ABSTRACT
Objective:
Insulin Icodec is a novel basal insulin analogue designed for once-weekly administration, therefore might propitiate reduction in the frequency of injections and facilitate treatment adherence. This study aimed to determine the glycemic control and safety profile of Insulin Icodec, compared with Glargine U100 in patients with diabetes mellitus type 2.
Materials and methods:
We performed a systematic review and meta-analysis of randomized controlled trials (RCT) data comparing Once-Weekly Insulin Icodec and Once-Daily Insulin Glargine U100 in patients with type 2 diabetes mellitus. PubMed, Embase, and Cochrane databases were searched for trials published up to May 14, 2022. Data were extracted from published reports and quality assessment was performed per Cochrane recommendations.
Results:
Three studies were included comprising 453 patients, 230 (50.77%) using Once-Weekly Insulin Icodec and 223 (49.22%) using Once-Daily Insulin Glargine U100. In the pooled data, Glycated Hemoglobin (MD -0.20% CI -0.33 to -0.07%; P=0.002) change from baseline demonstrated a significantly higher reduction in the Icodec group. Time with Glucose in Range (MD 6.60% CI 3.63 to 9.57%; P < 0.0001) and Insulin Dose Difference (MD 0.97UI CI 0.76 to 1.18UI; P < 0.0001) were higher in the Icodec group. There was no significant difference in fasting plasma glucose, body weight change, hypoglycemia or any adverse event evaluated.
Conclusions:
Once-Weekly Insulin Icodec was associated with a small reduction in Glycated Hemoglobin, as well as higher Time with Glucose in Range, with similar hypoglycemic adverse events, when compared with Once-Daily Insulin Glargine U100.
Keywords: Type 2 diabetes mellitus, insulin, insulin long-acting, insulin glargine, insulin icodec, glycemic control, glycated hemoglobin A
INTRODUCTION
Diabetes is a chronic and progressive illness requiring multiple strategies of interventions in order to reduce its burden (1). The progressive characteristic of the disease is clinically evidenced by the common need for optimization of pharmacological therapy, multiple times requiring the use of an external Insulin source. Insulin therapy in diabetes is a major cornerstone on its treatment, but the need for multiple daily subcutaneous applications and the risk of hypoglycemia becomes a challenge regarding patient adherence.
The necessity for optimizing treatment and reducing daily applications lead to the development of multiple insulin preparations and analogs in the past years, ranging from the first generation analogs, detemir and glargine-U100, to the second generation, glargine-U300 and degludec (2). Recently, a very innovative once weekly applied Insulin preparation was developed (3) and tested in a sequence of 3 phase 2 clinical trials (4-6), raising the expectations on this new type of treatment. Its use could help improve patient’s adherence to treatment as well as patient’s quality of life. However, an important issue that must be analyzed is effectiveness whether the new weekly applied insulin increases or diminishes the risk of hypoglycemia. Furthermore, several other phase 3 randomized clinical trials have started since, but there is no meta-analysis published on this subject to this date.
In light of this issue, we performed a systematic review and meta-analysis accessing the efficacy and safety of the weekly use of insulin Icodec in comparison to the daily insulin glargine, exploring populations with type 2 diabetes mellitus and their glycemic control.
MATERIALS AND METHODS
Eligibility criteria
Inclusion in this meta-analysis was restricted to studies that met all the following criteria: (1) randomized trials; (2) comparing the use once weekly insulin icodec to once daily insulin glargine; (3) enrolling patients with type 2 diabetes mellitus; (4) evaluating any of the desired outcomes; (5) articles written on English language. We excluded studies with (1) no control group; (2) overlapping studies population; clinical trial register entry only; (3) non-human studies and (4) studies reported only as abstracts.
Search strategy
We systematically searched PubMed, Embase and Cochrane central register of controlled trials from inception to May 2022 with the following search term: “Icodec”. The references from all included studies were also searched manually for any additional studies. Two authors (M.M.G and M.E.G) independently screened and extracted the data following predefined search criteria and quality assessment. The prospective meta-analysis protocol was registered on INPLASY with registration number INPLASY202250102 (DOI number: 10.37766/inplasy2022.5.0102). The systematic review was registered after the beginning of the review process.
Outcomes evaluated
The primary outcome was glycated hemoglobin (HbA1c). Outcomes included any adverse outcome, serious adverse event, any adverse event probably related to basal insulin, injection site reaction, hypersensitivity reaction, hypoglycemia alert, clinically significant or severe hypoglycemia, HBa1C (%) change from baseline, fasting plasma glucose (mg/dL) change from baseline, body weight (kg) change from baseline, time with glucose in range (%) and insulin dose difference (UI).
Time with Glucose in Range (TIR) varied between studies. Rosenstock (4) used a tight range (70-140 mg/dL), whereas Lingvay (5) and Bajaj (6) adopted 70-180 mg/dL. Percent TIR was calculated in the last two weeks of the trials, measured by continuous glucose measurement systems. Hypoglycemia alert (Level 1) was defined as Plasma Glucose < 70 mg/dL and > 54 mg/dL. While, clinically significant hypoglycemia determined as Plasma Glucose < 54 mg/dL and severe hypoglycemia as severe cognitive impairment requiring external assistance.
Risk of bias assessment
We evaluated the risk of bias in randomized studies using version 2 of the Cochrane Risk of Bias assessment tool (7). Two independent authors completed the risk of bias assessment (R.R.S and J.O.N.S). Disagreements were resolved through a consensus after discussing reasons for discrepancies. Each trial received a score of high, low, or unclear risk of bias in 5 domains: selection, performance, detection, attrition, and reporting biases. All included studies contributed to the evaluated outcomes through forest plots. Small-study effect was investigated by funnel-plot analysis of point estimates in relation to study weights.
The certainty of evidence was classified according to the Grading of recommendation Assessment, development and Evaluation (GRADE) method. The systematic review and meta-analysis were performed and reported in accordance with the Cochrane Collaboration Handbook for Systematic Review of Interventions and the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) statement guidelines (8,9).
Data analysis
Review Manager 5.3 (Cochrane Center, The Cochrane Collaboration, Denmark) was used for statistical analysis. Odds-ratios (OR) with 95% confidence intervals were used to compare treatment effect for categorical endpoints. Continuous outcomes were compared with mean differences. We assessed heterogeneity with I² statistics and Cochran Q test; p-values < 0.1 and I² > 25% were considered significant for heterogeneity. We used a fixed-effect model for outcomes with low heterogeneity (I² <2 5%). Otherwise, a DerSimonian and Laird random-effects model was used. We also performed sensitivity analyses by excluding individual studies to evaluate the impact of a single study in each outcome. No missing results were identified during data extraction.
