Abstract
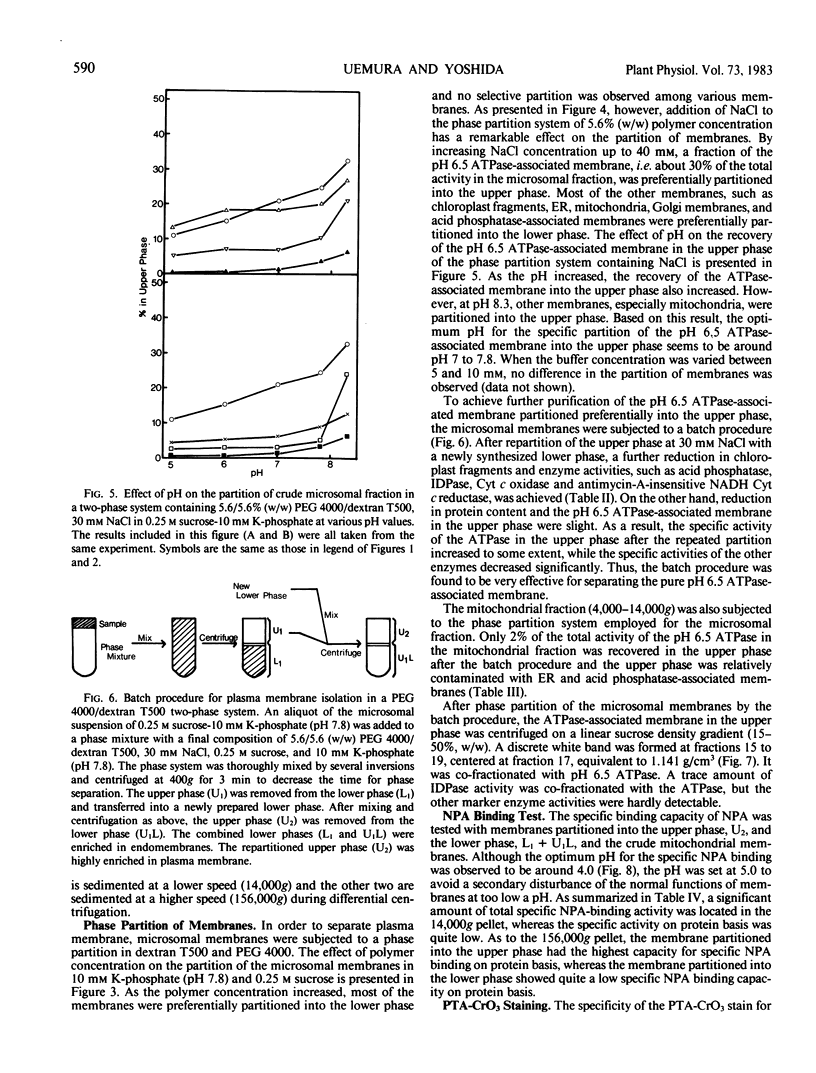
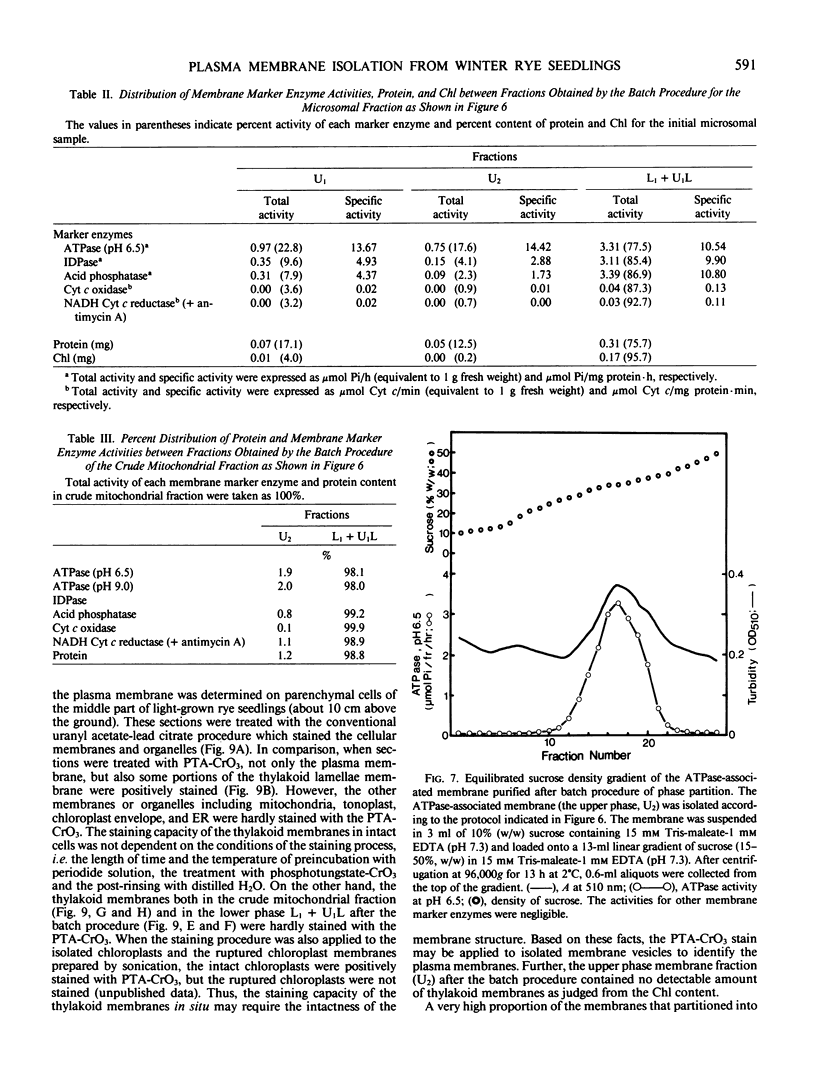
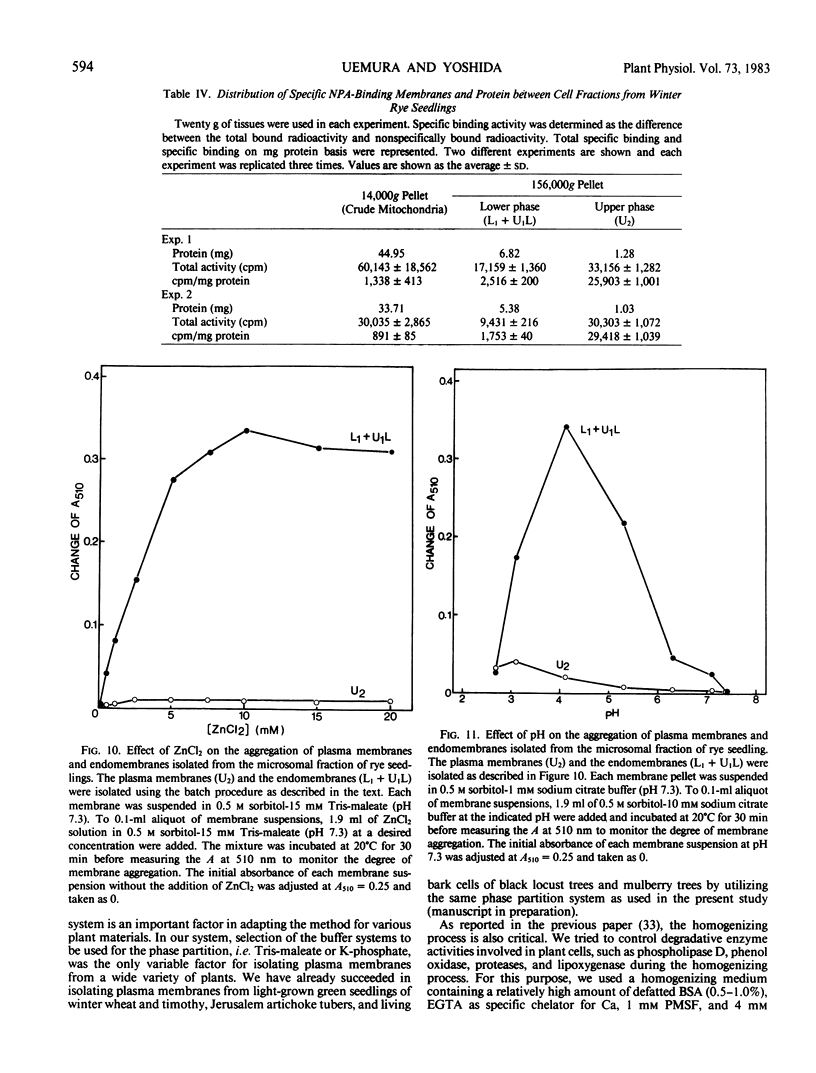
An effective method for the isolation of plasma membrane from light-grown winter rye seedlings (Secale cereale L. cv Puma) was established using a liquid two-polymer phase separation. The conditions for the specific partition of plasma membrane into the polyethylene glycol-enriched upper phase were examined, including variations in the polymer concentration, buffer system, pH, and NaCl addition in the phase partition system. The most effective phase partition system for the isolation of plasma membrane from winter rye consisted of 5.6/5.6% (w/w) polyethylene glycol 4000/dextran T500 in 0.25 molar sucrose-10 millimolar potassium phosphate-30 millimolar NaCl (pH 7.8), repeated once. When the isolated plasma membrane was centrifuged on a linear sucrose density gradient, a single band was found at the 34% (w/w) sucrose layer (1.141 grams per cubic centimeter) which co-fractionated with the pH 6.5-ATPase.
