Abstract
West Nile virus (WNV) is a re-emerging zoonotic pathogen with increasing incidence in Europe, producing a recent outbreak in 2020 in Spain with 77 human cases and eight fatalities. However, the factors explaining the observed changes in the incidence of WNV in Europe are not completely understood. Longitudinal monitoring of WNV in wild animals across Europe is a useful approach to understand the eco-epidemiology of WNV in the wild and the risk of spillover into humans. However, such studies are very scarce up to now. Here, we analysed the occurrence of WNV and Usutu virus (USUV) antibodies in 2102 samples collected between 2005 and 2020 from a population of feral horses in Doñana National Park. The prevalence of WNV antibodies varied between years, with a mean seroprevalence of 8.1% (range 0%–25%) and seasonally. Climate conditions including mean minimum annual temperatures and mean rainy days per year were positively correlated with WNV seroprevalence, while the annual rainfall was negatively. We also detected the highest incidence of seroconversions in 2020 coinciding with the human outbreak in southern Spain. Usutu virus-specific antibodies were detected in the horse population since 2011. The WNV outbreak in humans was preceded by a long period of increasing circulation of WNV among horses with a very high exposure in the year of the outbreak. These results highlight the utility of One Health approaches to better understand the transmission dynamics of zoonotics pathogens.
Keywords: Arboviruses, Emerging infectious diseases, Equus caballus, Flavivirus, Longitudinal studies
Graphical abstract
1. Introduction
West Nile virus (WNV) is a widespread zoonotic pathogen that belongs to the Flaviviridae family and can affect wild animals, livestock and humans [1]. Culex mosquitoes are considered the primary global vectors of WNV while birds act as reservoirs [2]. Most mammals, including humans, are considered dead-end hosts because they develop low levels of viremia, preventing mosquito infection when they feed on their blood [3]. WNV infections in humans rarely (<1% of cases) result in clinical disease but infection symptoms may range from fever and myalgia to meningoencephalitis that cause fatalities [4]. These same symptoms can be detected in horses, where approximately 20% of WNV infections are symptomatic with fever or severe neurological signs [5]. Mortality rates are up to 30% in horses with clinical disease, but this rate can reach 50% in unvaccinated animals [6].
WNV is considered a re-emerging pathogen in Europe, with an increasing but highly variable annual incidence [7]. In Spain, local circulation of WNV has been reported since 2003, mainly in southern Spain, through seroconversion of resident birds and horses [[8], [9], [10]], and the detection of the virus or its genome in mosquitoes and birds [11,12]. Human cases were sporadically detected in the region (one clinical case in 2004, two in 2010 and three in 2016) until 2020 when an outbreak occurred with 77 clinical cases and eight fatalities [13,14]. Various climate factors have been associated with the incidence of WNV in Europe, more importantly, temperature, precipitation, and Normalized Difference Vegetation Index (NDVI) (reviewed in [15,16]). However, longitudinal studies analysing the incidence of WNV in relation to climate conditions are scarce in the world [17] and, to our knowledge, none has been done in Europe. In addition, in the last years other flaviviruses with zoonotic potential are also circulating in Europe. One of them is Usutu virus (USUV), which was first detected in Europe in Austria in 2001 [18]. In Spain the virus was detected in mosquitoes in 2006 [19] and since 2009 the virus has been repeatedly reported in South-West Spain in mosquitoes [[20], [21], [22]]. While most infections in humans are asymptomatic, an increasing number of USUV positive blood donations and cases of encephalitis associated to USUV infections is being reported in Europe [18].
In this study we analysed the seroprevalence of WNV and occurrence of USUV antibodies in horses' sera over a period of 16 years in an area close to the 2020 WNV outbreak. These analyses allowed us to: i) identify the individual horse characteristics associated to WNV seroprevalence, ii) assess the relationship between different climate variables and WNV seroprevalence in horses, iii) study the association between WNV serology in horses and the occurrence of WNV disease cases in humans and iv) determine the exposure of the horses to USUV virus during the study.
2. Materials and methods
2.1. Study area, sampling and data collection
This study was conducted at the Doñana National Park (Spain). This is a highly preserved area where the Doñana Biological Station manages two large stances: the Reserva Biológica de Doñana (RBD) with 6794 ha and the Reserva Biológica del Guadiamar (RBG) with 3214 ha. Approximately 120–150 horses of the autochthonous Retuertas breed are kept in each of these areas under a free-ranging regime. Horses are individually marked with numbered ear tags and pass regular veterinary examinations. None of the horses have been vaccinated against WNV nor treated with ivermectin.
We collected 2102 blood samples from 768 individuals (ranging one to eleven samples/individual) over a 16-year (2005–2020) period. Blood samples were collected during the veterinary health controls, one or two times per year, except for 2006, when no samples were collected. Blood was drawn from the jugular vein using sterile syringes and was allowed to clot and then was placed at 4 °C overnight. Next morning, samples were centrifuged at 1700 xg for 15 min to separate the serum from the cellular fraction. Serum samples were stored at −80 °C until tested for the presence of antibodies against WNV and USUV.
Temperature and precipitation records for the study period were obtained from the closest meteorological station, located at the Palacio de Doñana (http://icts.ebd.csic.es/es/datos-meteorologicos?inheritRedirect=true). We analysed different climatic variables previously related with West Nile virus circulation [23,24], supplementary Table 1).
2.2. Antibody detection assays
From 2005 to 2009, we analysed all sera for WNV antibodies using a virus-neutralization test (VNT), that is the gold standard method for serological diagnosis of WNV recommended by the World Organization for Animal Health (WOAH, formerly OIE). The VNT was performed as described in [25], using in parallel WNV and USUV as antigens in order to identify cross-reactions and demonstrate WNV specificity in the serum samples analysed. Strains used as antigens in the VNT were: WNV strain Eg-101 and USUV SAAR-1776 (GenBank accession nos. AF260968 and AY453412, respectively). Only samples yielding positive neutralization (complete absence of cpe) at dilutions equal or higher than 1:10 were scored as positives, and the antibodies were only assigned as specific to WNV (or USUV) when VNT titre was at least fourfold higher than the titre obtained for the other virus [26]. If the titre differences did not reach this threshold the sample was scored as “undetermined flavivirus.”