Role of funding source
There was no funding source for this study. All the authors had full access to all data in the study. The corresponding author had final responsibility for the decision to submit for publication.
RESULTS
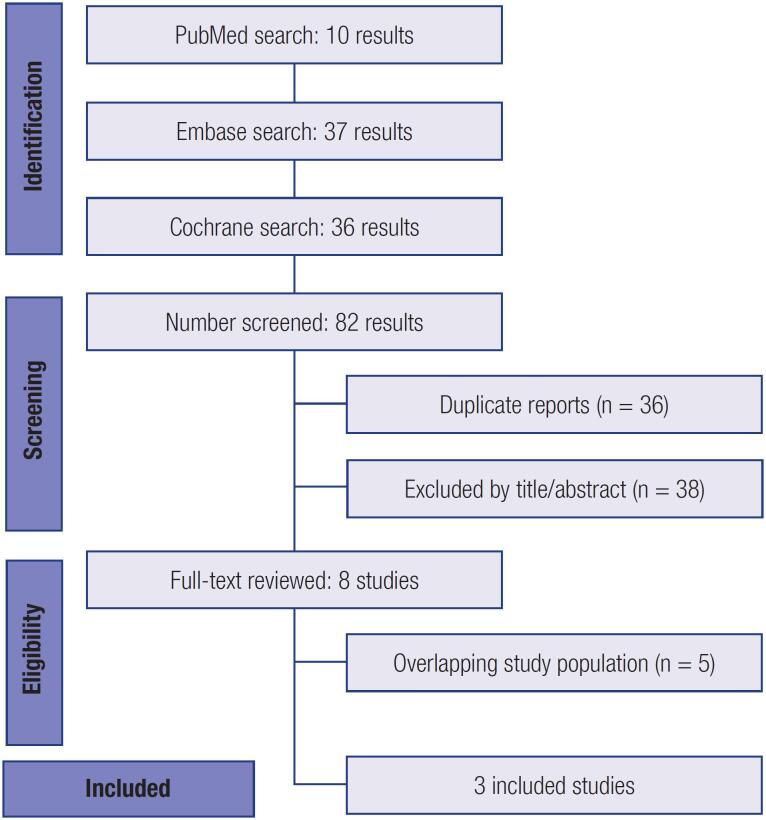
As detailed in Figure 1, the initial search yielded 82 results. After removal of duplicate records and studies with and exclusion criterion based on title/abstract review, 8 studies remained and were fully reviewed for the inclusion and exclusion criteria. Ultimately, a total of 453 patients from 3 Phase 2 Randomized Controlled Trials were included in this systematic review and meta-analysis (4-6), with exclusion of five studies due to overlapping population. Insulin Icodec Once-Weekly was prescribed to 230 (50,77%) patients and Insulin Glargine U100 Once-Daily to 223 (49,22%) patients. Studies characteristics are described in Table 1. Mean follow-up ranged from 16 to 26 weeks.
Figure 1. PRISMA flow diagram of study screening and selection.
Table 1. Baseline Characteristics of Included Studies in the Meta-analysis* .
| ROSENSTOCK (4) | BAJAJ (6) | LINGVAY (5) | ||
|---|---|---|---|---|
| Population | TDM2 using oral hypoglycemic drugs | TDM2 using oral hypoglycemic drugs | TDM2 using oral hypoglycemic drugs | |
| Intervention | Once weekly Icodec | Once weekly Icodec with Loading Dose | Once weekly Icodec Titration B |
|
| Control | Once daily Glargine U100 | Once daily Glargine U100 | Once daily Glargine U100 | |
| Study Design | RCT Phase 2 Double-blind |
RCT Phase 2 Open-label |
RCT Phase 2 Open-label |
|
| Follow-up | 26 weeks (+5) | 16 weeks (+5) | 16 weeks (+5) | |
| HbA1C for Inclusion | 7%-9,5% | 7%-10% | 7%-10% | |
| Target Fasting Glucose | 70-108 mg/Dl | 70-130 mg/dL | 80-130 mg/dL | |
| Previous Basal Insulin Therapy | No | Yes | No | |
| Number of Patients | ||||
| Intervention | n = 125 | n = 54 | n = 51 | |
| Control | n = 122 | n = 50 | n = 51 | |
| Total | N= 247 | N = 154 | N = 205 | |
| Male Sex | ||||
| Intervention | 70 (56,0) | 39 (72,2) | 53,8 | |
| Control | 69 (56,6) | 33 (66,0) | 52,9 | |
| Total | 139 (56,3) | 111 (72,1) | 53,7 | |
| Age | ||||
| Intervention | 59,7 (8,2) | 62,4 (7,2) | 61,4 (8,0) | |
| Control | 59,4 (9,5) | 60,5 (7,9) | 60,2 (8,1) | |
| Total | 59,6 (8,9) | 61,7 (7,8) | 60,7 (8,3) | |
| Diabetes Duration (years) | ||||
| Intervention | 10,5 (8,4) | 13,8 (7,7) | 9,2 (4,4) | |
| Control | 8,8 (6,1) | 14,8 (8,1) | 11,8 (6,8) | |
| Total | 9,7 (7,4) | 15,1 (8,1) | 10,1 (6,0) | |
| Body Mass Index | ||||
| Intervention | 31,1 (4,9) | 30,2 (4,3) | 30,8 (3,8) | |
| Control | 31,4 (4,4) | 30,3 (5,0) | 30,6 (4,7) | |
| Total | 41,3 (4,6) | 29,8 (4,5) | 31,3 (4,5) | |
| HbA1C (%) | ||||
| Intervention | 8,09 (0,7) | 7,8 (0,7) | 8,2 (0,9) | |
| Control | 7,96 (0,6) | 7,9 (0,7) | 8,2 (0,8) | |
| Total | 8,02 (0,6) | 7,9 (0,7) | 8,1 (0,8) | |
| Fasting Plasma Glucose (mg/dL) | ||||
| Intervention | 182 (42) | 142 (34) | 177 (41) | |
| Control | 180 (42) | 148 (36) | 168 (42) | |
| Total | 181 (42) | 144 (37) | 175 (39) | |
| Diabetes Complication | ||||
| Intervention | 27 (21,6) | NA | NA | |
| Control | 22 (18,0) | NA | NA | |
| Total | 49 (19,8) | NA | NA | |
| Atherosclerotic Cardiovascular Disease | ||||
| Intervention | 8 (6,4) | NA | NA | |
| Control | 5 (4,1) | NA | NA | |
| Total | 13 (5,3) | NA | NA | |
Absolute Number (Percentage) and Mean (Standard Deviation).
