Practice Patterns
Figure 2 shows the patient treatment flow from biopsy to initiation of treatment. Per the local standard of care, a patient with a new diagnosis of cancer is seen in Gynecologic Oncology and has bloodwork and an ultrasound performed, followed by a presentation at a multidisciplinary tumor board to determine a treatment plan. If the patient is a surgical candidate, they are added to the surgery list and operated on by one of the three gynecologic oncologists. If the patient is not an operative candidate, they are referred to medical oncology and receive a minimum of six cycles of chemotherapy. If RT is planned, a patient will undergo at least six cycles before the radiation oncology referral and may undergo subsequent cycles of a second chemotherapy agent until they reach the top of the queue. While they await a radiation oncology appointment, some patients are re-evaluated by gynecologic oncology to determine if they are now a surgical candidate after initial treatment with chemotherapy. One patient in our cohort underwent neoadjuvant chemotherapy followed by surgery, with another planned and awaiting treatment.
FIG 2.
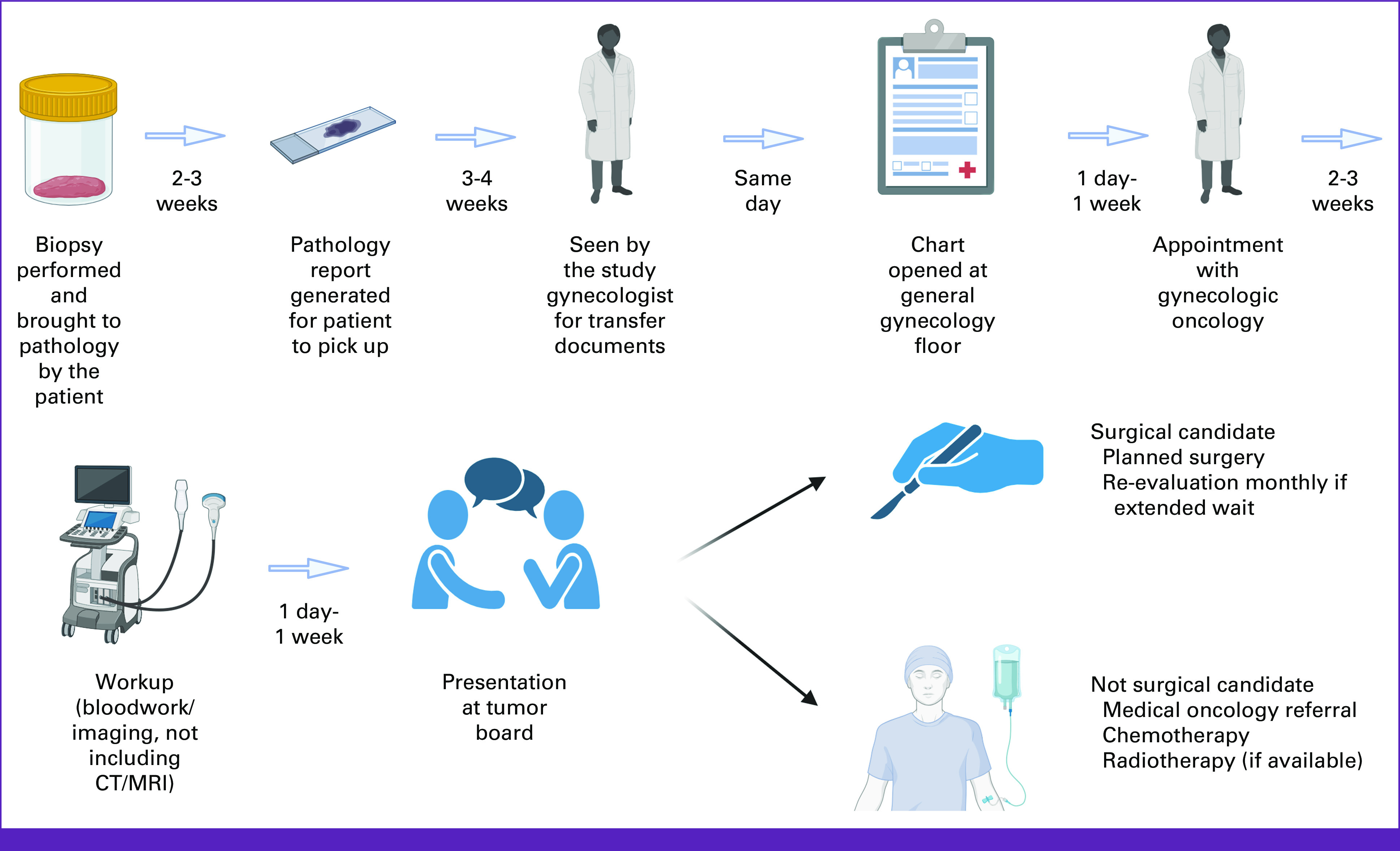
Patient treatment flow from time of biopsy to time of first treatment. CT, computed tomography; MRI, magnetic resonance imaging.
