Abstract
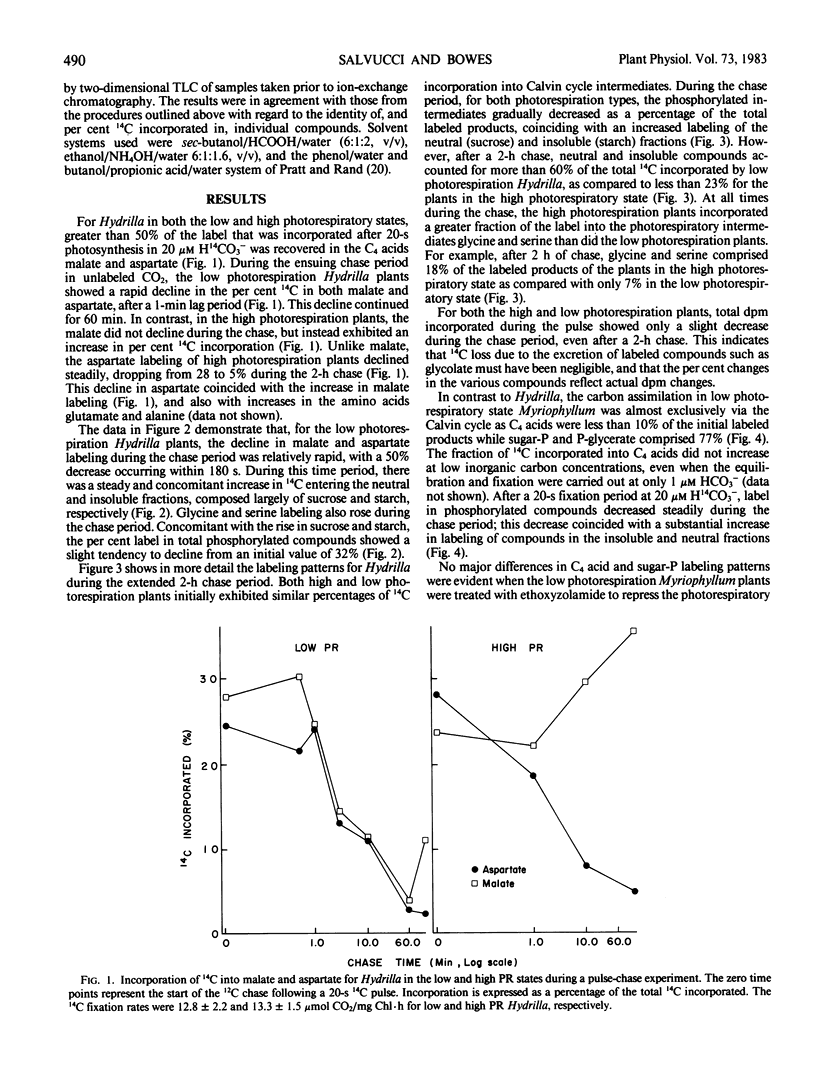
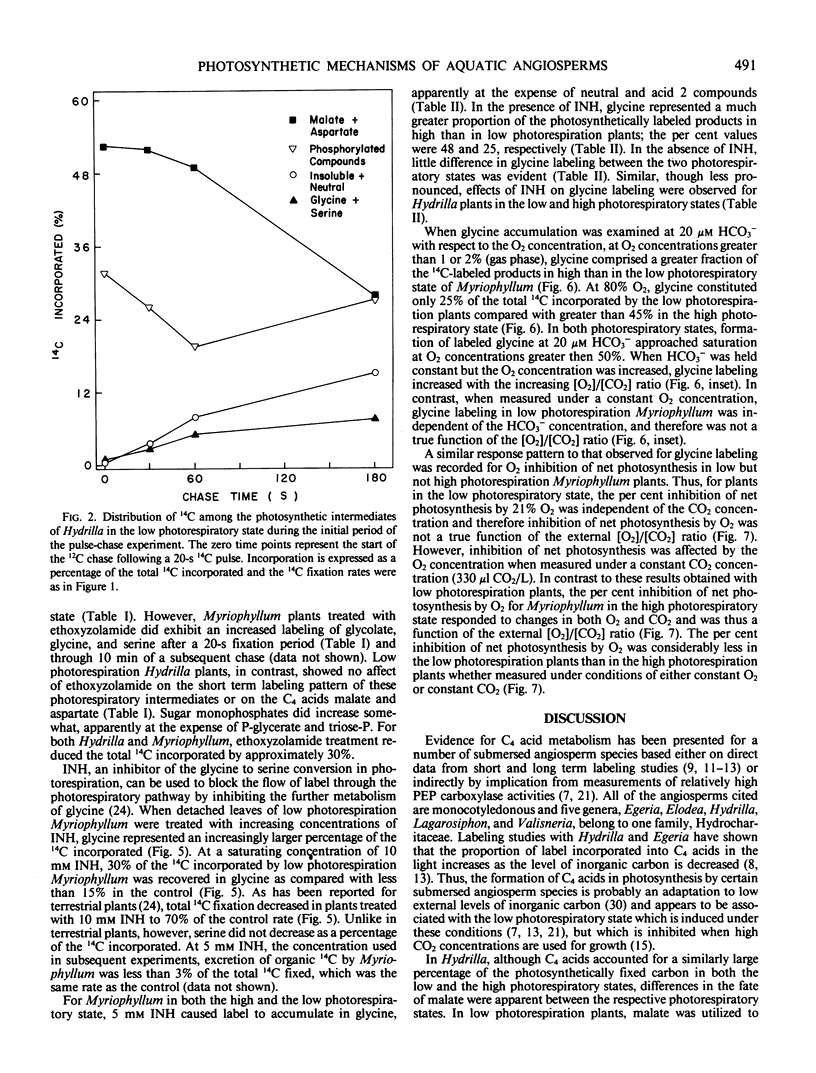
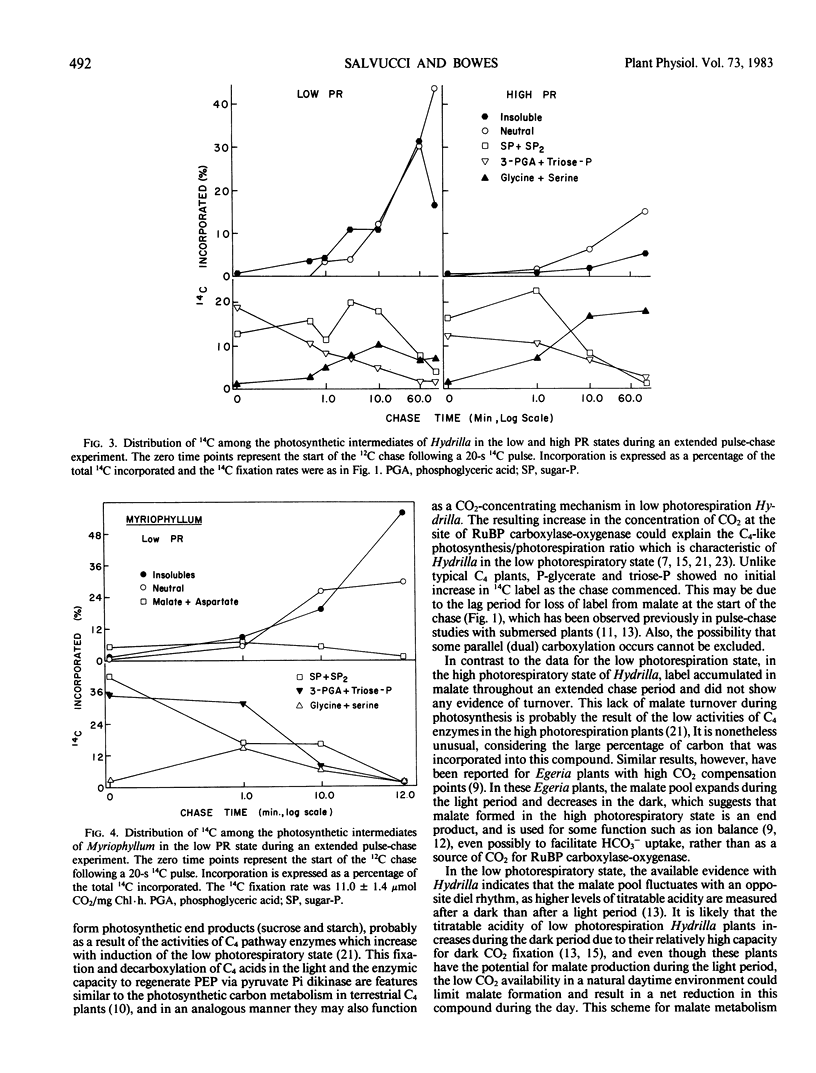
The submersed angiosperms Myriophyllum spicatum L. and Hydrilla verticillata (L.f.) Royal exhibited different photosynthetic pulse-chase labeling patterns. In Hydrilla, over 50% of the 14C was initially in malate and aspartate, but the fate of the malate depended upon the photorespiratory state of the plant. In low photorespiration Hydrilla, malate label decreased rapidly during an unlabeled chase, whereas labeling of sucrose and starch increased. In contrast, for high photorespiration Hydrilla, malate labeling continued to increase during a 2-hour chase. Thus, malate formation occurs in both photorespiratory states, but reduced photorespiration results when this malate is utilized in the light. Unlike Hydrilla, in low photorespiration Myriophyllum, 14C incorporation was via the Calvin cycle, and less than 10% was in C4 acids.