HBA1C: glycated hemoglobin; NA: not available; RCT: randomized controlled trial; TDM2: diabetes mellitus type 2.
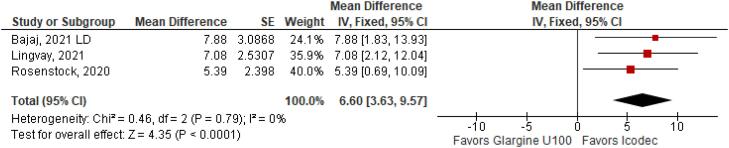
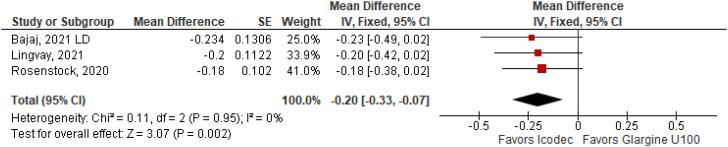
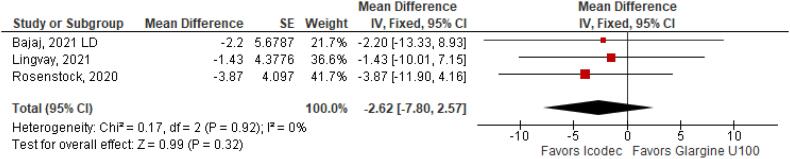
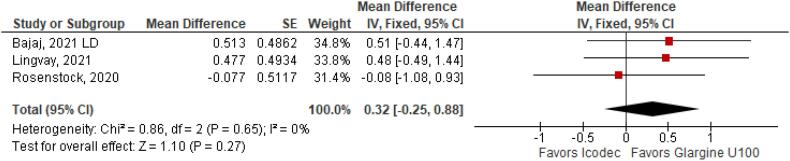
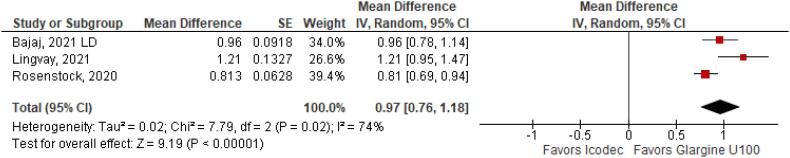
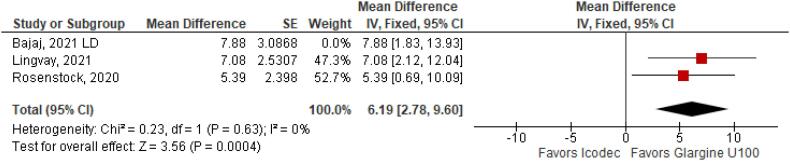
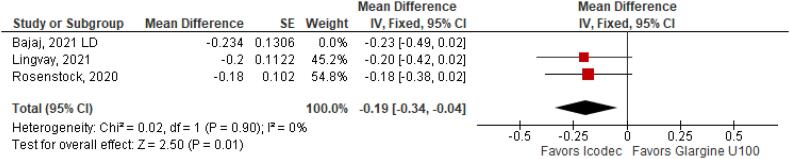
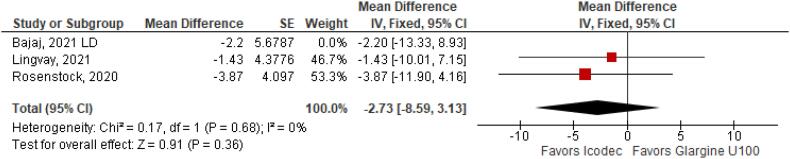
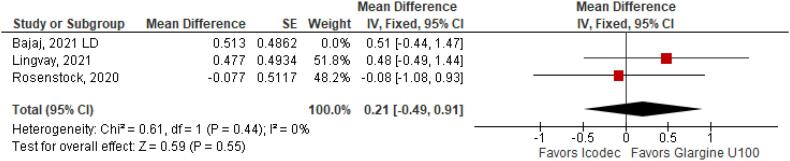
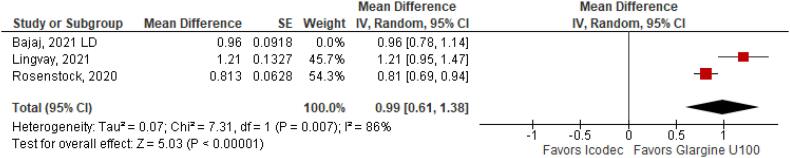
With regards to the glycemic control, Time with Glucose in Range (MD 6.60% CI 3.63 to 9.57%; P < 0.0001; I2 = 0%; Figure 2) was higher in the Icodec group, as well as greater reduction in Glycated Hemoglobin (MD -0.20% CI -0.33 to -0.07%; P = 0.002; I2 = 0%; Figure 3) between patients who received Icodec and Glargine U100. There was no significant difference in Fasting Plasma Glucose (MD -2.62 mg/dL CI -7.80 to 2.57 mg/dL; P = 0.32; I2 = 0%; Figure 6) and body weight (MD 0.3 2kg CI -0.25 to 0.88 kg; P = 0.27; I2 = 0%; Figure 7) change from baseline. However, the insulin dose difference (MD 0,97 UI CI 0.76 to 1.18 UI; P = 0.02; I2 = 74%; Figure 8) was higher in the Icodec group.