DISCUSSION
Our cohort of 30 women diagnosed with invasive cervical cancer as part of the larger MULHER cervical cancer screening study in Mozambique had significant delays from the time of biopsy to initiation of treatment (median, 10.4 months). In addition, several barriers to initiating recommended cancer treatment were identified. We found that the median time was over 1 year from diagnosis to initiation of RT, which is likely due to limited capacity and extremely long wait times for RT, limiting expedient access to standard-of-care treatment. As a result, patients are often experiencing long delays in care or care that is not standardized. Because of long wait times to initiate RT simulation and treatment, it is common practice in Mozambique to first begin treatment with chemotherapy, as shown in our study. Patients typically receive a minimum of six cycles and often 9-12 cycles of chemotherapy (most often carboplatin and paclitaxel) before having an evaluation with the radiation oncology providers. Patients may receive a second-line chemotherapy before their RT appointment if the first line is determined to be ineffective.
The government of Mozambique provides health care services to the majority of population through the Ministry of Health National Healthcare System, and this care is offered for free. A small proportion of population are able to afford private insurance and access to private clinics for treatment.14 As previously mentioned, while Mozambique has made great strides in obtaining a linear accelerator and initiating a radiation oncology program, there is very limited access to the country's one machine, which serves its entire population of 32 million, including many patients with breast and prostate cancers in addition to cervical cancer. Because of these long wait times, patients often receive chemotherapy and/or subsequent surgery when chemoradiation would offer them a better chance of cure.15 There is currently no brachytherapy available in the country. While the typically published recommendation is one linear accelerator per 450 patients per year or one linear accelerator per 500,000 population in low-resource countries,16 Mozambicans must determine how best to serve their current population of 32 million with the one available machine. As such, difficult discussions regarding the best way to triage patients as they present to radiation oncology are ongoing to maximize the use of this limited, life-saving resource.
In 2002, in recognition of the increasing cancer burden faced by low- and middle-income countries (LMICs), the WHO called for tailoring cancer therapies toward a country's available resources and this led to the creation of resource-stratified guidelines for screening, prevention, and treatment of cancer.17 Building on this initial effort, ASCO launched ASCO International in 2013 and has since released resource-stratified guidelines covering the full spectrum of cervical cancer screening, prevention, and treatment.18,19 These guidelines provide recommendations across all stages of cervical cancer and all resource settings (basic, limited, enhanced, and maximal).19 For instance, the guidelines suggest that providers can consider neoadjuvant chemotherapy followed by extrafascial hysterectomy for patients with stage IB2, IIA2, or IIIA disease.19 Ongoing training with and utilization of resource-tailored recommendations with in-country providers present a method standardizing treatment for these patients and ensuring that the limited resources that are available are being maximized for each individual patient.