From 2010 to 2020 sera was first analysed with an ELISA kit [27], that was made available commercially at that time (Ingezim West Nile Compac, Ingenasa, Spain), as an initial screening for WNV antibodies. Then, in positive and doubtful samples VNT was carried out as described above to confirm the results and specificity to WNV or USUV. Between 2010 and 2014 a third flavivirus, Bagaza virus (BAGV), was used in the VNT in parallel to WNV and USUV because Bagaza was found circulating in Cádiz in 2010 [28]. However, none of the sera tested was positive for Bagaza.
2.3. Statistical analysis
We used generalized linear mixed-effects models (GLMM) with a ‘logit’ link function and binomial distribution to test for the effect of horse sex (male and female), age class (foals:<1 year; young: 1–5 years; adult: 5–15 years; and aged: >15 years), locality (RBD and RBG), and season (spring, summer, and autumn) on the horse serological status (positive or negative for the presence of antibodies against WNV). The year of sampling and horse identity (ID) was included as a random factor to account for the temporal stratification and for the multiple samples coming from the same individuals. The statistical significance of factors and post-hoc test was tested with ANOVA type II chi-square tests and Least-squares means respectively. Prevalence was estimated from the back-transformed lsmeans estimates to control for the effects of the variables included in the statistical models. USUV occurrence was not tested statistically because the ELISA used is specific to WNV but not to USUV, and consequently some sera with USUV antibodies may have remained undetected.
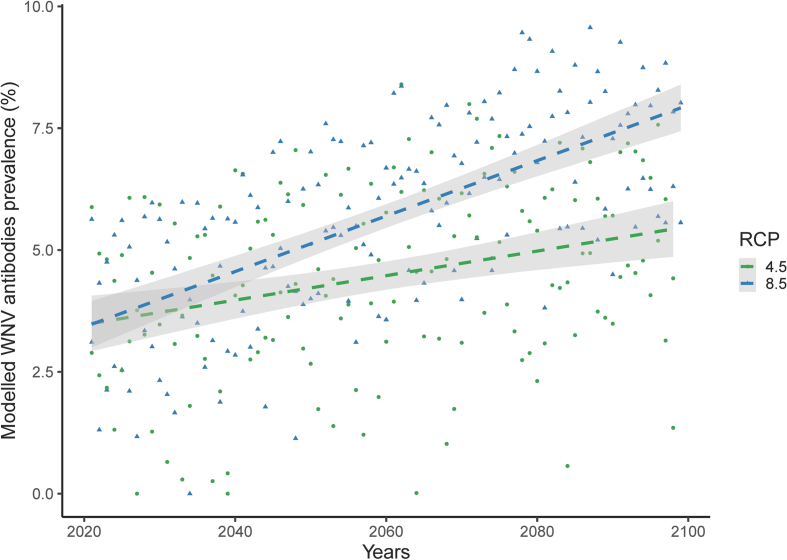
In addition, we ran independent GLMM models to analyse the relationship between climate factors and WNV seroconversion. We analysed data from 609 individuals, with each individual contributing only one sample. The samples included in the analysis were either collected from foals during their first year of life (485 individuals) or from individuals sampled in two consecutive years that were seronegative in the first capture and consequently the year of seroconversion can be determined from the second capture (124 individuals). Note that in this case the horses can be again seronegative or had seroconverted between first and second capture. The serological status was included as the response variable, while sex, locality, and season and climate variables (supplementary Table 1) were included as the independent variables. We used forward stepwise selection to fit a final model that included only the climate predictor variables that were significantly related to the response variable. To illustrate the estimated effects of climate change on WNV seroprevalence in horses we projected the results of the GLMM using climatic variables estimated for the period 2020–2100 (Supplementary Fig. 1). We considered two different greenhouse gas emission scenarios representative concentration pathways (RCP 4.5) and (RCP 8.5).The former assumes that global annual emissions will peak around 2040, with emissions declining thereafter, while in the latter emissions continue to rise throughout the twenty-first century [29].
The collinearity between independent variables of GLMM was tested with the variance inflation factor (VIF) [30]. VIF values were lower than five in all the cases. Overdispersion did not deviate significantly from one as estimated by the Pearson statistic. All statistical analyses and figures were done in R (v. 3.6.3; The R Foundation for Statistical Computing Platform 2020) using the packages: arm, car, lme4, MuMIn, multcomp, MASS, Matrix, Rcpp, stats, nortest, lmerTest, multcompView, emmeans, lsmeans, optimx texreg, mctest, dplyr, and ggplot2.
