Abstract
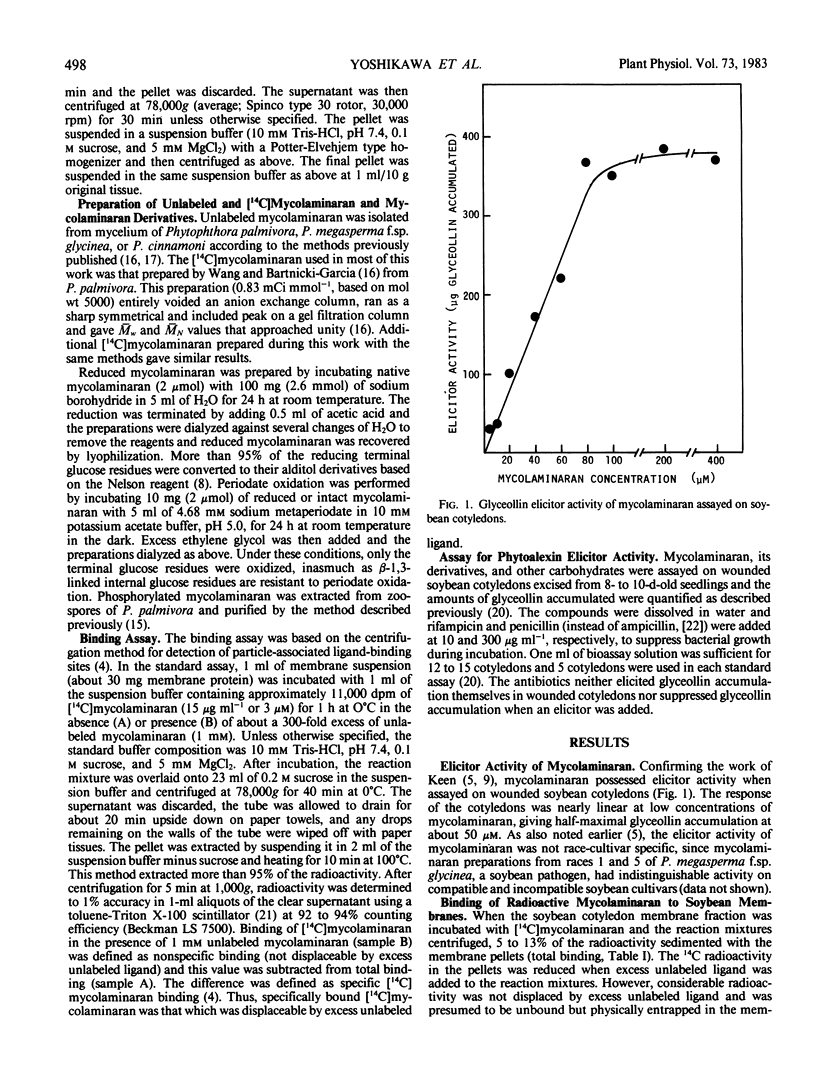
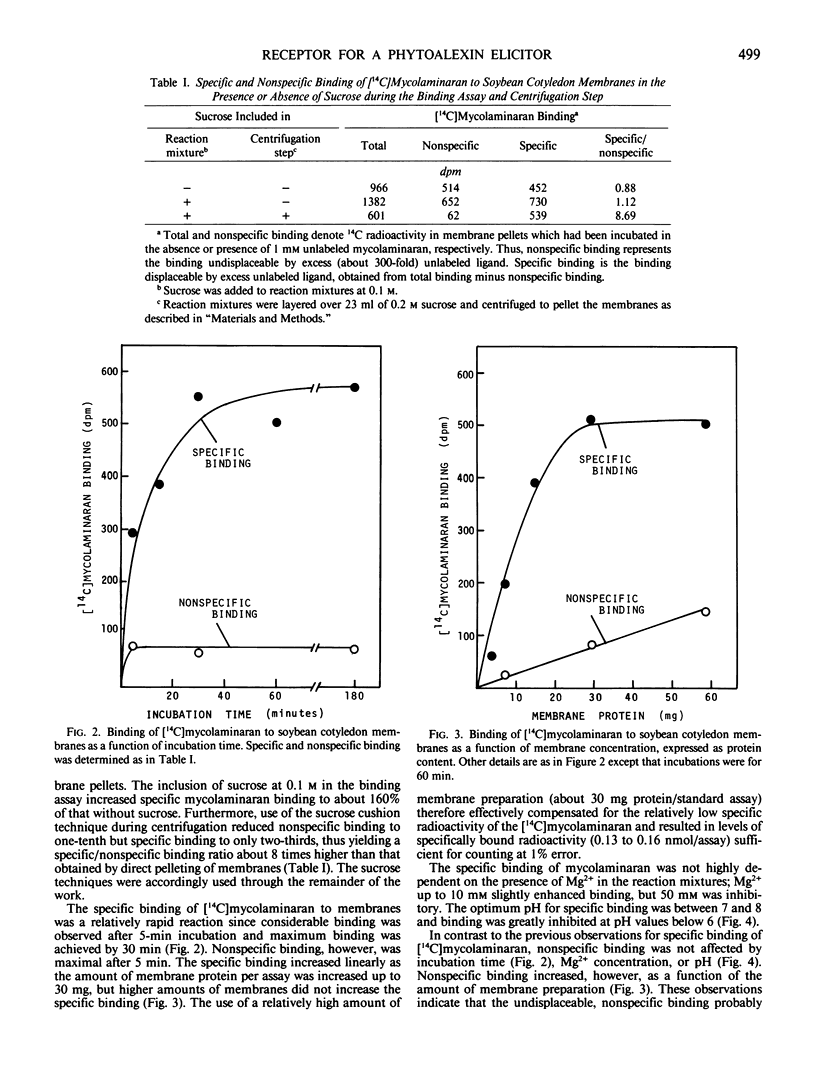
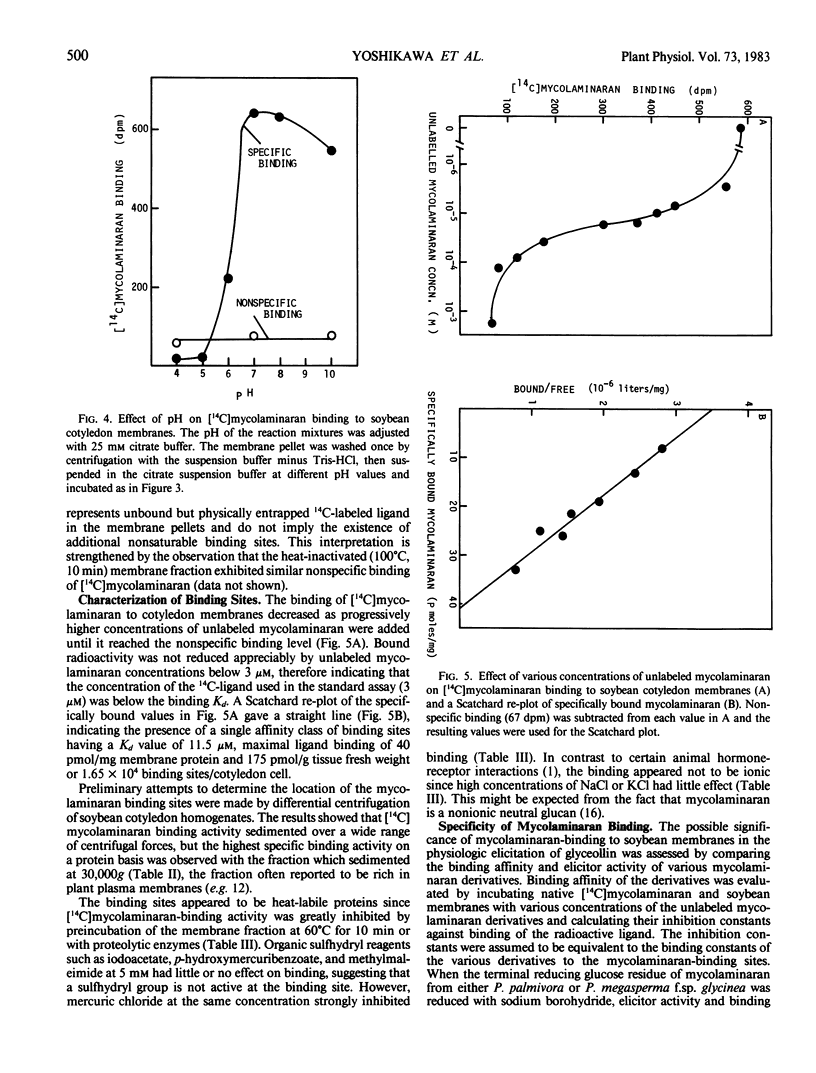
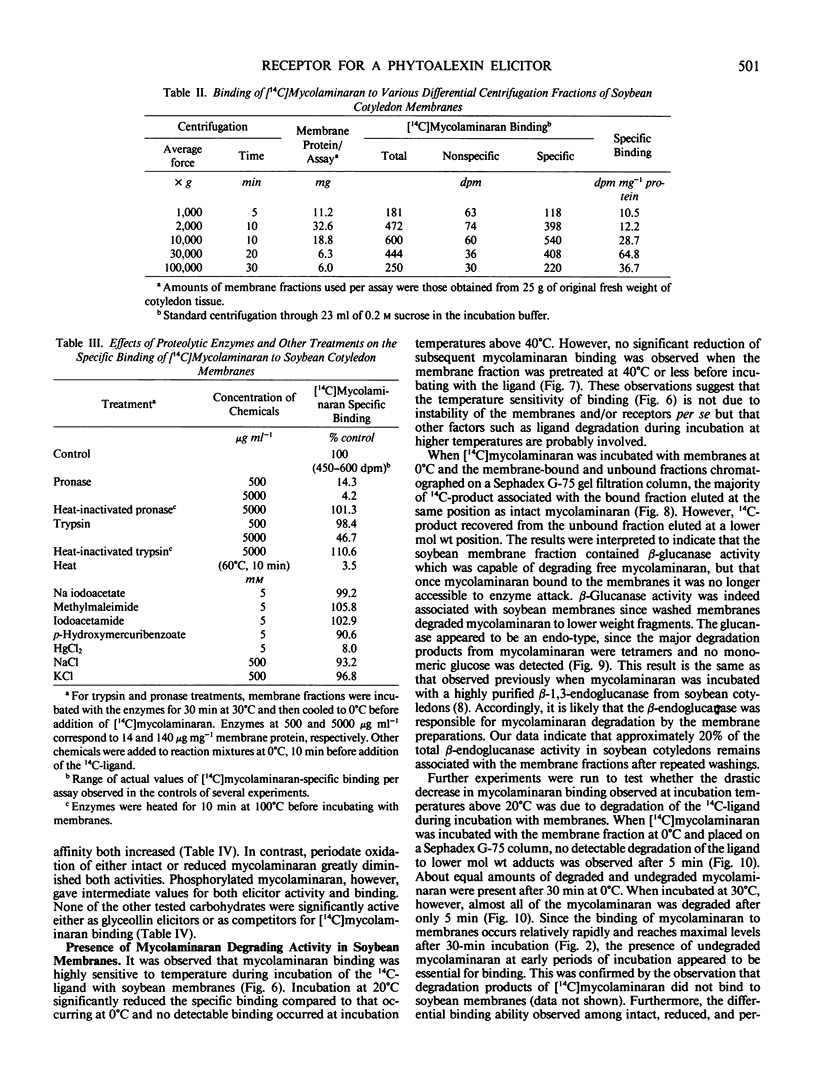
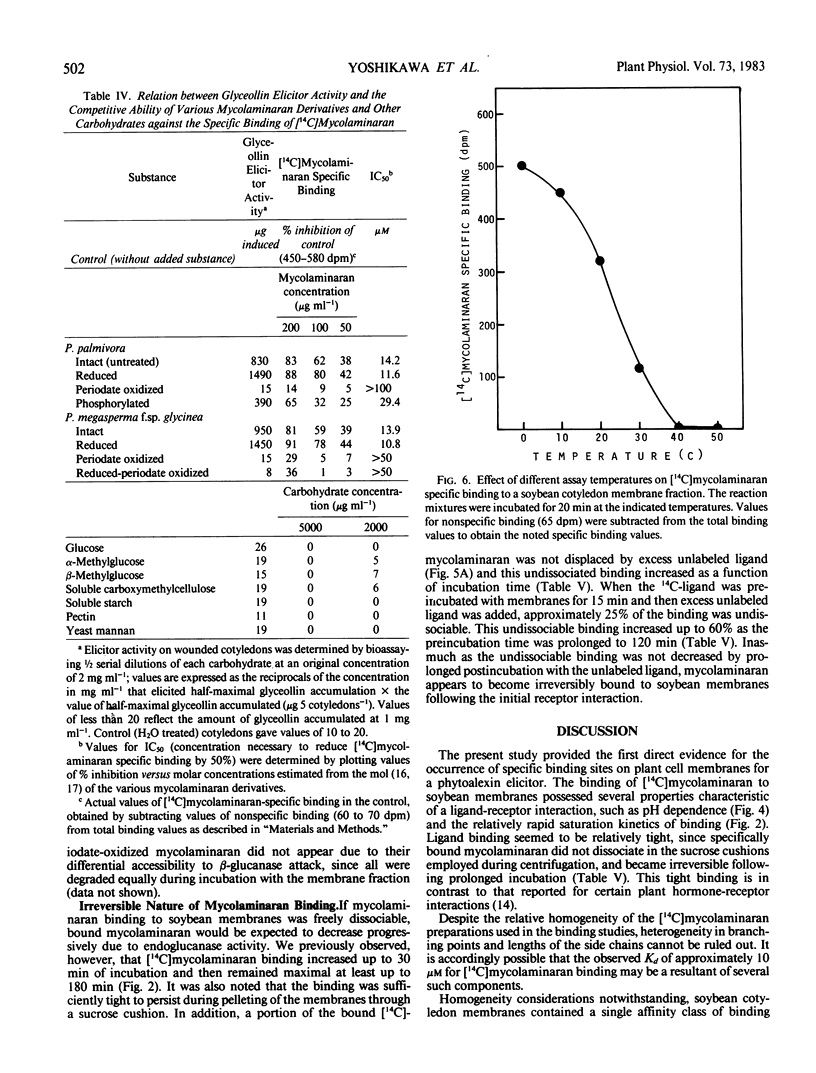
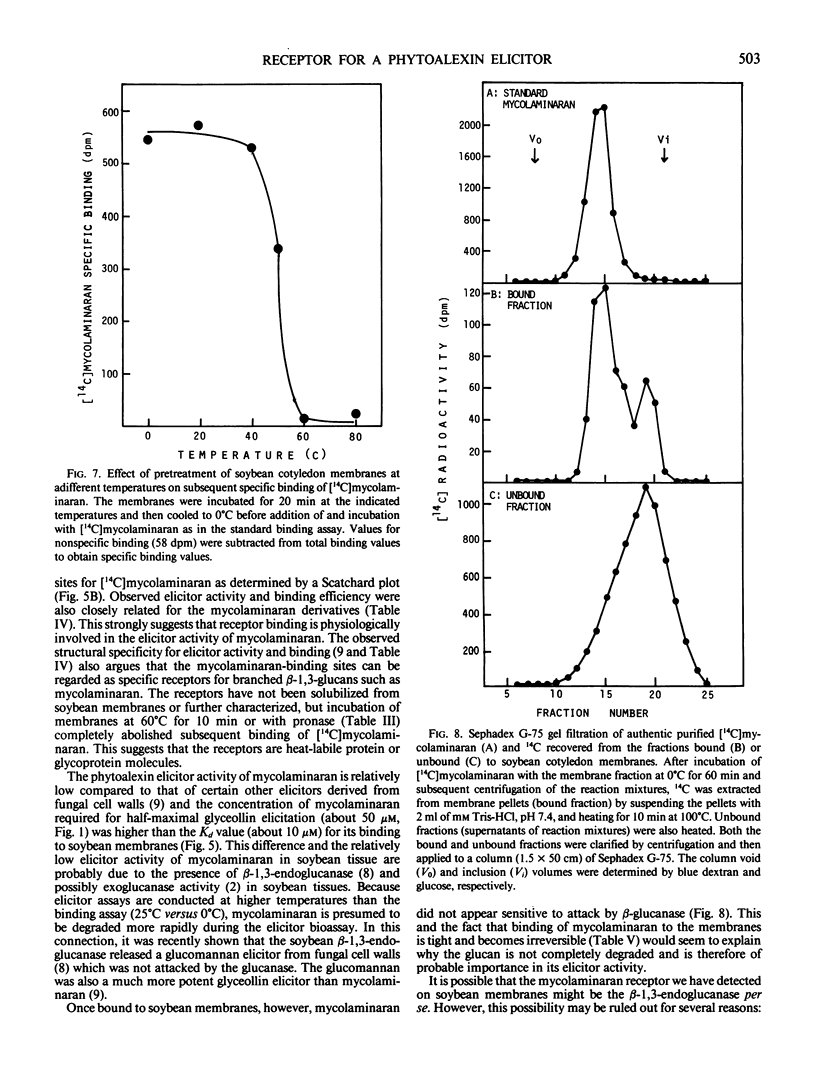
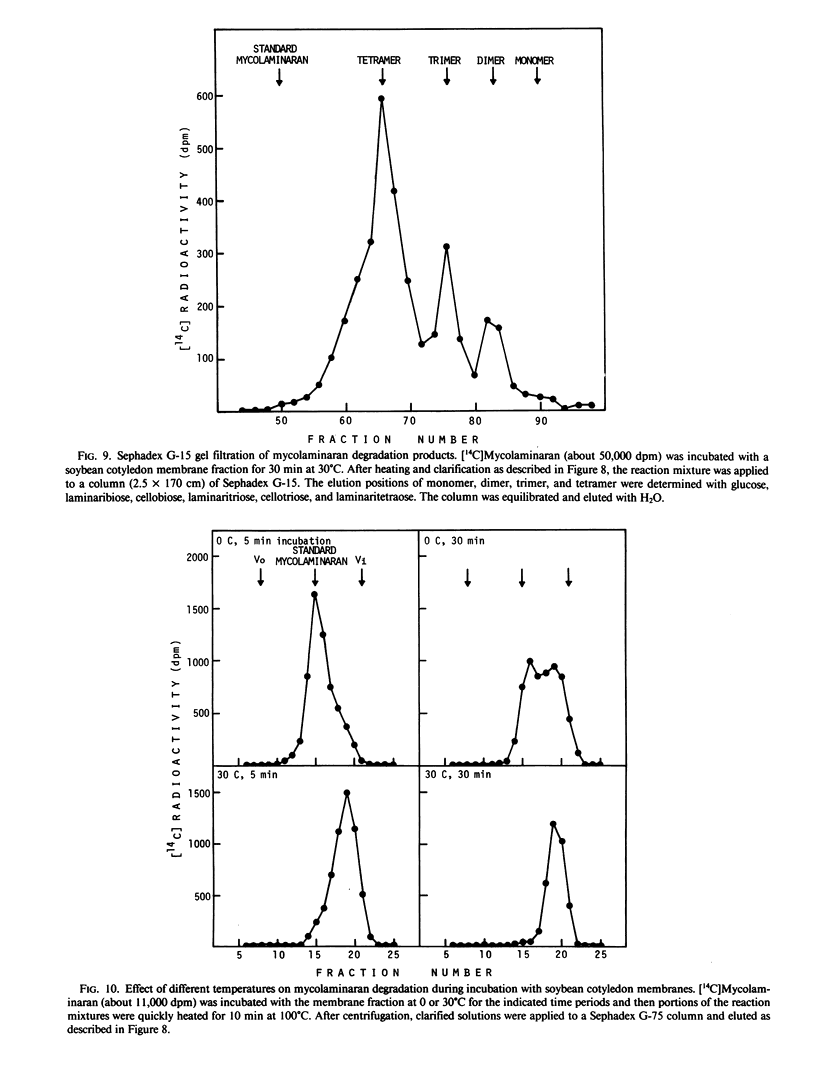
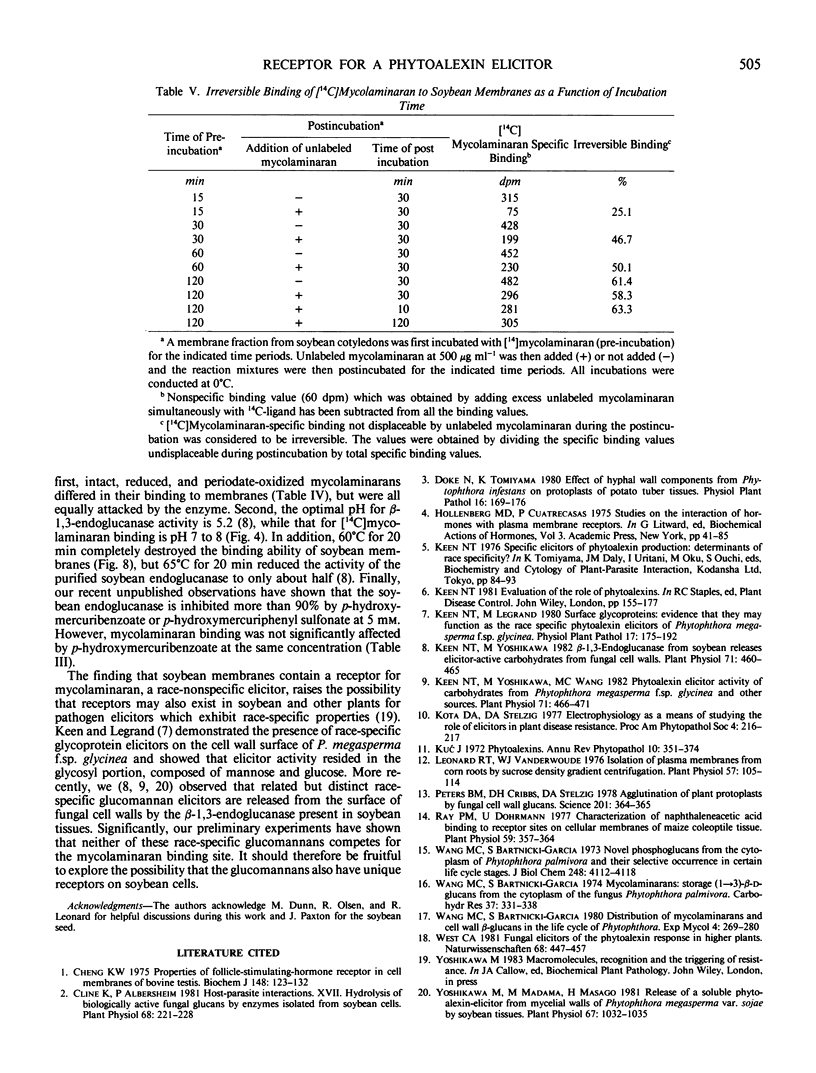
Soybean membrane preparations specifically bound [14C]mycolaminaran, a branched β-1,3-glucan produced by Phytophthora sp. which elicits production of the phytoalexin glyceollin in soybean tissues. A Scatchard plot of the binding data disclosed the presence of a single affinity class of binding sites with a Kd value