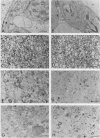
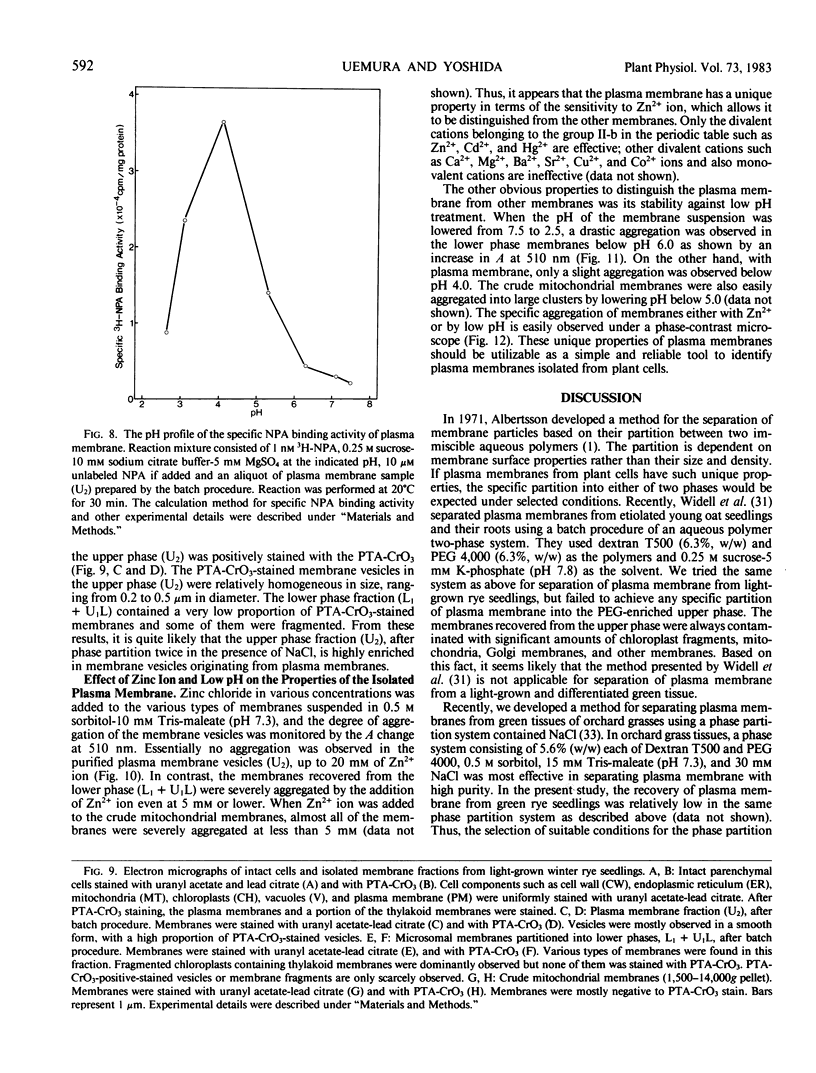
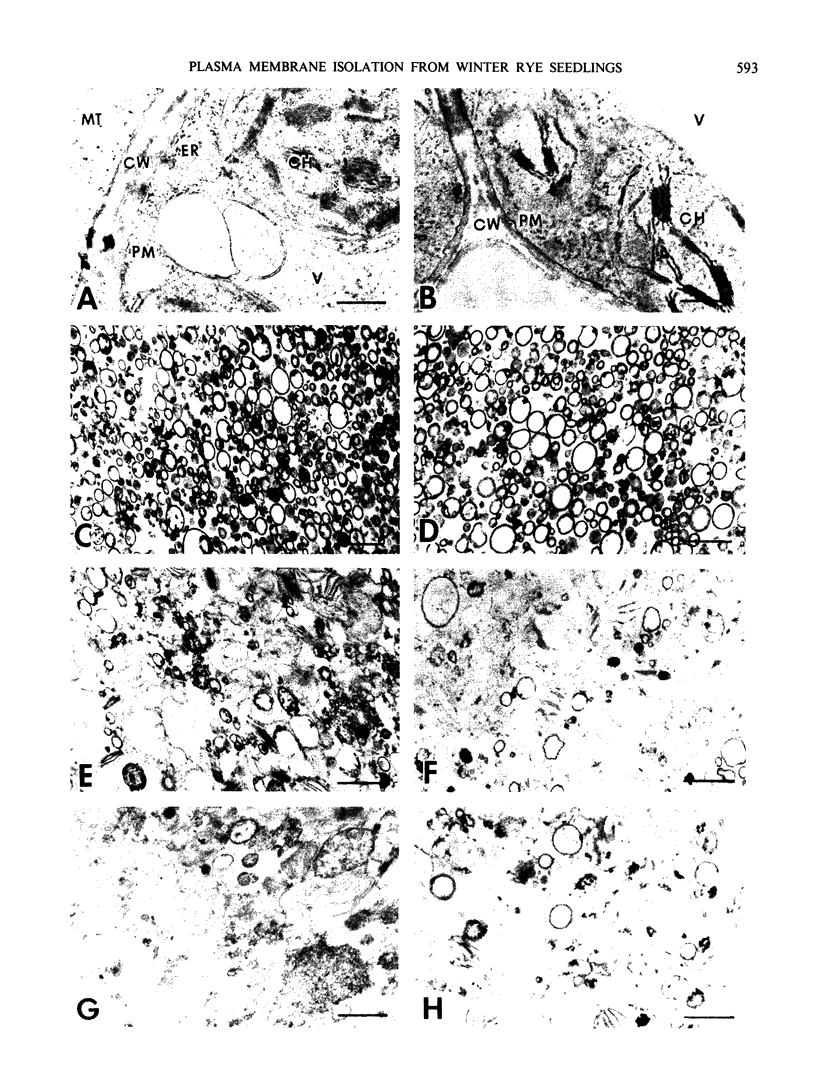
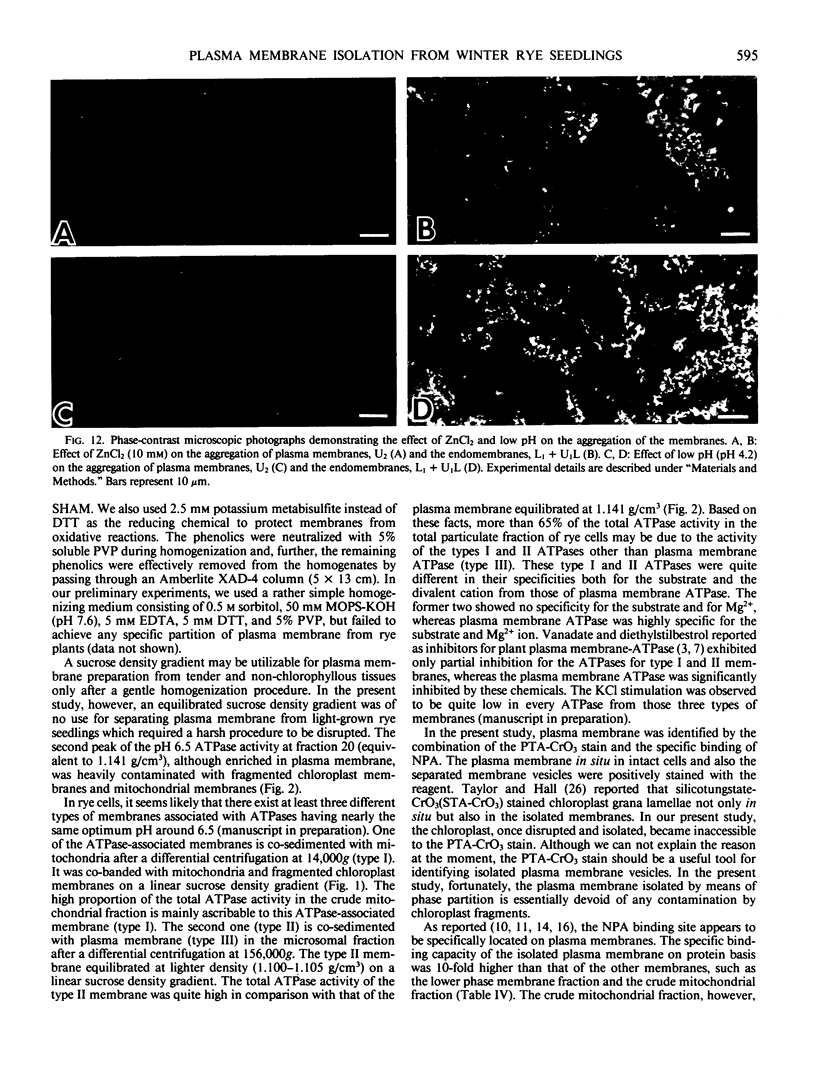
Identification of plasma membrane was performed by the combination of phosphotungstic acid-chromic acid stain and specific binding of N-1-naphthylphthalamic acid. Based on morphometrical observations after phosphotungstic acid-chromic acid stain, the isolated plasma membrane consisted mostly of vesicles of high purity. The isolated plasma membrane also showed extremely high specificity for N-1-naphthylphthalamic acidbinding, 10-fold higher than other membranes. It was also confirmed that there is a distinct difference in properties between plasma membrane and other membranes. The endomembranes such as from chloroplasts, mitochondria, and endoplasmic reticulum were observed to be highly sensitive to Zn2+ ion and lower pH, which resulted in an abrupt aggregation of membranes. On the contrary, plasma membrane was very stable to these treatments and no aggregation was observed. These unique properties of isolated plasma membrane are generally observed in a wide variety of plant species and can be utilized for the assessment of the purity of preparations of isolated plasma membranes and for their identification.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke N. E., Hodges T. K. Inhibition of adenosine triphosphatase activity of the plasma membrane fraction of oat roots by diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):48–52. doi: 10.1104/pp.63.