3. Results
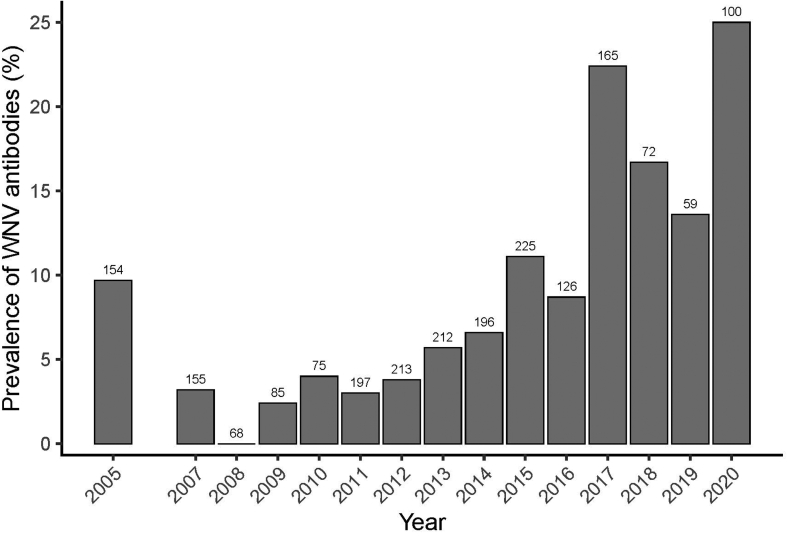
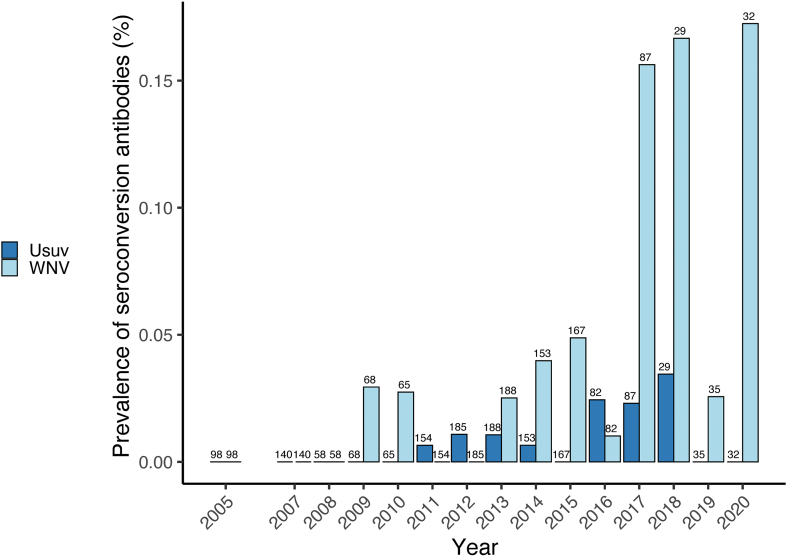
Of the 2102 samples tested, 462 were analysed only by VNT between 2005 and 2009, of which 22 were positives. From 2010 to 2020 we tested 1640 samples by ELISA, 51 were identified as doubtful and 275 were identified as positive. Positive and doubtful samples were subjected to VNT analysis showing that in total 259 were positives (Table 1). Prevalence of WNV antibodies detected by VNT varied between years, ranging from 0.00% in 2008 to 25.00% in 2020 (Fig. 1).
Table 1.
Number (and percentage) of samples with antibodies specific for WNV, USUV or for undetermined flavivirus according to VNT titres against WNV and USUV.
| TITRES | WNV | USUV | FLAVIVIRUS |
|---|---|---|---|
| 1:10 | 10 (5.49) | 10 (32.26) | 10 |
| 1:20 | 21 (11.54) | 12 (38.71) | 16 (34.78) |
| 1:40 | 36 (19.78) | 4 (12.90) | 5 (10.87) |
| 1:80 | 26 (14.29) | 1 (3.23) | 5 (10.87) |
| 1:160 | 41 (22.53) | 3 (9.68) | 8 (17.39) |
| 1:320 | 23 (12.64) | 0 (0) | 2 (4.35) |
| 1:640 | 23 (12.64) | 0 (0) | 0 (0) |
| >1:640 | 2 (1.10) | 1 (3.23) | 0 (0) |
| TOTAL | 182 (70.27) | 31(11.96) | 46 (17.76) |
Fig. 1.
WNV antibody prevalence in feral horses monitored in Doñana between 2005 and 2020. Numbers above bars indicate sample sizes.
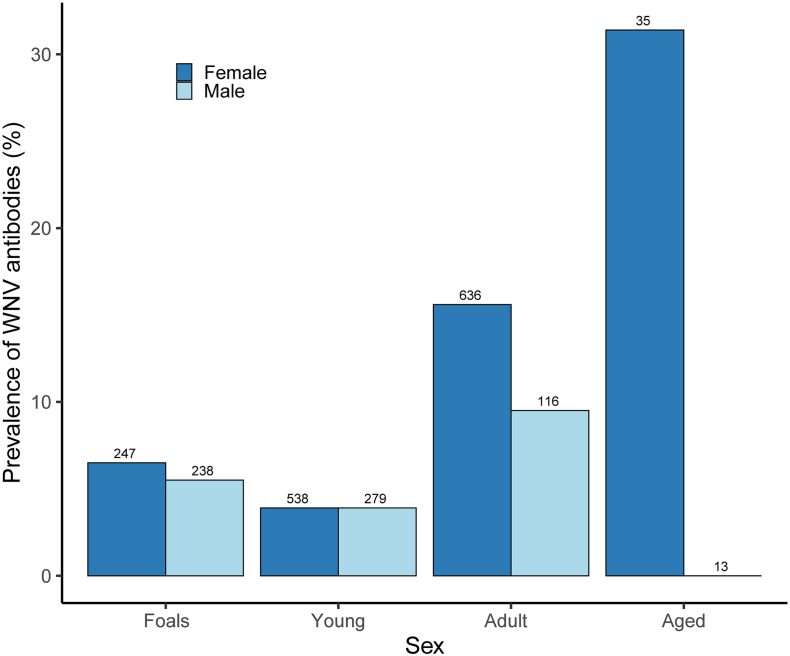
Overall, the prevalence of WNV antibodies varied according to the age of the horses (χ2 = 33.99, df = 3, p < 0.01, Table 2) and between seasons (χ2 = 7.91, df = 2, p = 0.02 Table 2). In particular, the highest prevalence of WNV antibodies was found in aged and adult horses, being significantly different from the other age categories and period being significantly higher in autumn (Table 2 and supplementary Table 2). Surprisingly, no male horses of the aged category (N = 13) were detected with antibodies against WNV, while 11 of 35 aged females had WNV antibodies (Fig. 2). >90% of the sera with USUV antibodies were from adult female horses, and at RBD we detected more than double of horses with antibodies against USUV than at RBG.
Table 2.