To aid in capacity building efforts for cancer treatment, the IGCS has launched the Gynecologic Oncology Global Fellowship,20 a 2-year training program designed for LMICs that do not currently have formal training programs in gynecologic oncology. Institutions in select LMICs are partnered with international mentors from high-resource areas who provide hands-on surgical training. This is complemented with a web-based curriculum and monthly telementoring tumor board conferences via Project Extension for Community Healthcare Outcomes.21,22 By increasing the number of physicians trained in gynecologic oncology, the IGCS seeks to increase access to life-saving subspecialty care for patients living in LMICs.
As global health efforts increase the number and reach of cervical cancer screening programs in LMICs, there will be a commensurate increase in the number of women diagnosed with invasive cancer as part of the screening program. In Mozambique, expanded screening and advanced detection of women with cervical cancer could put an additional burden on a fragile health care system and greater demands on the one linear accelerator in the country. Our review of this patient cohort demonstrates that significant barriers exist in achieving optimal care for these patients, even in a group of women who were being closely followed as part of a large, well-resourced research study. Therefore, screening programs must take these barriers into account and attempt to address cancer treatment issues concurrently with prevention efforts. This may require optimizing alternative treatments to standard-of-care chemoradiation such as neoadjuvant chemotherapy and surgery as recommended in the aforementioned ASCO guidelines.
The strength of our study is that we were able to prospectively follow a cohort of women diagnosed as part of a larger research study with more available resources for follow-up. Research staff were able to assist patients navigating the complex health care system, and data could be collected to identify points of delay. Our study has several limitations, which included the small size of our cervical cancer cohort. Issues with accurate chart/reporting severely limit providers' ability to follow a patient across the spectrum of their oncologic journey and often contribute to treatment delays as the workup must be started anew or details of previous treatment are obtained from patient's verbal histories. This was true of our cohort as well although patient data up to the point of cancer diagnosis were obtained by study nurses and entered into REDCap. We, therefore, stipulate that patient records are even less accurate/available to guide care for the most women seen at Maputo Central Hospital and Xai-Xai Provincial Hospital who did participate in such a study. At present, the Mozambican public health system does not use an electronic medical record and does not have regular access to stable internet services. While the percentage of Mozambicans with internet access has doubled from 15% to 32% from 2015 to 2021, over two thirds of the population still do not have access to the internet.23 As such, the health system relies on a paper charting system. Patients are assigned a medical record number after paying a registration fee of 15 Mozambican metical (MZN), the equivalent of $0.23 in US dollars. The patient is then responsible for bringing this registration card with them to future appointments, and, if forgotten, a new number will be assigned. As a result, patients often have multiple medical charts across the hospital with different record identification numbers. Furthermore, there is a lack of a centralized charting facility/system and, as such, records are frequently lost or misplaced. As noted above, the majority of patients in our study did not have medical records available for review.
On the basis of our findings, further study is needed to better understand the barriers and facilitators to timely diagnosis and treatment of cervical cancer in LMICs. Previous research has shown that late stage at diagnosis is a factor associated with loss to follow-up among patients with cancer24; thus, by improving screening and early detection of invasive cancer, our team hopes to be able to improve follow-up and therefore survival. To that end, our team will be launching a trial in Mozambique to better understand structural and psychosocial barriers contributing to delays in diagnosis and treatment initiation for women living with HIV (WLWH), a group that is at particularly high risk for the development of cervical cancer. In this study, we plan to use a mixed methods approach to measure and identify barriers and facilitators to timely diagnosis and initiation of treatment for women referred for diagnostic cervical biopsies or LEEP. We will also evaluate the performance of a novel point-of-care digital histopathology platform in WLWH undergoing biopsies/LEEP. As shown in our study and others,25 lengthy pathology turnaround times because of a lack of pathology services and reliable charting system often contribute to delays in care. The shortage in trained pathologists and pathology services is a major barrier to cancer care in LMICs. It is our hope that this novel technology may be able to lessen the reliance on traditional pathology routes such that more patients are able to be screened or diagnosed with earlier-stage disease and to be treated accordingly.