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barland P., Schroeder E. A. A new rapid method for the isolation of surface membranes from tissue culture cells. J Cell Biol. 1970 Jun;45(3):662–668. doi: 10.1083/jcb.45.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boss W. F., Ruesink A. W. Isolation and Characterization of Concanavalin A-labeled Plasma Membranes of Carrot Protoplasts. Plant Physiol. 1979 Dec;64(6):1005–1011. doi: 10.1104/pp.64.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Boehm C., Theuvenet A. P. Sugar transport and potassium permeability in yeast plasma membrane vesicles. Biochim Biophys Acta. 1976 May 21;433(3):583–596. doi: 10.1016/0005-2736(76)90283-2. [DOI] [PubMed] [Google Scholar]
- Galbraith D. W., Northcote D. H. The isolation of plasma membrane from protoplasts of soybean suspension cultures. J Cell Sci. 1977 Apr;24:295–310. doi: 10.1242/jcs.24.1.295. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Narayanan K. R., Mudge K. W., Poovaiah B. W. Demonstration of auxin binding to strawberry fruit membranes. Plant Physiol. 1981 Dec;68(6):1289–1293. doi: 10.1104/pp.68.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan K. R., Mudge K. W., Poovaiah B. W. In vitro auxin binding to cellular membranes of cucumber fruits. Plant Physiol. 1981 Apr;67(4):836–840. doi: 10.1104/pp.67.4.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin D. S., Spanswick R. M. Labeling and isolation of plasma membranes from corn leaf protoplasts. Plant Physiol. 