Results of the GLMM analysing relationships between the seroprevalence of WNV antibodies and the characteristics of horses (sex, age, period, and locality of sampling) (N = 2102). Post-hoc tests of differences of means between groups are shown. Different letters indicate groups showing significant differences.
| Variable | Category | Estimate | Std. error | Z value | PR(>|Z|) | Post HOC |
|---|---|---|---|---|---|---|
| Intercept | −1.72 | 0.569 | −3.025 | 0.002* | ||
| Age | Adult | 0(a) | A | |||
| Aged | 0.678 | 0.678 | −0.810 | 0.418 | A | |
| Young | −1.841 | 0.330 | −5.573 | <0.001*** | B | |
| Foals | −1.075 | 0.361 | −2.978 | 0.003** | B | |
| Sex | Female | 0(a) | A | |||
| Male | −0.316 | 0.389 | −0.810 | 0.417 | A | |
| Period | Autumn | 0(a) | A | |||
| Spring | −0.722 | 0.887 | −0.814 | 0.415 | AB | |
| Summer | −0.960 | 0.341 | −2.812 | 0.005** | B | |
| Locality | RBD | 0(a) | A | |||
| RBG | −0.218 | 0.342 | −0.639 | 0.523 | A |
Reference category.
Fig. 2.
WNV antibody prevalence in feral horses monitored in Doñana according to sex and age class. Numbers above bars indicate sample sizes.
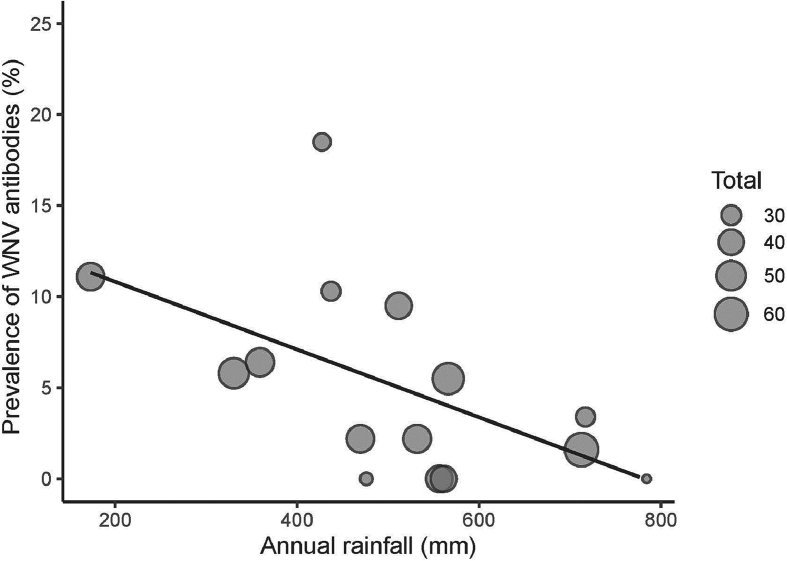
Using the dataset of horses sampled in two consecutive years or during their first year of life, we found that the seroprevalence of WNV antibodies was not significantly related to host sex, locality, season and annual maximum temperature (Table 3). In contrast, annual minimum temperature (χ2 = 5.74, df = 1, p = 0.02) was positively correlated with WNV seroprevalence (Fig. 3). The prevalence of antibodies was also negatively associated with annual rainfall (χ2 = 10.20, df = 1, p < 0.01) (Fig. 4) while number of rainy days show a positive relationship with seroprevalence (χ2 = 5.01, df = 1, p = 0.03). According to the minimum temperatures, annual rainfall and number of rainy days in the study area forecasted by the scenarios RCP 4.5 and RCP 8.5 (Supplementary Fig. 1), we projected the variation in the prevalence of WNV antibodies under climate change scenarios for the period 2020–2100. The model predictions indicate that mean WNV seroprevalence in the study area will increase around 3% in the more favourable and 6% in the less favourable climate change scenarios (Fig. 4).
Table 3.
Results of the GLMM analysing the relationships between the seroprevalence of WNV infection in foals and sex, period and locality of sampling, and climate variables (mean annual temperature, mean maximum annual temperature and annual rainfall) (N = 485).
| Variables | Category | Estimate | Std. error | Z Value | PR(>|Z|) |
|---|---|---|---|---|---|
| (Intercept) | −10.830 | 7.503 | −1.443 | 0.148 | |
| Sex | Female | 0(a) | |||
| Male | −0.071 | 0.392 | −0.182 | 0.855 | |
| Period | Autumn | 0(a) | |||
| Spring | 0.704 | 0.751 | 0.937 | 0.348 | |
| Summer | −0.149 | 0.734 | −0.204 | 0.838 | |
| Locality | RBD | 0(a) | |||
| RBG | −0.229 | 0.443 | −0.516 | 0.605 | |
| Climate | |||||
| Annual minimum temperature | 0.956 | 0.399 | 2.395 | 0.016* | |
| Annual maximum temperature | 0.579 | 0.324 | 1.785 | 0.074· | |
| Annual rainfall | −0.2.994 | 0.937 | −3.194 | 0.001** | |
| Mean Rainy days | 7.596 | 3.393 | 2.239 | 0.025* |
Reference category.
Fig. 3.
Bubble plot showing the relationship between WNV seroprevalence (%) and annual minimum mean temperature (A) or annual rainfall (mm) (B). N is the total number of foal horses tested.
Fig. 4.
Percentage of change of prevalence of WNV antibodies in the period 2020–2100 in relation to the reference period 2005–2020 according to the relationships between temperature, rainfall and number of rainy days and prevalence of WNV antibodies reported in our study and the climatic projections for scenarios RCP 4.5 and RC 8.5.
From 416 individuals sampled in more than one occasion 82 were positive for WNV antibodies. 66 horses seroconverted for WNV and 14 seroreverted (Fig. 5 and supplementary Table 3). Of the 342 individuals that were tested on more than one occasion by VNT for antibodies against USUV from 2010 to 2020, 31 were positive, of which 11 seroconverted and 5 seroreverted (Fig. 5 and supplementary Table 3).
Fig. 5.
Seroconversions to WNV and USUV for feral horses tested in two consecutive years according to the year of second sampling. Numbers above bars indicate sample sizes.