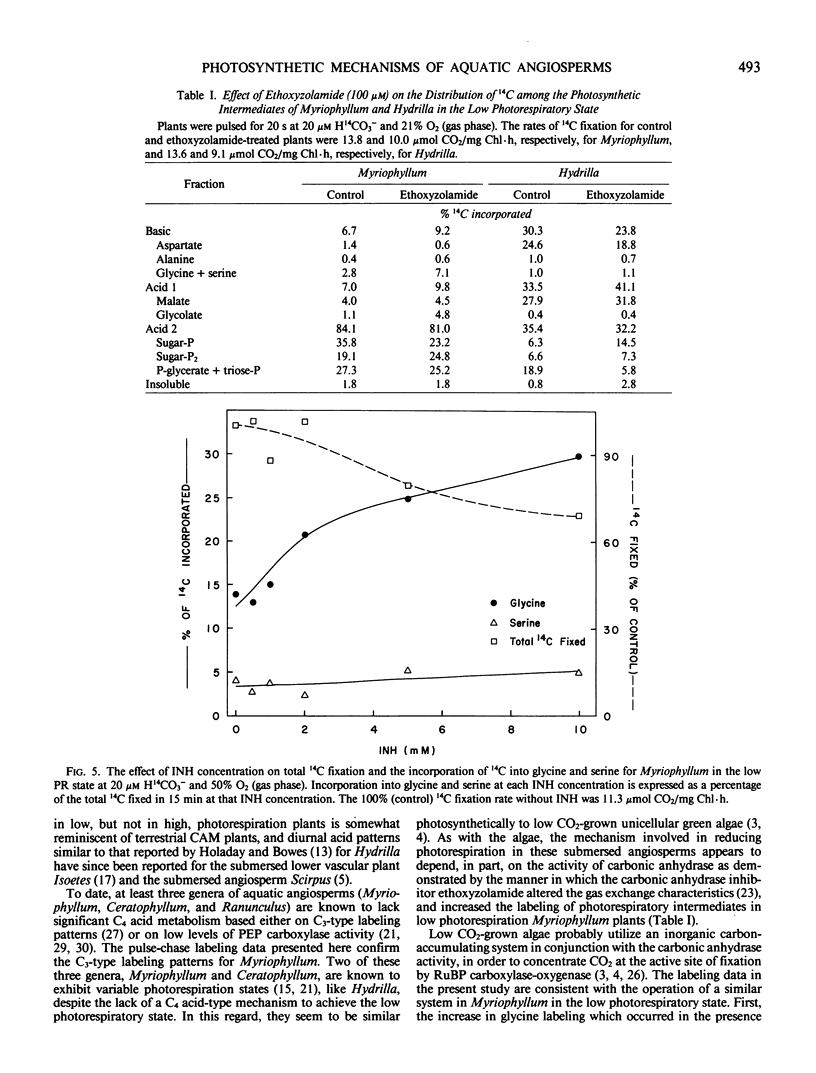
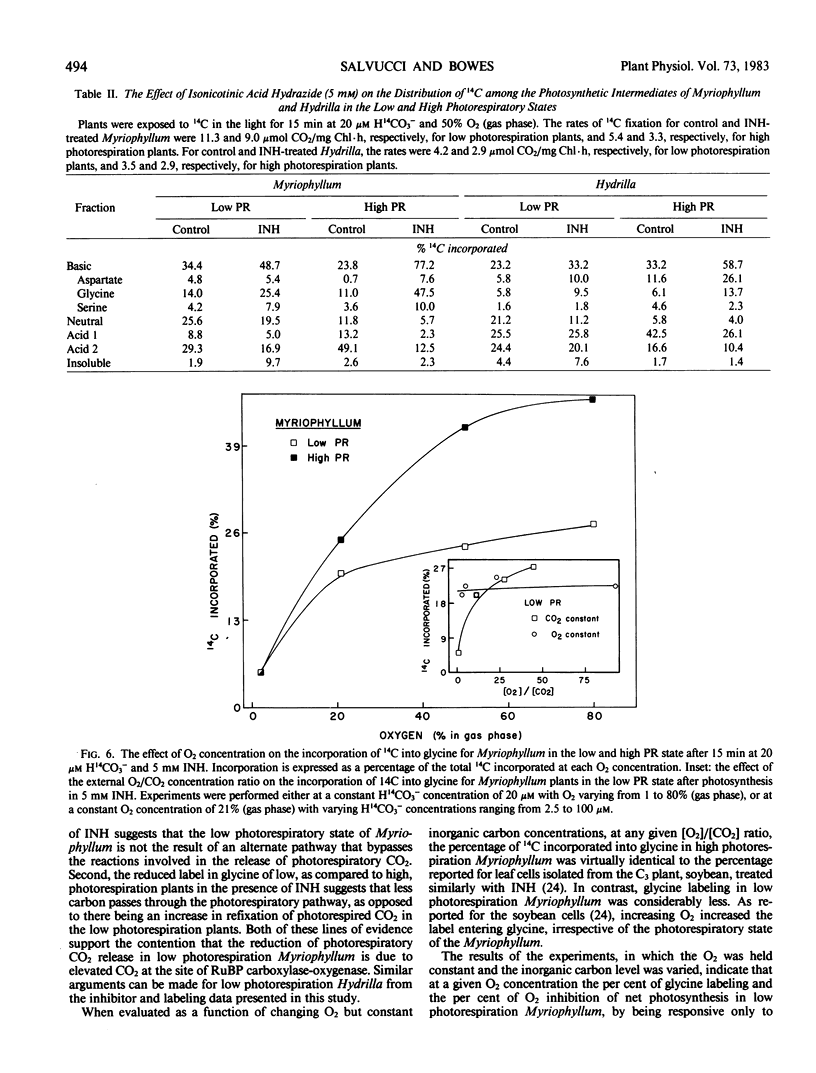
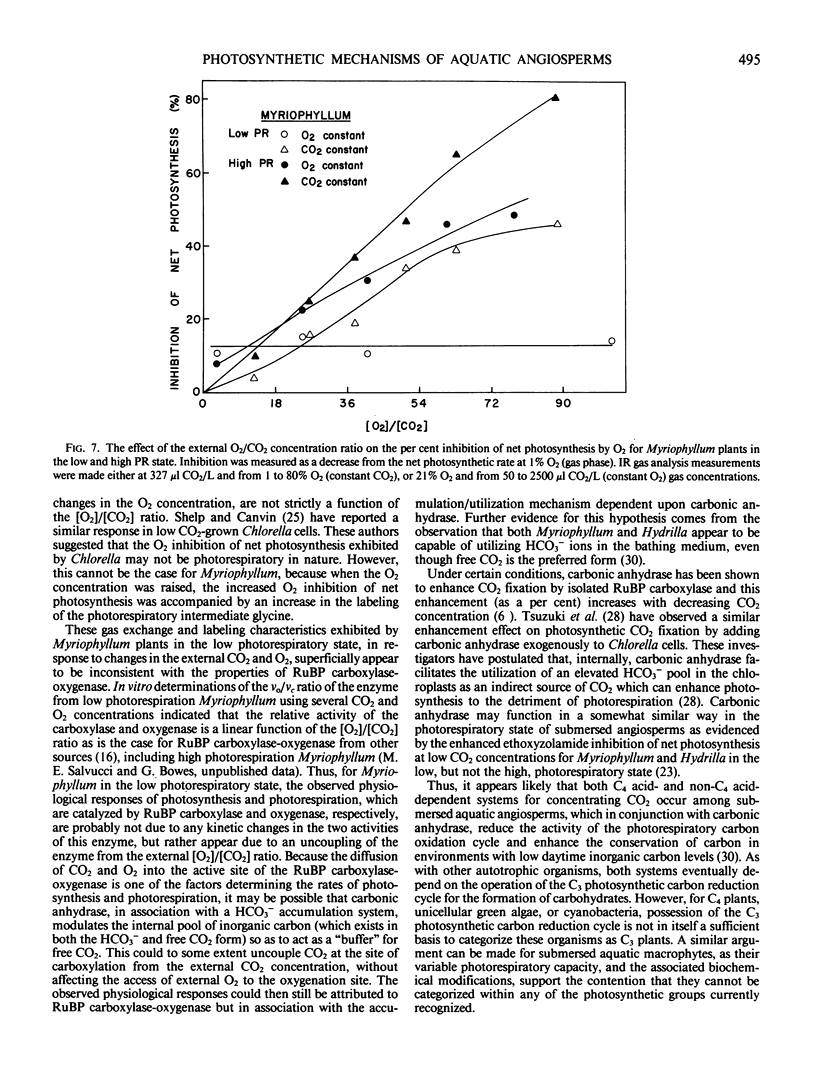
Ethoxyzolamide, a carbonic anhydrase inhibitor and a repressor of the low photorespiratory state, increased the label in glycolate, glycine, and serine of Myriophyllum. Isonicotinic acid hydrazide increased glycine labeling of low photorespiration Myriophyllum from 14 to 25%, and from 12 to 48% with high photorespiration plants. Similar trends were observed with Hydrilla. Increasing O2 increased the per cent [14C]glycine and the O2 inhibition of photosynthesis in Myriophyllum. In low photorespiration Myriophyllum, glycine labeling and O2 inhibition of photosynthesis were independent of the CO2 level, but in high photorespiration plants the O2 inhibition was competitively decreased by CO2. Thus, in low but not high photorespiration plants, glycine labeling and O2 inhibition appeared to be uncoupled from the external [O2]/[CO2] ratio.