of 11.5 micromolar for the glucan. To assess the physiologic importance of mycolaminaran binding in phytoalexin elicitation, several derivatives of mycolaminaran were prepared. Reduced mycolaminaran had slightly greater elicitor activity and binding affinity than the native substance, while periodinated mycolaminaran was virtually devoid of either elicitor activity orbinding capability. Phosphorylated mycolaminaran, on the other hand, gave values for both elicitor activity and membrane binding which were intermediate between the native and periodinated preparations. No other tested carbohydrates competed with the binding of [14C]mycolaminaran. Soybean membrane preparations contained β-1,3-endoglucanase activity that degraded mycolaminaran and reduced both its efficiency as a phytoalexin elicitor and its membrane binding at temperatures above 0°C. Once [14C]mycolaminaran bound to membranes, however, it was not appreciably susceptible to glucanase attack and could not be displaced with excess unlabeled ligand. Taken collectively, the observations suggest that the membrane binding sites are mycolaminaran-specific receptors which are physiologically involved in the initiation of phytoalexin production in soybean cotyledons. Because the binding of mycolaminaran to membranes was abolished by heat and proteolytic enzymes, the receptor is probably a protein(s) or glycoprotein(s).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng K. W. Properties of follicle-stimulating-hormone receptor in cell membranes of bovine testis. Biochem J. 1975 Jul;149(1):123–132. doi: 10.1042/bj1490123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K., Albersheim P. Host-Pathogen Interactions : XVII. HYDROLYSIS OF BIOLOGICALLY ACTIVE FUNGAL GLUCANS BY ENZYMES ISOLATED FROM SOYBEAN CELLS. Plant Physiol. 1981 Jul;68(1):221–228. doi: 10.1104/pp.68.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M., Wang M. C. Phytoalexin Elicitor Activity of Carbohydrates from Phytophthora megasperma f.sp. glycinea and Other Sources. Plant Physiol. 