4. Discussion
4.1. Longitudinal analyses of WNV antibodies in feral horses
Since 2003 different studies have provided evidence of local circulation of WNV in different areas of Spain [9,31,32]. Our results confirm the continuous circulation of WNV in the Guadalquivir marshes between 2005 and 2020 based on the prevalence of antibodies in foal horses and seroconversions. Seropositive foals were found all the years except for 2008, but in that year WNV was found in mosquitoes captured in the same study area [20]. Our results indicate an important exposure of feral horses to WNV in Doñana, with a mean annual seroprevalence of antibodies of 8.1% but an important interannual variation (range 0%–25%). Other studies reported similar prevalences in horses in Spain (8.3%) in 2010–2019 [31], but also lower values (1.35%) between 2011 and 2013 [33] and higher values (19.7%) between 2018 and 2019 [34]. Interestingly, higher prevalence in the last years have been reported in the present and previous studies, not only in wildlife but also in human cases [13], suggesting that the circulation of WNV is more and more frequent and has become endemic in Spain.
Seroprevalence in autumn was higher, probably due to the transmission of WNV during the summer that may explain the higher seroprevalence in autumn. A similar effect of the season was reported in longitudinal and cross-sectional studies in birds [8,9]. Finally, no significant differences were found between localities, which could be due to the low distance between them (lower than 7 km at some points).
4.2. Intrinsic individual factors associated with WNV prevalence
The higher WNV seroprevalence found in older animals, especially females, is a pattern reported [34,35] that could be expected based on their longer exposure to the bites of infected mosquitoes. The fact that antibodies were not found in aged males could be due to various reasons. For example, males in our populations are stallions and these individuals may be more likely to die as a result of WNV disease than mares or geldings [23]. These effects could be driven by differences in the testosterone level [36] and as a result we found antibodies in aged females but not in aged males [35]. Unfortunately, we have no information on the causes of mortality in our population, which merits further study in the future. The number of USUV seropositives detected in these same horses follows the same pattern, where >90% of seropositives were detected in adult female horses, as occurs for WNV. However, the specificity of the ELISA kit used not exclude an underestimation of the number of USUV positives. In addition, we should consider the possibility that detection of antibodies in foals could be due in part to maternal antibody transfer. Turner et al. [37] analysed the antibodies of foals from WNV vaccinated dams and found drastic reduction by 90 days of age. Although we cannot exclude the maternal origin of the antibodies, these results suggest that the impact in our results should be considered low as horses were usually sampled >4 months after birth.
4.3. Effects of climate variables
Minimum annual temperature was positively related to WNV seroprevalence in foals. Temperature plays an important role in the transmission of WNV by affecting the growth rates of mosquito populations, increasing their biting habits and survival, as well as reducing virus extrinsic incubation period and increasing virus load in vectors [38,39]. Higher spring - summer temperatures have been also associated to larger number of WNV disease cases in Europe [40,41]. In contrast, the effect of rainfall on WNV incidence remains controversial [42,43]. Accordingly, we found that while accumulated rainfall was negatively associated with the WNV seroprevalence, the mean number of rainy days had a positive association. Hahn et al. [17] also found that lower than normal annual precipitation increased the average WNV disease incidence. In our system, lower annual precipitation would reduce the availability of surface water for horses, birds, and mosquitoes, favouring a higher spatial overlap in the permanent water resources and thus favouring the enzootic cycle of WNV. But disease patterns also depend on mosquito ecology [39]. For example, Cx. pipiens does not need large volumes of water to reproduce, so rainfall more spread across the year but not necessarily more abundant rainfall may favour the maintenance of these populations for longer periods of time favouring WNV transmission. In fact, Roiz et al. [44] showed that winter rainfall was positively associated to Cx. pipiens abundance. Unfortunately, there is not information available yet on the effect of climate on Culex perexiguus abundance [24]. This mosquito species is the main vector of WNV in South West Spain [45]. This is a highly ornitophilic mosquito species [45] that breeds in the rice fields but also in small freshwater ponds [46]. Due to its foraging behaviour is very important in WNV amplification but also a bridge vector between birds and horses [45]. During the 2020 WNV outbreak in Andalucía, most of the positive pools of WNV were of Cx. perexiguus (97%) and only one pool (3%) was of Cx. pipiens [14].
Climate projections for southern Spain indicate that temperature will increase, and rainfall will decrease [47]. Consequently, based on the projections made here, we can expect even for the RCP 4.5 an increase in WNV mean prevalence over 3%. Larger increases are expected for the scenario RCP 8.5 with WNV mean prevalence reaching 6% or even 8% which would double the average values recorded during the study period.
4.4. Seroconversions and seroreversions in re-tested horses
Seroconversion was very frequent in re-tested feral horses during the studied period, since almost 80% of the detected positive horses were seronegative in previous seasons and seroconverted. Similar results were found in common coot Fulica atra [8]. Furthermore, we also found evidence of the occurrence of seroreversions affecting 17% of the positive individuals re-tested. Seroreversion of antibodies against WNV have been previously reported in both feral and domestic horses [10,48] and also in common coot [9], suggesting that the level of WNV antibodies may fall below the detection limit after an undetermined period post-infection and, consequently, serology of older animals may underestimate lifelong exposure to WNV. It should be noted that between 2017 and 2020 the proportion of seroconversions has sharply increased, reaching the highest value in 2020, matching with the WNV in humans outbreak in southern Spain [13].
Finally, many studies warn about the spread not only of WNV but also of USUV in a large part of the European continent over the last two decades, posing a new risk for human health [49,50]. In the study region, the first detection of USUV virus was in 2009 [20]. Antibodies to USUV have been detected in different species of wild birds, horses and red deer [7]. However, we have detected around 3% of seroconversion for USUV since 2011 to 2018, despite the techniques used to detect antibodies against this virus are designed and optimized to WNV. The results obtained suggest an active circulation of USUV in the study region at least since 2009, suggesting that this flavivirus is also endemic to western Andalucía and should be also considered as a potential cause for unknown origin cases of meningitis in humans in the region.