Figure 2. Mean Difference of Time with Glucose in Range (%) for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
Figure 3. Mean Difference of HBa1C (%) Change from Baseline for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
Figure 6. Mean Difference of Fasting Plasma Glucose (mg/dL) for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
Figure 7. Mean Difference of Body Weight (kg) Change from Baseline for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
Figure 8. Mean Difference of Insulin Dose Difference (UI) for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
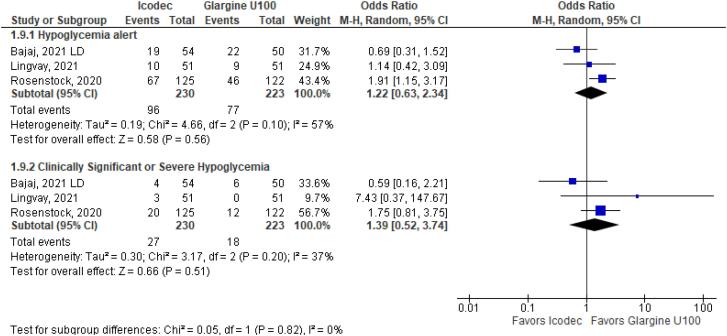
As for the safety endpoints, hypoglycemic alert (OR 1.22 CI 0.63 to 2.34; P = 0.56; I2 = 57%; Figure 4) and clinically significant or severe hypoglycemia (OR 1.39 CI 0.52 to 3.74; P = 0.51; I2 = 37%; Figure 4) had no significant difference in the once-weekly insulin icodec group, compared with once-daily insulin Glargine U100.
Figure 4. Odds Ratio of Hypoglycemia for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
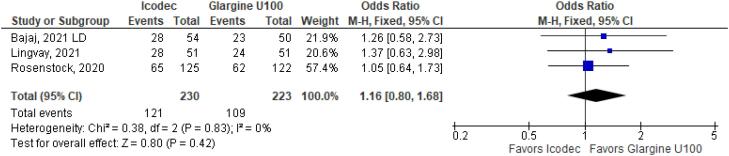
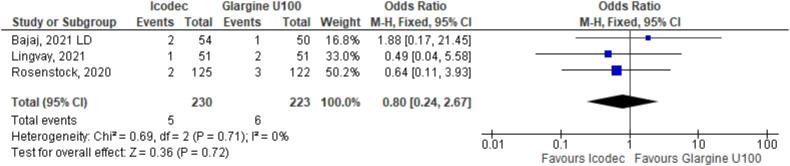
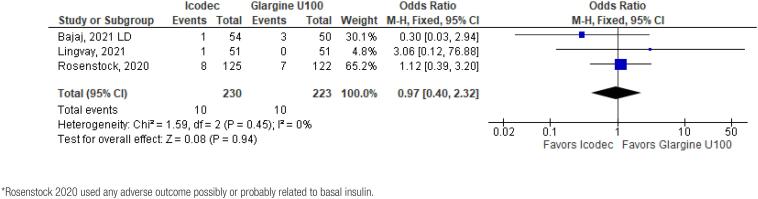
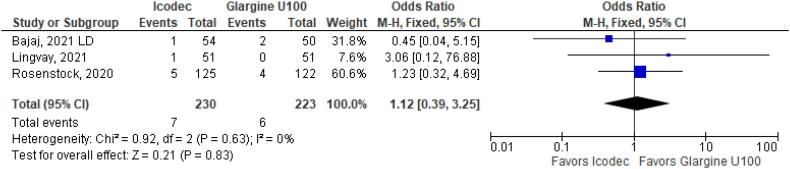
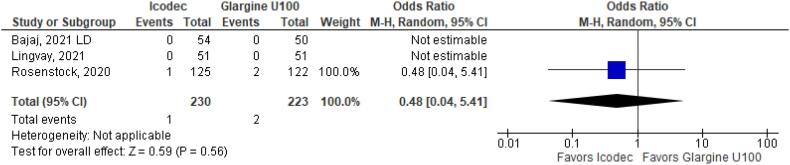
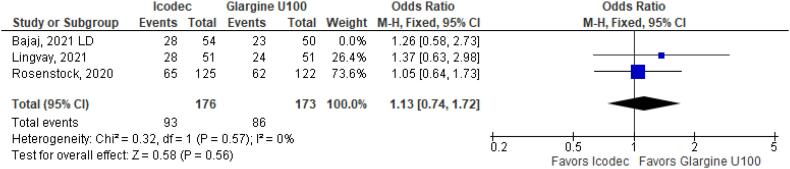
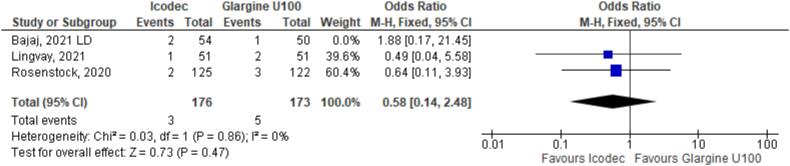
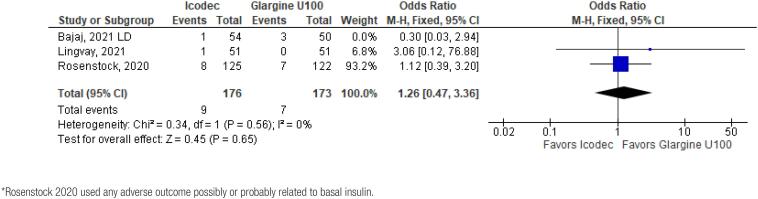
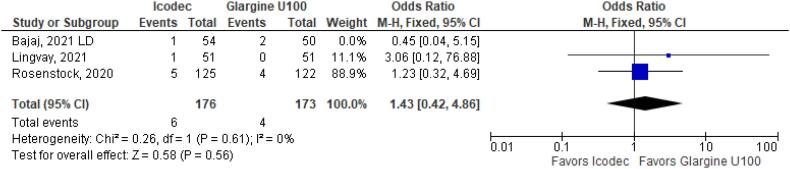
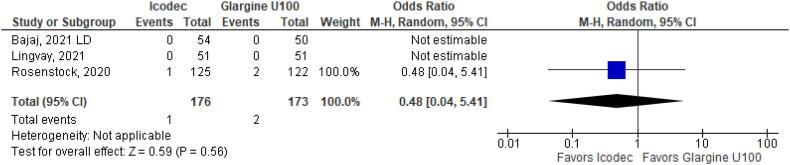
In addition, any adverse event (OR 1.16 CI 0.80 to 1.68; P = 0.42; I2 = 0%; Figure 5), serious adverse event (OR 0.80 CI 0.24 to 2.67; P = 0.72; I2 = 0%; Figure 9) and adverse event probably related to basal insulin (OR 0.97 CI 0.40 to 2.32; P = 0.94; I2 = 0%; Figure 10) showed no statistically significant difference. Injection site reaction (OR 1.12 CI 0.39 to 3.25; P = 0.83; I2 = 0%; Figure 11) and hypersensitity reaction (OR 0.48 CI 0.04 to 5.41; P = 0.56; Figure 12) also were not significant.