1980 Jun;65(6):1053–1057. doi: 10.1104/pp.65.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Travis R. L., Booz M. L. Partial Characterization of a Potassium-stimulated Adenosine Triphosphatase from the Plasma Membrane of Meristematic and Mature Soybean Root Tissue. Plant Physiol. 1979 Mar;63(3):573–577. doi: 10.1104/pp.63.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L. Isolation of plasma membrane from tissue culture--L cells. Methods Enzymol. 1974;31:156–162. [PubMed] [Google Scholar]
- Widell S., Lundborg T., Larsson C. Plasma membranes from oats prepared by partition in an aqueous polymer two-phase system : on the use of light-induced cytochrome B reduction as a marker for the plasma membrane. Plant Physiol. 1982 Nov;70(5):1429–1435. doi: 10.1104/pp.70.5.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S. Freezing injury and phospholipid degradation in vivo in woody plant cells: I. Subcellular localization of phospholipase d in living bark tissues of the black locust tree (robinia pseudoacacia L.). Plant Physiol. 1979 Aug;64(2):241–246. doi: 10.1104/pp.64.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Uemura M., Niki T., Sakai A., Gusta L. V. Partition of membrane particles in aqueous two-polymer phase system and its practical use for purification of plasma membranes from plants. Plant Physiol. 1983 May;72(1):105–114. doi: 10.1104/pp.72.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]