5. Conclusion
This study provides further evidence that West Nile virus (WNV) is endemic in Spain since a long time. The highest incidence of WNV seroconversions in horses in 2020, overlapping with the large WNV outbreak in humans highlights the suitability of feral horses as a valuable model for studying the environmental factors influencing WNV circulation in the wild. On the long-term (2005–2020), temperature and precipitation were the environmental factors more related to WNV seroprevalence in horses. Consequently, according to future climate change scenarios, we can expect the incidence of WNV in animals to increase in the following decades. This may also imply a higher risk of spillover to humans, but the final impact on human health will depend on the understanding of the factors modulating intensity of spillover from wild animals in to humans [14] and our capacity to design and implement effective management policies to reduce WNV intensity of infection in mosquitoes and/or the exposure of humans to infected mosquitoes, as is the objective of the new Regional and National Plans for the control of vectors and vector borne pathogens.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The personnel of ICTS-RBD and a many veterinarians and students collaborated in sample collection and processing. Francisco Miranda, Olaya García and other Laboratory of Ecophysiology technicians and students helped with the ELISA analyses. This study has been partially funded by the European Union projects EDEN (10284), EDENEXT (261504) and EuroWestNile (261391), Spanish Ministry of Science projects CGL2009-11445, CGL2012-30759, CGL2015-65055-P, E-RTA2015-00002-CO2-01, PGC2018-095704-B-I00, PID2020-116768RR-C21, PID2021-123761OB-I00, PID2020-118921RJ-100, co-financed by FSE funds, projects P07-RNM-02511 and P11-RNM-7038 from the Andalucian Government, PLEC2021-007968 project (acronym NEXTHREAT) funded by MCIN/AEI/10.13039/5011000110333, CSIC's Global Health Platform (PTI Salud Global) and European Union NextGenerationEU/PRTR funds.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100578.
Appendix A. Supplementary data
Supplementary material
Data availability
Data will be made available on request.
References
- 1.Beck C., Jimenez-Clavero M.A., Leblond A., Durand B., Nowotny N., Leparc-Goffart I., Zientara S., Jourdain E., Lecollinet S. Flaviviruses in europe: complex circulation patterns and their consequences for the diagnosis and control of west nile disease. Int. J. Environ. Res. Public Health. 2013;10:6049–6083. doi: 10.3390/ijerph10116049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reisen W.K., Fang Y., Martinez V.M. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J. Med. Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- 3.Root J.J., Bosco-Lauth A.M. West Nile Virus Associations in Wild Mammals: an update. Viruses. 2019;11 doi: 10.3390/V11050459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen L.R., Marfin A.A. West Nile virus: a primer for the clinician. Ann. Intern. Med. 2002;137:173–179. doi: 10.7326/0003-4819-137-3-200208060-00009. [DOI] [PubMed] [Google Scholar]
- 5.Venter M., Pretorius M., Fuller J.A., Botha E., Rakgotho M., Stivaktas V., Weyer C., Romito M., Williams J. West Nile virus lineage 2 in horses and other animals with neurologic disease, South Africa, 2008-2015. Emerg. Infect. Dis. 2017;23:2060–2064. doi: 10.3201/EID2312.162078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertram F.M., Thompson P.N., Venter M. Epidemiology and clinical presentation of west nile virus infection in Horses in South Africa, 2016–2017. Pathog. 2021;10(2020):20. doi: 10.3390/PATHOGENS10010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilibic-Cavlek T., Savic V., Petrovic T., Toplak I., Barbic L., Petric D., Tabain I., Hrnjakovic-Cvjetkovic I., Bogdanic M., Klobucar A., Mrzljak A., Stevanovic V., Dinjar-Kujundzic P., Radmanic L., Monaco F., Listes E., Savini G. Emerging trends in the epidemiology of West Nile and Usutu virus infections in Southern Europe. Front. Vet. Sci. 2019;6:437. doi: 10.3389/FVETS.2019.00437/XML/NLM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Figuerola J., Soriguer R., Rojo G., Tejedor C.G., Jimenez-Clavero M.A. Seroconversion in wild birds and local circulation of West Nile Virus, Spain. Emerg. Infect. Dis. 2007;13:1915. doi: 10.3201/EID1312.070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Figuerola J., Jiménez-Clavero M.A., López G., Rubio C., Soriguer R., Gómez-Tejedor C., Tenorio A. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Vet. Microbiol. 2008;132:39–46. doi: 10.1016/J.VETMIC.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez-Clavero M.A., Llorente F., Sotelo E., Soriguer R., Gómez-Tejedor C., Figuerola J., et al. Vet. Rec. 2010;167:379–380. doi: 10.1136/VR.C3155. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Clavero M.A., Sotelo E., Fernandez-Pinero J., Llorente F., Blanco J.M., Rodriguez-Ramos J., Perez-Ramirez E., Höfle U. West Nile virus in golden eagles, Spain, 2007. Emerg. Infect. Dis. 2008;14:1489–1491. doi: 10.3201/EID1409.080190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vázquez A., Sánchez-Seco M.P., Ruiz S., Molero F., Hernández L., Moreno J., Magallanes A., Tejedor C.G., Tenorio A. Putative new lineage of West Nile Virus, Spain. Emerg. Infect. Dis. 2010;16:549. doi: 10.3201/EID1603.