1983 Mar;71(3):466–471. doi: 10.1104/pp.71.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen N. T., Yoshikawa M. beta-1,3-Endoglucanase from Soybean Releases Elicitor-Active Carbohydrates from Fungus Cell Walls. Plant Physiol. 1983 Mar;71(3):460–465. doi: 10.1104/pp.71.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Vanderwoude W. J. Isolation of plasma membranes from corn roots by sucrose density gradient centrifugation: an anomalous effect of ficoll. Plant Physiol. 1976 Jan;57(1):105–114. doi: 10.1104/pp.57.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters B. M., Cribbs D. H., Stelzig D. A. Agglutination of plant protoplasts by fungal cell wall glucans. Science. 1978 Jul 28;201(4353):364–365. doi: 10.1126/science.201.4353.364. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. C., Bartnicki-Garcia S. Novel phosphoglucans from the cytoplasm of Phytophthora palmivora and their selective occurrence in certain life cycle stages. J Biol Chem. 1973 Jun 10;248(11):4112–4118. [PubMed] [Google Scholar]
- Yoshikawa M., Matama M., Masago H. Release of a Soluble Phytoalexin Elicitor from Mycelial Walls of Phytophthora megasperma var. sojae by Soybean Tissues. Plant Physiol. 1981 May;67(5):1032–1035. doi: 10.1104/pp.67.5.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Yamauchi K., Masago H. De Novo Messenger RNA and Protein Synthesis Are Required for Phytoalexin-mediated Disease Resistance in Soybean Hypocotyls. Plant Physiol. 1978 Mar;61(3):314–317. doi: 10.1104/pp.61.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer U., Ebel J., Mulheirn L. J., Lyne R. L., Grisebach H. Induction of phytoalexin synthesis in soybean. Dimethylallylpyrophosphate:trihydroxypterocarpan dimethylallyl transferase from elicitor-induced cotyledons. FEBS Lett. 1979 May 1;101(1):90–92. doi: 10.1016/0014-5793(79)81301-0. [DOI] [PubMed] [Google Scholar]