Figure 5. Odds Ratio of Any Adverse Outcome for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100.
Figure 9. Forrest Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Serious Adverse Event.
Figure 10. Forrest Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Any Adverse Event Probably related to Basal Insulin*.
Figure 11. Forrest Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Injection Site Reaction.
Figure 12. Forrest Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Hypersensitivity Event.
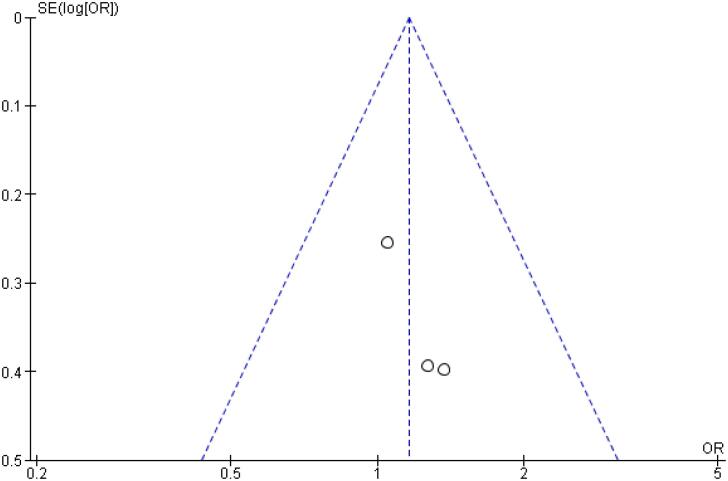
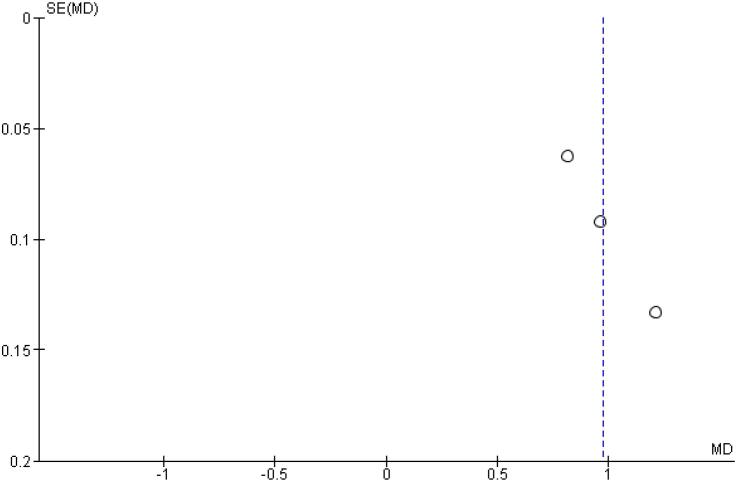
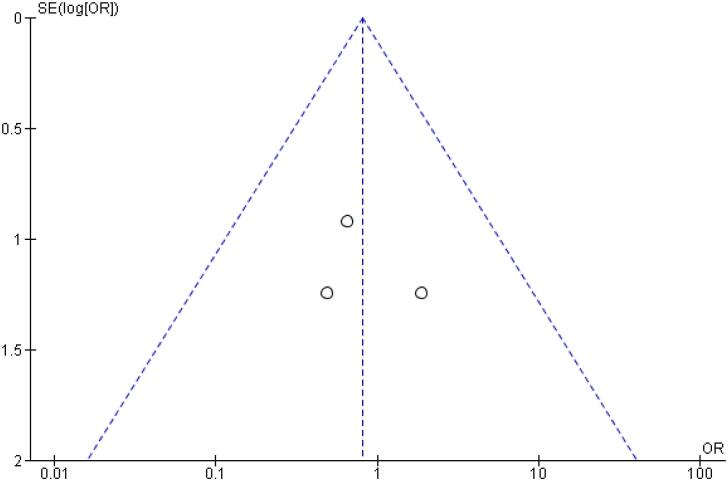
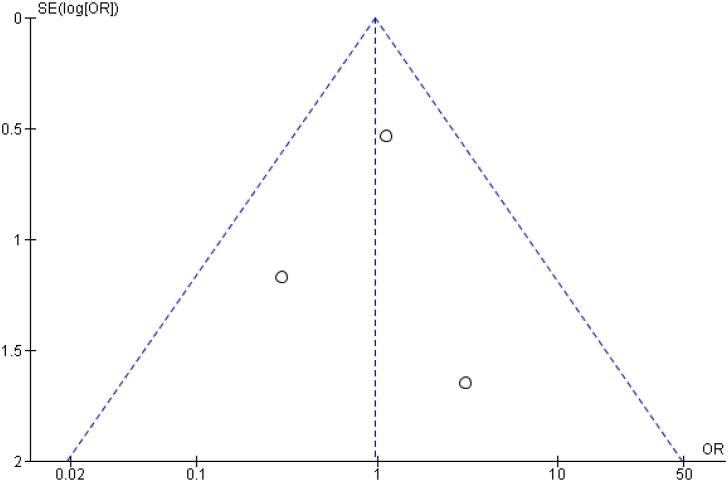
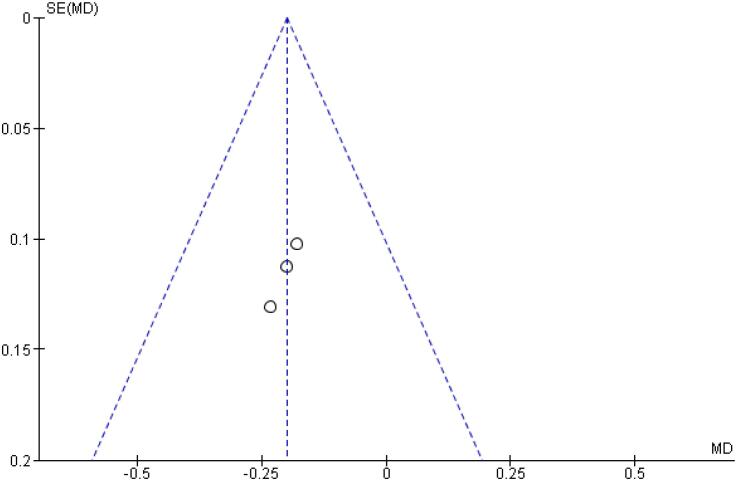
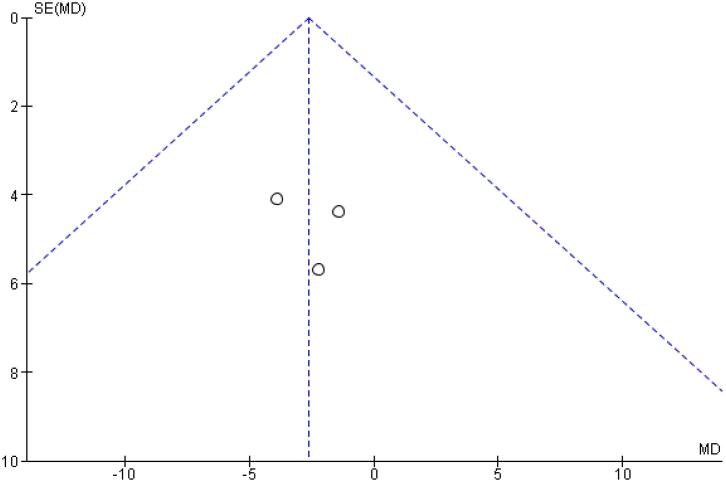
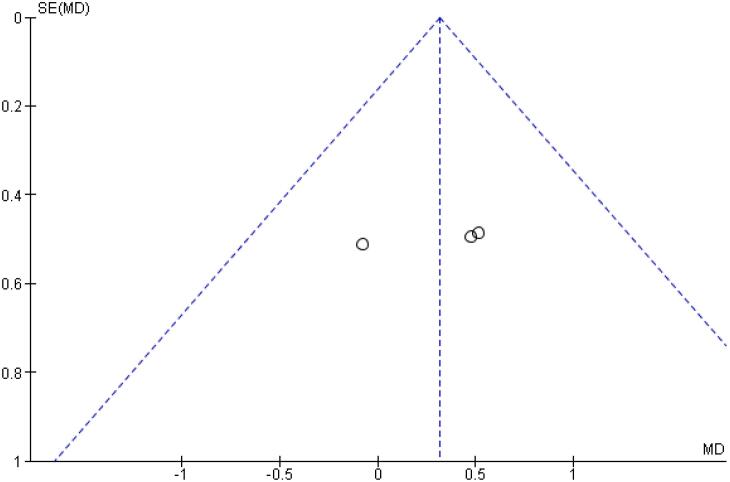
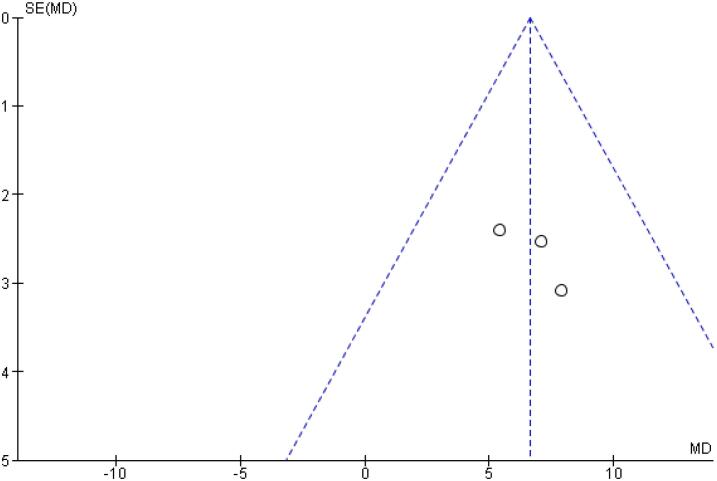
Table 2 outlines individual appraisal of each RCT included in the meta-analysis. Overall, all studies were deemed at low risk of bias. There was also no evidence of small-study effect by funnel plots. There was a symmetrical distribution of studies with similar weights around the meta-analysis point estimate (Figure 13–24).
Table 2. Risk of bias assessment of studies included in the meta-analysis.
Figure 13. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Any Adverse Outcome.
Figure 24. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Insulin Dose Difference (UI).
Figure 14. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Serious Adverse Event.
Figure 15. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Any Adverse Event Probably related to Basal Insulin.