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Alarcón L.G.S.M., Fernández-Martínez B., Moros M.J.S., Vázquez A., Pachés P.J., Villacieros E.G., Martín M.B.G., Borras J.F., Lorusso N., Aceitero J.M.R., Moro E., de Celis A., Oyonarte S., Mahillo B., González L.J.R., Sánchez-Seco M.P., Rodríguez B.S., Catalán U.A., Contreras S.R., Pérez-Olmeda M., Soria F.S. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Eurosurveillance. 2021;26:2002010. doi: 10.2807/1560-7917.ES.2021.26.19.2002010/CITE/PLAINTEXT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Figuerola J., Jiménez-Clavero M.Á., Ruíz-López M.J., Llorente F., Ruiz S., Hoefer A., Aguilera-Sepúlveda P., Jiménez-Peñuela J., García-Ruiz O., Herrero L., Soriguer R.C., Delgado R. Fernández, Sánchez-Seco M.P., la Puente J. Martínez-de, Vázquez A. A One Health view of the West Nile virus outbreak in Andalusia (Spain) in 2020. Emerg. Microbes Infect. 2022;11:2570–2578. doi: 10.1080/22221751.2022.2134055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brugueras S., Fernández-Martínez B., Martínez-de la Puente J., Figuerola J., Porro T.M., Rius C., Larrauri A., Gómez-Barroso D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environ. Res. 2020;191 doi: 10.1016/J.ENVRES.2020.110038. [DOI] [PubMed] [Google Scholar]
- 16.Giesen C., Herrador Z., Fernandez-Martinez B., Figuerola J., Gangoso L., Vazquez A., Gómez-Barroso D. A systematic review of environmental factors related to WNV circulation in European and Mediterranean countries. One Heal. 2023;16 doi: 10.1016/J.ONEHLT.2022.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn M.B., Monaghan A.J., Hayden M.H., Eisen R.J., Delorey M.J., Lindsey N.P., Nasci R.S., Fischer M. Meteorological conditions associated with increased incidence of West Nile virus disease in the United States, 2004–2012. Am. J. Trop. Med. Hyg. 2015;92:1013. doi: 10.4269/AJTMH.14-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vilibic-Cavlek T., Petrovic T., Savic V., Barbic L., Tabain I., Stevanovic V., Klobucar A., Mrzljak A., Ilic M., Bogdanic M., Benvin I., Santini M., Capak K., Monaco F., Listes E., Savini G. Epidemiology of usutu virus: the European scenario. Pathog. (Basel, Switzerland). 2020;9:1–19. doi: 10.3390/PATHOGENS9090699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Busquets N., Alba A., Allepuz A., Aranda C., Núñez J.I. Usutu virus sequences in Culex pipiens (Diptera: Culicidae), Spain. Emerg. Infect. Dis. 2008;14:861. doi: 10.3201/EID1405.071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vázquez A., Ruiz S., Herrero L., Moreno J., Molero F., Magallanes A., Sánchez-Seco M.P., Figuerola J., Tenorio A. West Nile and Usutu viruses in Mosquitoes in Spain, 2008–2009. Am. J. Trop. Med. Hyg. 2011;85:178. doi: 10.4269/AJTMH.2011.11-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roiz D., Vázquez A., Ruiz S., Tenorio A., Soriguer R., Figuerola J. Evidence that passerine birds act as amplifying hosts for usutu virus circulation. Ecohealth. 2019;16:734–742. doi: 10.1007/S10393-019-01441-3/FIGURES/2. [DOI] [PubMed] [Google Scholar]
- 22.Bravo-Barriga D., Ferraguti M., Magallanes S., Aguilera-Sepúlveda P., Llorente F., Pérez-Ramírez E., Vázquez A., Guerrero-Carvajal F., Sánchez-Seco M.P., Jiménez-Clavero M.Á., Mora-Rubio C., Marzal A., Frontera E., de Lope F. Identification of Usutu Virus Africa 3 lineage in a survey of mosquitoes and birds from urban areas of Western Spain. Transbound. Emerg. Dis. 2023;2023:1–10. doi: 10.1155/2023/6893677. [DOI] [Google Scholar]
- 23.Epp T., Waldner C., West K., Townsend H. Factors associated with West Nile virus disease fatalities in horses. Can. Vet. J. 2007;48:1137. /pmc/articles/PMC2034420/ (accessed February 18, 2022) [PMC free article] [PubMed] [Google Scholar]
- 24.Roiz D., Ruiz S., Soriguer R., Figuerola J. Climatic effects on mosquito abundance in Mediterranean wetlands. Parasit. Vectors. 2014;7:1–13. doi: 10.1186/1756-3305-7-333/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Llorente F., García-Irazábal A., Pérez-Ramírez E., Cano-Gómez C., Sarasa M., Vázquez A., Jiménez-Clavero M.Á. Influence of flavivirus co-circulation in serological diagnostics and surveillance: A model of study using West Nile, Usutu and Bagaza viruses. Transbound. Emerg. Dis. 2019;66:2100–2106. doi: 10.1111/TBED.13262. [DOI] [PubMed] [Google Scholar]
- 26.Calisher C.H., Karabatsos N., Dalrymple J.M., Shope R.E., Porterfield J.S., Westaway E.G., Brandt W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989;70:37–43. doi: 10.1099/0022-1317-70-1-37/CITE/REFWORKS. [DOI] [PubMed] [Google Scholar]
- 27.Sotelo E., Llorente F., Rebollo B., Camuñas A., Venteo A., Gallardo C., Lubisi A., Rodríguez M.J., Sanz A.J., Figuerola J., Jiménez-Clavero M.Á. Development and evaluation of a new epitope-blocking ELISA for universal detection of antibodies to West Nile virus. J. Virol. Methods. 2011;174:35–41. doi: 10.1016/J.JVIROMET.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Agüero M., Fernández-Pinero J., Buitrago D., Sánchez A., Elizalde M., Miguel E.S., Villalba R., Llorente F., Jiménez-Clavero M.Á. Bagaza virus in partridges and pheasants, Spain, 2010. Emerg. Infect. Dis. 2011;17:1498. doi: 10.3201/EID1708.110077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinshausen M., Smith S.J., Calvin K., Daniel J.S., Kainuma M.L.T., Lamarque J., Matsumoto K., Montzka S.A., Raper S.C.B., Riahi K., Thomson A., Velders G.J.M., van Vuuren D.P.P. The RCP greenhouse gas concentrations and their extensions from 1765 to 2300. Clim. Chang. 2011;109:213–241. doi: 10.1007/S10584-011-0156-Z/TABLES/5. [DOI] [Google Scholar]