Surbhi Grover
Honoraria: Varian Medical Systems
Consulting or Advisory Role: GenesisCare
Research Funding: Varian Medical Systems
Kathleen Schmeler
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at International Gynecologic Cancer Society 2022 Annual Meeting, September 29-October 1, 2022, New York, NY.
SUPPORT
Supported in part by the National Institutes of Health (NIH) through MD Anderson's Cancer Center Support Grant P30CA016672, USAID-PEER (Partnerships for Enhanced Engagement in Research)/NASEM, award number AID-OAA-A-11-00012, the Giles-O'Malley Foundation, the Joe Family, and the NIH/National Cancer Institute under award number T32 CA101642 Training of Academic Gynecologic Oncologists.
AUTHOR CONTRIBUTIONS
Conception and design: Samantha Batman, Siro Daud, Celso Bila, Arlete Mariano, Marcelo Vieira, Georgia Fontes-Cintra, Jean Claude Batware, Surbhi Grover, Ellen Baker, Cesaltina Lorenzoni, Kathleen Schmeler, Mila P. Salcedo
Administrative support: Celso Bila, Celda De Jesus, Elvira Luis, Cesaltina Lorenzoni, Mila P. Salcedo
Provision of study materials or patients: Ricardina Rangeiro, Siro Daud, Edgar Tsambe, Celso Bila, Nafissa Osman, Hira Atif, Celda De Jesus, Marcelo Vieira, Andre Lopes, Jean Claude Batware, Cesaltina Lorenzoni
Collection and assembly of data: Samantha Batman, Ricardina Rangeiro, Eliane Monteiro, Dercia Changule, Siro Daud, Edgar Tsambe, Celso Bila, Nafissa Osman, Carla Carrilho, Andrea Neves, Hira Atif, Celda De Jesus, Arlete Mariano, Renato Moretti-Marques, Marcelo Vieira, Andre Lopes, Jean Claude Batware, Elvira Luis, Ellen Baker, Cesaltina Lorenzoni, Kathleen Schmeler, Mila P. Salcedo
Data analysis and interpretation: Samantha Batman, Siro Daud, Celso Bila, Arlete Mariano, Marcelo Vieira, Jean Claude Batware, Surbhi Grover, Ellen Baker, Bryan Fellman, Jane Montealegre, Jose Jeronimo, Elizabeth Chiao, Cesaltina Lorenzoni, Kathleen Schmeler, Mila P. Salcedo
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Surbhi Grover
Honoraria: Varian Medical Systems
Consulting or Advisory Role: GenesisCare
Research Funding: Varian Medical Systems
Kathleen Schmeler
Patents, Royalties, Other Intellectual Property: UpToDate
No other potential conflicts of interest were reported.
REFERENCES
- 1.https://data.worldbank.org/indicator/SP.POP.TOTL.FE.ZS?locations=MZ Mozambique. The World Bank.
- 2.https://gco.iarc.fr/today/data/factsheets/populations/508-mozambique-fact-sheets.pdf Global cancer Mozambique. World Health Organization.
- 3.https://www.cdc.gov/globalhivtb/where-we-work/Mozambique.pdf Mozambique: CDC Division of Global HIV and TB Country Profile. Centers for Disease Control & Prevention.
- 4. Osman N. S10.2 cervical cancer screening: Benefits and challenges of HPV self-sampling in Mozambique. Sex Transm Infect. 2021;97:A14 LP-A14. suppl 1. [Google Scholar]
- 5. Patel JD, Galsky MD, Chagpar AB, et al. Role of American Society of Clinical Oncology in low- and middle-income countries. J Clin Oncol. 2011;29:3097–3102. doi: 10.1200/JCO.2011.35.6378. [DOI] [PubMed] [Google Scholar]
- 6. Salcedo MP, Oliveira C, Andrade V, et al. The Capulana study: A prospective evaluation of cervical cancer screening using human papillomavirus testing in Mozambique. Int J Gynecol Cancer. 2020;30:1292–1297. doi: 10.1136/ijgc-2020-001643. [DOI] [PubMed] [Google Scholar]
- 7.https://igcs.org/mentorship-and-training-mozambique/ Training site in Mozambique. International Gynecologic Cancer Society.