Figure 16. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Injection Site Reaction.
Figure 17. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Hypersensitivity Reaction.
Figure 18. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Hypoglycemia Alert.
Figure 19. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Clinically Significant or Severe Hypoglycemia.
Figure 20. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for HBa1C (%) Change from Baseline.
Figure 21. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Fasting Plasma Glucose (mg/dL) Change from Baseline.
Figure 22. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Body Weight (kg) Change from Baseline.
Figure 23. Funnel Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 for Time with Glucose in Range (%).
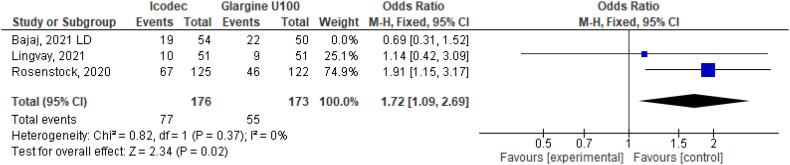
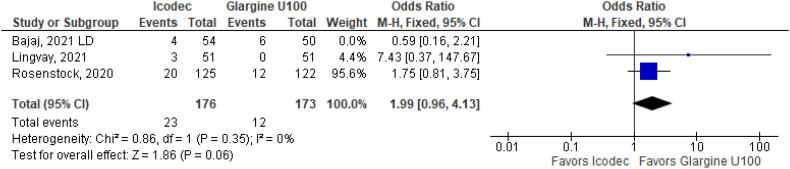
We conducted a sensitivity analysis, to assess the impact of individual studies in our results. When Bajaj study (6) was excluded from the analysis, Once-Weekly Insulin Icodec presented with higher odds ratio for Hypoglycemia Alert (OR 1.72 CI 1.09 to 2.69; P = 0.02; I2 = 0%; Figure 35). Nevertheless, Clinically Significant or Severe Hypoglycemia (OR 1.99 CI 0.96 to 4.13; P = 0.06; I2 = 0%; Figure 36) was still not significant after excluding Bajaj study (6). In addition, only Rosenstock study (4) contributed to hypersensitivity reaction outcome, since there were no events registered in Bajaj (6) or Lingvay (5) studies No more outcomes were altered by individual studies. Sensitivity analysis excluding Bajaj study is contained in Figures 25–36.