- 30.Zuur A.F., Ieno E.N., Elphick C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010;1:3–14. doi: 10.1111/J.2041-210X.2009.00001.X. [DOI] [Google Scholar]
- 31.Napp S., Llorente F., Beck C., Jose-cunilleras E., Soler M., Pailler-garcía L., Amaral R., Aguilera-sepúlveda P., Pifarré M., Molina-lópez R., Obón E., Nicolás O., Lecollinet S., Jiménez-clavero M.Á., Busquets N. Widespread circulation of flaviviruses in horses and birds in northeastern spain (Catalonia) between 2010 and 2019. Viruses. 2021;13 doi: 10.3390/V13122404/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferraguti M., Martínez-de la Puente J., Jiménez-Clavero M.Á., Llorente F., Roiz D., Ruiz S., Soriguer R., Figuerola J. A field test of the dilution effect hypothesis in four avian multi-host pathogens. PLoS Pathog. 2021;17 doi: 10.1371/JOURNAL.PPAT.1009637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abad-Cobo A., Llorente F., Del Barbero M.C., Cruz-López F., Forés P., Jiménez-Clavero M. Serosurvey reveals exposure to West Nile virus in asymptomatic horse populations in Central Spain Prior to Recent Disease Foci, Transbound. Emerg. Dis. 2017;64:1387–1392. doi: 10.1111/TBED.12510. [DOI] [PubMed] [Google Scholar]
- 34.Guerrero-Carvajal F., Bravo-Barriga D., Martín-Cuervo M., Aguilera-Sepúlveda P., Ferraguti M., Jiménez-Clavero M.Á., Llorente F., Alonso J.M., Frontera E. Serological evidence of co-circulation of West Nile and Usutu viruses in equids from western Spain. Transbound. Emerg. Dis. 2021;68:1432–1444. doi: 10.1111/TBED.13810. [DOI] [PubMed] [Google Scholar]
- 35.Selim A., Megahed A., Kandeel S., Alouffi A., Almutairi M.M. West Nile virus seroprevalence and associated risk factors among horses in Egypt. Sci. Report. 2021;2021 111. 11:1–9. doi: 10.1038/s41598-021-00449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folstad I., Karter A.J. Parasites, Bright males, and the immunocompetence handicap. Am. Nat. 1992;139:603–622. doi: 10.1086/285346. [DOI] [Google Scholar]
- 37.Turner J.L., Waggoner J.W., Rose S.S., Arns M.J., Hankins K.G., Tuttle J. West Nile Virus antibody titers and total immunoglobulin G concentrations in foals from mares vaccinated in Late Gestation. J. Equine Vet. Sci. 2008;28:17–21. doi: 10.1016/J.JEVS.2007.11.009. [DOI] [Google Scholar]
- 38.Kilpatrick A.M., Meola M.A., Moudy R.M., Kramer L.D. Temperature, viral genetics, and the transmission of west nile virus by culex pipiens mosquitoes. PLoS Pathog. 2008;4 doi: 10.1371/JOURNAL.PPAT.1000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paz S., Semenza J.C. Environmental drivers of West Nile fever epidemiology in Europe and Western Asia—A Review. Int. J. Environ. Res. Public Health. 2013;10:3543–3562. doi: 10.3390/IJERPH10083543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camp J.V., Nowotny N. The knowns and unknowns of West Nile virus in Europe: what did we learn from the 2018 outbreak? Expert Rev. Anti-Infect. Ther. 2020;18:145–154. doi: 10.1080/14787210.2020.1713751. [DOI] [PubMed] [Google Scholar]
- 41.Marini G., Manica M., Delucchi L., Pugliese A., Rosà R. Spring temperature shapes West Nile virus transmission in Europe. Acta Trop. 2021;215 doi: 10.1016/J.ACTATROPICA.2020.105796. [DOI] [PubMed] [Google Scholar]
- 42.Vandyk J.K., Rowley W.A. Response of Iowa mosquito populations to unusual precipitation patterns as measured by New Jersey light trap collections. J. Am. Mosq. Control Assoc. 1995;11:200–205. http://www.ncbi.nlm.nih.gov/pubmed/7595446. [PubMed] [Google Scholar]
- 43.Chase J.M., Knight T.M. Drought-induced mosquito outbreaks in wetlands. Ecol. Lett. 2003;6:1017–1024. doi: 10.1046/J.1461-0248.2003.00533.X. [DOI] [Google Scholar]
- 44.Roiz D., Vazquez A., Rosà R., Muñoz J., Arnoldi D., Rosso F., Figuerola J., Tenorio A., Rizzoli A. Blood meal analysis, flavivirus screening, and influence of meteorological variables on the dynamics of potential mosquito vectors of West Nile virus in northern Italy. J. Vector Ecol. 2012;37:20–28. doi: 10.1111/J.1948-7134.2012.00196.X. [DOI] [PubMed] [Google Scholar]
- 45.Muñoz J., Ruiz S., Soriguer R., Alcaide M., Viana D.S., Roiz D., Vázquez A., Figuerola J. Feeding patterns of potential West Nile virus vectors in south-west Spain. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roiz D., Ruiz S., Soriguer R., Figuerola J. Landscape effects on the presence, abundance and diversity of mosquitoes in Mediterranean wetlands. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.AEMet Agencia Estatal de Meteorología, Gobierno de España, El Tiempo. 2013. http://www.aemet.es/es/serviciosclimaticos/cambio_climat/result_graficos?opc4=0&opc6=0 (accessed June 20, 2022)
- 48.Cabre O., Grandadam M., Lou Marié J., Gravier P., Prangé A., Santinelli Y., Rous V., Bourry O., Durand J.P., Tolou H., Davoust B. West Nile Virus in horses, sub-Saharan Africa. Emerg. Infect. Dis. 2006;12:1958–1960. doi: 10.3201/EID1212.060042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clé M., Beck C., Salinas S., Lecollinet S., Gutierrez S., Van de Perre P., Baldet T., Foulongne V., Simonin Y. Usutu virus: A new threat? Epidemiol. Infect. 2019;147:1–11. doi: 10.1017/S0950268819001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salinas S., Constant O., Desmetz C., Barthelemy J., Lemaitre J.M., Milhavet O., Nagot N., Foulongne V., Perrin F.E., Saiz J.C., Lecollinet S., Van de Perre P., Simonin Y. Deleterious effect of Usutu virus on human neural cells. PLoS Negl. Trop. Dis. 2017;11 doi: 10.1371/JOURNAL.PNTD.0005913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.