- 8. Sengayi-Muchengeti M, Joko-Fru WY, Miranda-Filho A, et al. Cervical cancer survival in sub-Saharan Africa by age, stage at diagnosis and Human Development Index: A population-based registry study. Int J Cancer. 2020;147:3037–3048. doi: 10.1002/ijc.33120. [DOI] [PubMed] [Google Scholar]
- 9.https://www.cancerresearchuk.org/about-cancer/cervical-cancer/survival#:∼:text=Survival for all stages of cervical cancer&text=more than 80 out of,years or more after diagnosis Survival. Cancer Research UK.
- 10.World Health Organization 2019. https://www.who.int/publications/i/item/9789241550598 WHO guidelines for the use of thermal ablation for cervical pre-cancer lesions.
- 11.World Health Organization . Interpretation of “WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention”. ed 2. 2021. https://www.who.int/publications/i/item/9789240030824 [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.https://www.trade.gov/country-commercial-guides/mozambique-healthcare Mozambique—Healthcare. International Trade Administration.
- 15. Tewari KS, Monk BJ. New strategies in advanced cervical cancer: From angiogenesis blockade to immunotherapy. Clin Cancer Res. 2014;20:5349–5358. doi: 10.1158/1078-0432.CCR-14-1099. [DOI] [PubMed] [Google Scholar]
- 16.https://www.cocir.org/fileadmin/Publications_2019/19107_COC_Radiotherapy_Age_Profile_web4.pdf Radiotherapy age profile & density. European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry.
- 17. Hunter N, Dempsey N, Tbaishat F, et al. Resource-stratified guideline-based cancer care should be a priority: Historical context and examples of success. Am Soc Clin Oncol Educ Book. 2020;40:217–226. doi: 10.1200/EDBK_279693. [DOI] [PubMed] [Google Scholar]
- 18. Shastri SS, Temin S, Almonte M, et al. Secondary prevention of cervical cancer: ASCO resource–stratified guideline update. JCO Glob Oncol. 2022;8:e2200217. doi: 10.1200/GO.22.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chuang LT, Temin S, Camacho R, et al. Management and care of women with invasive cervical cancer: American Society of Clinical Oncology resource-stratified clinical practice guideline. JCO Glob Oncol. 2016;2:311–340. doi: 10.1200/JGO.2016.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.https://igcs.org/mentorship-and-training/global-curriculum/ Global curriculum & mentorship program. International Gynecologic Cancer Society.
- 21. Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–2207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Varon ML, Baker E, Byers E, et al. Project ECHO cancer initiative: A tool to improve care and increase capacity along the continuum of cancer care. J Cancer Educ. 2021;36:25–38. doi: 10.1007/s13187-021-02031-0. [DOI] [PubMed] [Google Scholar]
- 23.https://blogs.worldbank.org/digital-development/moving-mozambique-toward-digital-future Moving Mozambique toward a digital future. World Bank Blogs.
- 24. Habinshuti P, Hagenimana M, Nguyen C, et al. Factors associated with loss to follow-up among cervical cancer patients in Rwanda. Ann Glob Heal. 2020;86:117. doi: 10.5334/aogh.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mumba JM, Kasonka L, Owiti OB, et al. Cervical cancer diagnosis and treatment delays in the developing world: Evidence from a hospital-based study in Zambia. Gynecol Oncol Rep. 2021;37:100784. doi: 10.1016/j.gore.2021.100784. [DOI] [PMC free article] [PubMed] [Google Scholar]