Figure 35. Odds Ratio of Hypoglycemia Alert for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 36. Odds Ratio of Clinically Significant or Severe Hypoglycemia for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 25. Mean Difference of Time with Glucose in Range (%) for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 26. Mean Difference of HBa1C (%) Change from Baseline for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 27. Mean Difference of Fasting Plasma Glucose (mg/dL) Change from Baseline for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 28. Mean Difference of Body Weight (kg) Change from Baseline for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 29. Mean Difference of Insulin Dose Difference (UI) for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 30. Odds Ratio of Any Adverse Outcome for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 31. Odds Ratio of Serious Adverse Event for Forrest Plot of Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 32. Odds Ratio of Any Adverse Event Probably related to Basal Insulin for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6)*.
Figure 33. Odds Ratio of Injection Site Reaction for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
Figure 34. Odds Ratio of Hypersensitivity Reaction for Once-Weekly Icodec vs. Once-Daily Insulin Glargine U100 without Bajaj study (6).
DISCUSSION
In this systematic review and meta-analysis of 3 Phase 2 RCTs and 454 patients, we compared Once-Weekly Insulin Icodec with Once-Daily Insulin Glargine U100 in patients with Diabetes Mellitus Type 2. The main findings were as follows: (1) There was a 0.20% grater reduction of Glycated Hemoglobin from baseline in the Icodec group. (2) Insulin Icodec increased Time with Glucose in Range with a mean difference of 6.60%. (3) Patients in the Icodec group needed a higher weekly dose when compared with the Glargine group, with a mean difference of 0.97 UI. (4) There was no difference in regards to safety endpoints.
Insulin Icodec has stable pharmacokinetic and pharmacodynamic profiles supporting once-weekly administration. This can be attributed to a strong reversible albumin binding, slow receptor-mediated clearance and reduced enzymatic degradation (3,10). After subcutaneous injection, Icodec is absorbed and into the circulation and binds to albumin to form an essentially inactive depot. Thus, Icodec molecules slowly reaches insulin receptors to stimulate glucose lowering at target tissues. After each weekly injection, the pool of albumin-bound Icodec gradually increases, until steady state is reached after approximately 1 month when the full glucose lowering effect is achieved and insulin clearance matches administered insulin dose (3,10).
Concerning the frequency of injections, the reduction to weekly insulin could proportionate the same results observed with the advent of injectable once-weekly glucagon-like peptide 1 (GLP-1) (11). Studies comparing once-weekly and once-daily injectable GLP-1 showed increase in treatment adherence and patient satisfaction (11,12). The same benefits were seen in patients who never had injectable treatment and the ones previously treated who switched from daily to weekly injections (12). Although patient satisfaction is an important outcome for this new treatment, it was not evaluated in the included studies, but similar benefits can emerge from it. Furthermore, fewer injections might facilitate treatment initiation in patients with type 2 diabetes who have not previously taken insulin, by reducing clinical inertia and promoting better acceptance of insulin therapy (4).
The recommended mean TIR by the International Consensus is > 70% (13). In addition, it is known that each 5% incremental in TIR is associated with clinically significant benefits (13-16). After evaluating 1.440 participants, Beck and cols. (14) found that a reduction of 10% in TIR increased the hazard ratio of retinopathy in 64% and microalbuminuria in 40% (P < 0.001). Therefore, Once-Weekly Insulin Icodec presented with a notable 6.60% increase in TIR compared with Once-Daily Insulin Glargine U100, result that could indicate a potential reduction in diabetes complications.
The included studies were only Phase 2 RCTs, not powered to detect significant differences between treatments for any endpoint. This limited our data to a restrict sample of patients and excluding several subgroup of interest, such as patients with chronic kidney disease and patients using sulfonylureas. In this context, there is a need for larger Phase 3 RCTs to demonstrate the effect and safety profile of Insulin Icodec with greater quality of evidence. To this date, there are 22 RCTs registered on NIH clinical trials registry, 3 of them being the subject of study of the meta-analysis and 8 trials are already completed and awaiting the results (CT.gov).
Lingvay study (5) evaluated three different Icodec titration algorithms, divided by prebreakfast self-measured glucose target and weekly insulin dose adjustment. We included Icodec titration B in our analysis, since this group used a 28 unit/week adjustment and a prebreakfast target of 80-130 mg/dL which aligns with ADA recommendations (17). Moreover, titration C adopted a different target than Glargine U100 group, thus could add bias to the analysis, and titration A used a 21 unit/week adjustment, which differed from Rosenstock and Bajaj studies. In this context, a similar target of 70-130 mg/dL was used by Bajaj study (6), while Rosenstock study (4) adopted a tighter target, ranging from 70-108 mg/dL. Regarding insulin dose adjustment, all three studies applied a 28 unit/week adjustment.
Furthermore, Bajaj study (6) included patients with TDM2 already using basal insulin therapy, in contrast to Rovenstock (4) and Lingvay (5). As a result, there could be changes in the response to insulin therapy if a patient has been previously treated, thus could have influenced our results. In the Escalada study (18), insulin naive patients who initiated treatment with Glargine U100 had a slight increase in hypoglycemia, while insulin experienced patients who switched from any basal insulin to Glargine U100 showed less episodes of the outcome. Therefore, patients initiating treatment could be more susceptible to hypoglycemic episodes, due to an initial adapting period and the lack of knowledge in hypoglycemia prevention. Moreover, once weekly titration might difficult insulin dose adjustment, especially for naive patients, predisposing them to hypoglycemic episodes in the beginning of the treatment.
In addition, Bajaj study (6) compared two separate Icodec treatment regiments, with and without an initial loading dose. Since Insulin Icodec has a half-life of 1 week and reaches its steady state around 3-4 weeks, the cessation of a previous basal insulin could decompensate glycemic control (5,6). Therefore, an initial loading dose could minimize the impact of this transition. In this sense, we chose to include the loading dose group in our meta-analysis (6).