These data indicate that the low photorespiratory states of Hydrilla and Myriophyllum are mediated by different mechanisms, the former being C4-like, while the latter resembles that of low CO2-grown algae. Both may require carbonic anhydrase to enhance the use of inorganic carbon for reducing photorespiration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroote D., Kennedy R. A. Photosynthesis In Elodea canadensis Michx: Four-Carbon Acid Synthesis. Plant Physiol. 1977 Jun;59(6):1133–1135. doi: 10.1104/pp.59.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday A. S., Bowes G. C(4) Acid Metabolism and Dark CO(2) Fixation in a Submersed Aquatic Macrophyte (Hydrilla verticillata). Plant Physiol. 1980 Feb;65(2):331–335. doi: 10.1104/pp.65.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeley J. E., Bowes G. Gas Exchange Characteristics of the Submerged Aquatic Crassulacean Acid Metabolism Plant, Isoetes howellii. Plant Physiol. 1982 Nov;70(5):1455–1458. doi: 10.1104/pp.70.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci M. E., Bowes G. Induction of reduced photorespiratory activity in submersed and amphibious aquatic macrophytes. Plant Physiol. 1981 Feb;67(2):335–340. doi: 10.1104/pp.67.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servaites J. C. Chemical inhibition of the glycolate pathway in soybean leaf cells. Plant Physiol. 1977 Oct;60(4):461–466. doi: 10.1104/pp.60.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelp B. J., Canvin D. T. Photorespiration and Oxygen Inhibition of Photosynthesis in Chlorella pyrenoidosa. Plant Physiol. 1980 May;65(5):780–784. doi: 10.1104/pp.65.5.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley R. A., Naylor A. W. Photosynthesis in Eurasian Watermilfoil (Myriophyllum spicatum L.). Plant Physiol. 1972 Jul;50(1):149–151. doi: 10.1104/pp.50.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van T. K., Haller W. T., Bowes G. Comparison of the photosynthetic characteristics of three submersed aquatic plants. Plant Physiol. 1976 Dec;58(6):761–768. doi: 10.1104/pp.58.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]