Early glucose control has been associated with a reduction of microvascular complications and MACE due to glycemic legacy (metabolic memory) (19). A 10-year post-intervention follow-up of the UKPDS showed cardiovascular benefit among patients with newly diagnosed T2DM who had been assigned to intensive glycaemic control (20). Knowing the highly prevalent clinical inertia for insulin initiation, with a delay of up to 4.9 years, a potential reduction in this time might lead to an improvement in these outcome (5). Furthermore, tighter goals might be sought in selected patients, such as short diabetes duration, long life expectancy and without evidence of cardiovascular disease, despite HbA1c recommended target being ≤ 7%. Therefore, the possibility of decreasing clinical inertia, associated with a greater reduction in HbA1c without an increase in hypoglycemia, could show great promise in TDM2 management (19).
Considering the reduction of only 0.2% in glycated hemoglobin and that the costs entailed to Once-Weekly Icodec were not disclosed, a cost benefit analysis should be conducted in a public health context (19). In this regard, Perez-Nieves and cols. evaluated the impact of basal insulin in patient adherence and heath costs. A reduction in total costs was observed with basal insulin due to higher adherence besides an initial large cost by reducing complications and hospitalizations over a period of 3 years (21).
Although methodological rigor was followed throughout the conduction if the study, the present review may have other limitations. Our research strategy included only studies in English and did not cover small data bases for the presence of RCT comparing insulin Icodec and Glargine U100 in patients with TDM2. Also, the authors of the original studies were not contacted for additional data. Nevertheless, we believe that none of this limitations would change the conclusions of this review.
In conclusion, Once-Weekly Insulin Icodec was associated with a small reduction in Glycated Hemoglobin, as well as higher TIR, with similar hypoglycemic events, in comparison to Glargine U100. The results from Phase 3 RCTs, as well as individual studies for patients with previous basal insulin therapy, could help measure the efficacy and safety of Insulin Icodec henceforth.
Acknowledgements:
the authors would like to thank Rhanderson Cardoso, MD, for the support in conducting the research.
Footnotes
Data sharing agreement: because this meta-analysis was based on data extracted from previously published research, all of the data and study materials are available in the public domain. The authors of this meta-analysis do not have access to patient-level data of the individual studies. Researchers with an interest in individual-level data from the studies included in this meta-analysis are encouraged to contact the corresponding author from each individual study for such requests.
REFERENCES
- 1.American Diabetes Association Introduction: Standards of Medical Care in Diabetes – 2022. Diabetes Care. 2022;45(Suppl 1):S1–S2. doi: 10.2337/dc22-Sint. [DOI] [PubMed] [Google Scholar]
- 2.Retnakaran R, Zinman B. The ongoing evolution of basal insulin therapy over 100 years and its promise for the future. Diabetes Obes Metab. 2022;24(S1):17–26. doi: 10.1111/dom.14552. [DOI] [PubMed] [Google Scholar]
- 3.Hövelmann U, Brøndsted L, Kristensen NR, Ribel-Madsen R, Devries JH, Heise T, et al. 237-or: insulin icodec: an insulin analog suited for once-weekly dosing in type 2 diabetes. Diabetes. 2020;69(Suppl 1):237-OR–237-OR. [Google Scholar]
- 4.Rosenstock J, Bajaj HS, Janež A, Silver R, Begtrup K, Hansen MV, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. 2020;383(22):2107–2116. doi: 10.1056/NEJMoa2022474. [DOI] [PubMed] [Google Scholar]
- 5.Lingvay I, Buse JB, Franek E, Hansen MV, Koefoed MM, Mathieu C, et al. A randomized, open-label comparison of once-weekly insulin icodec titration strategies versus once-daily insulin glargine U100. Diabetes Care. 2021;44(7):1595–1603. doi: 10.2337/dc20-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bajaj HS, Bergenstal RM, Christoffersen A, Davies MJ, Gowda A, Isendahl J, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: a phase 2 randomized controlled trial. Diabetes Care. 2021;44(7):1586–1594. doi: 10.2337/dc20-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898–l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 8.Cochrane Training Cochrane handbook for systematic reviews of interventions. [Accessed on: June 3, 2022]. Available from: https://training.cochrane.org/handbook .
- 9.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishimura E, Pridal L, Glendorf T, Hansen BF, Hubálek F, Kjeldsen T, et al. Molecular and pharmacological characterization of insulin icodec: a new basal insulin analog designed for once-weekly dosing. BMJ Open Diab Res Care. 2021;9(1):e002301. doi: 10.1136/bmjdrc-2021-002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mody R, Huang Q, Yu M, Zhao R, Patel H, Grabner M, et al. Adherence, persistence, glycaemic control and costs among patients with type 2 diabetes initiating dulaglutide compared with liraglutide or exenatide once weekly at 12‐month follow‐up in a real‐world setting in the United States. Diabetes Obes Metab. 2019;21(4):920–929. doi: 10.1111/dom.13603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takase T, Nakamura A, Yamamoto C, Nomoto H, Miya A, Dannoura M, et al. Improvement in treatment satisfaction after switching from liraglutide to dulaglutide in patients with type 2 diabetes: A randomized controlled trial. J Diabetes Investig. 2019;10(3):699–705. doi: 10.1111/jdi.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–405. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, et al. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018;41(11):2370–2376. doi: 10.2337/dc18-1131. [DOI] [PubMed] [Google Scholar]
- 16.Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020;22(2):72–78. doi: 10.1089/dia.2019.0251. [DOI] [PubMed] [Google Scholar]
- 17.American Diabetes Association 6. Glycemic targets: standards of medical care in diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S73–S84. doi: 10.2337/dc21-S006. [DOI] [PubMed] [Google Scholar]
- 18.Escalada J, Bonnet F, Wu J, Bonnemaire M, Gupta S, Cambron-Mellott JM, et al. Reduced hypoglycemia risk in type 2 diabetes patients switched to/initiating insulin glargine 300 vs 100 u/ml: a European Real-World Study. Adv Ther. 2020;37(9):3863–3877. doi: 10.1007/s12325-020-01436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valensi P, Prévost G, Schnell O, Standl E, Ceriello A. Targets for blood glucose: What have the trials told us. Eur J Prev Cardiol. 2019;26(2 Suppl):64–72. doi: 10.1177/2047487319885456. [DOI] [PubMed] [Google Scholar]
- 20.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Nieves M, Boye KS, Kiljanski J, Cao D, Lage MJ. Adherence to Basal Insulin Therapy Among People with Type 2 Diabetes: A Retrospective Cohort Study of Costs and Patient Outcomes. Diabetes Ther. 2018;9:1099–1111. doi: 10.1007/s13300-018-0421-5. [DOI] [PMC free article] [PubMed] [Google Scholar]