Abstract
Fatty acid synthesis was compared in cell-free extracts of epidermis and parenchyma of Allium porrum L. leaves. Parenchyma extracts had the major fatty acid synthetase (FAS) activity (70-90%) of the whole leaf; palmitic acid was also the major fatty acid synthesized when acetyl-coenzyme A (CoA) was the primer, but when acetyl-acyl carrier protein (ACP) was employed, C18:0 and C16:0 were synthesized in equal proportion. With the epidermal FAS system when either acetyl-CoA or acetyl-ACP was tested in the presence of labeled malonyl-CoA, palmitic acid was the only product synthesized. Specific activities of the FAS enzyme activities were determined in both tissue extracts.
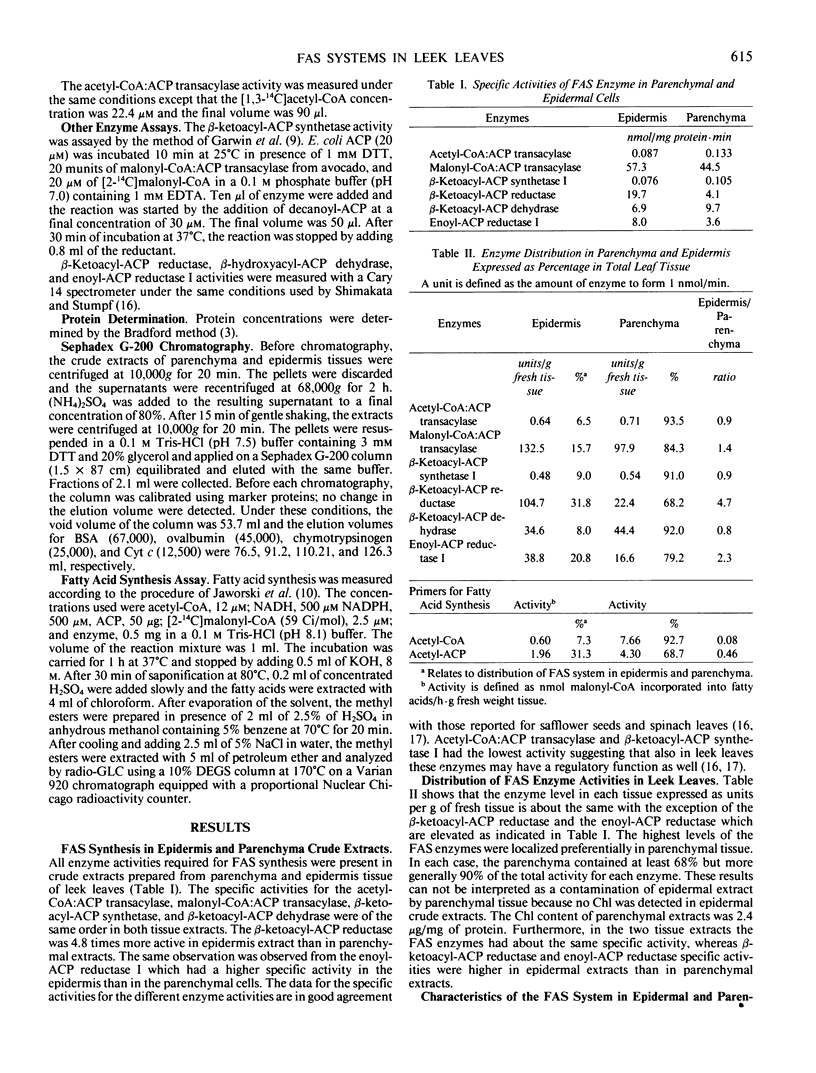
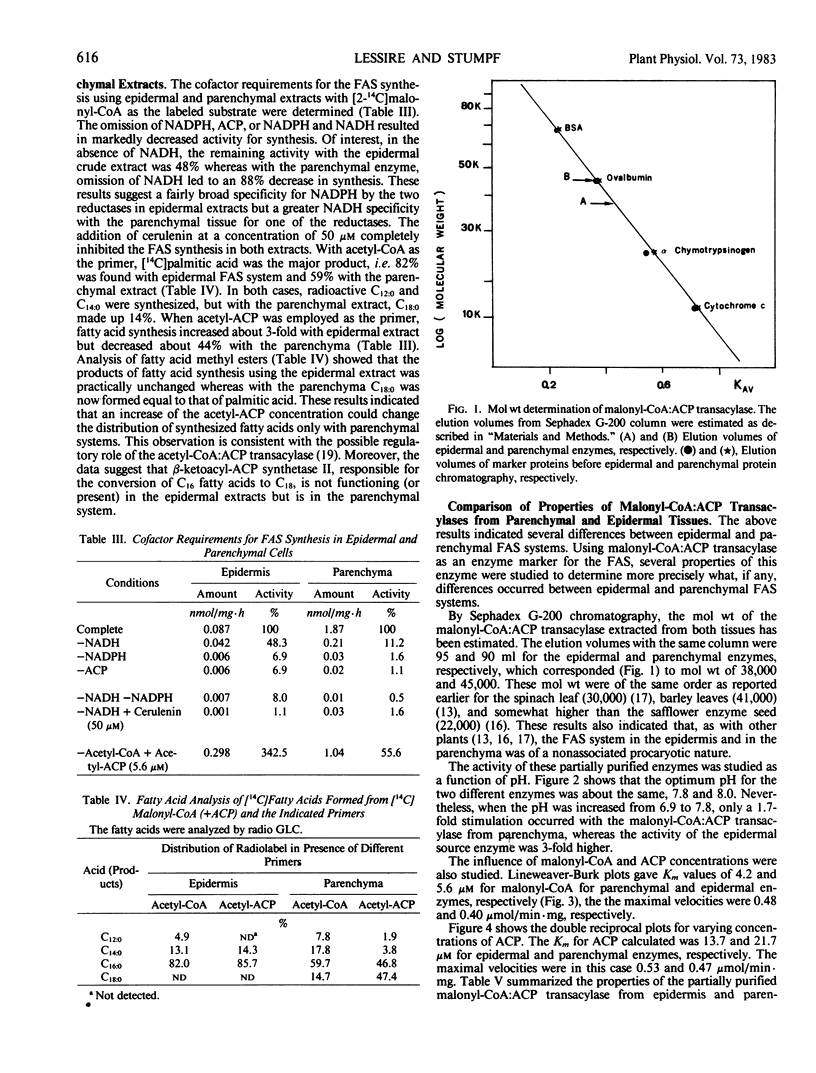
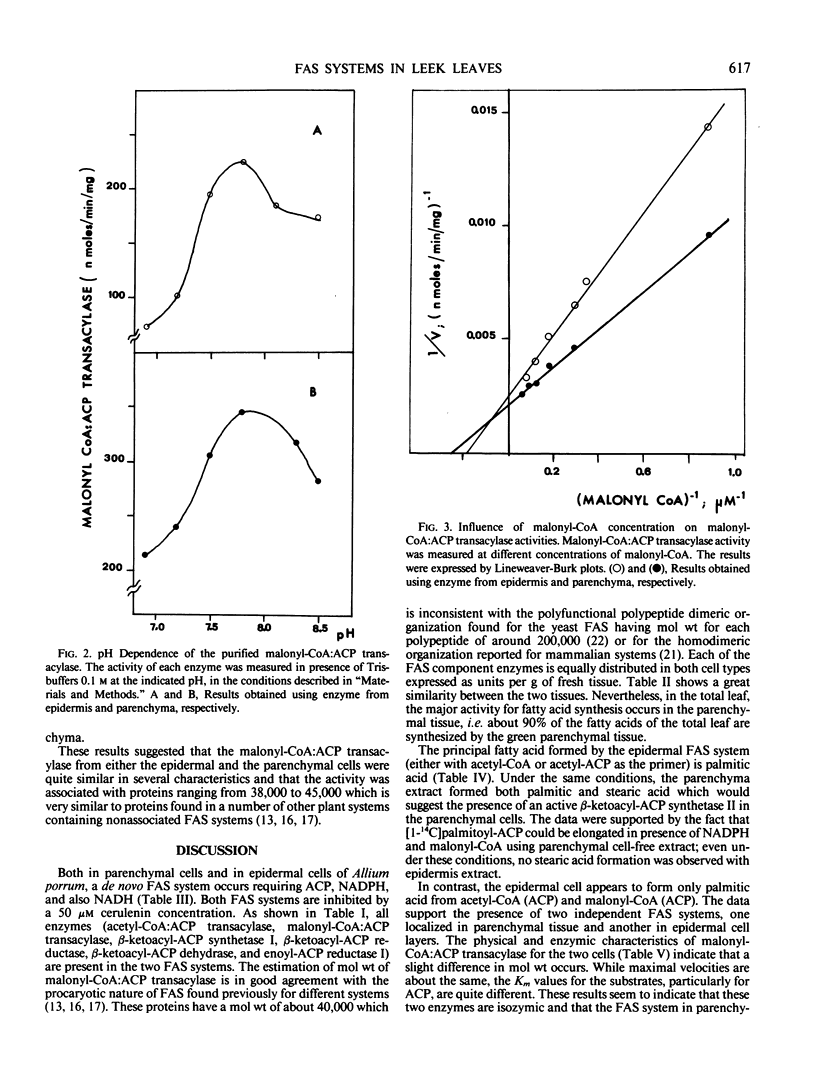
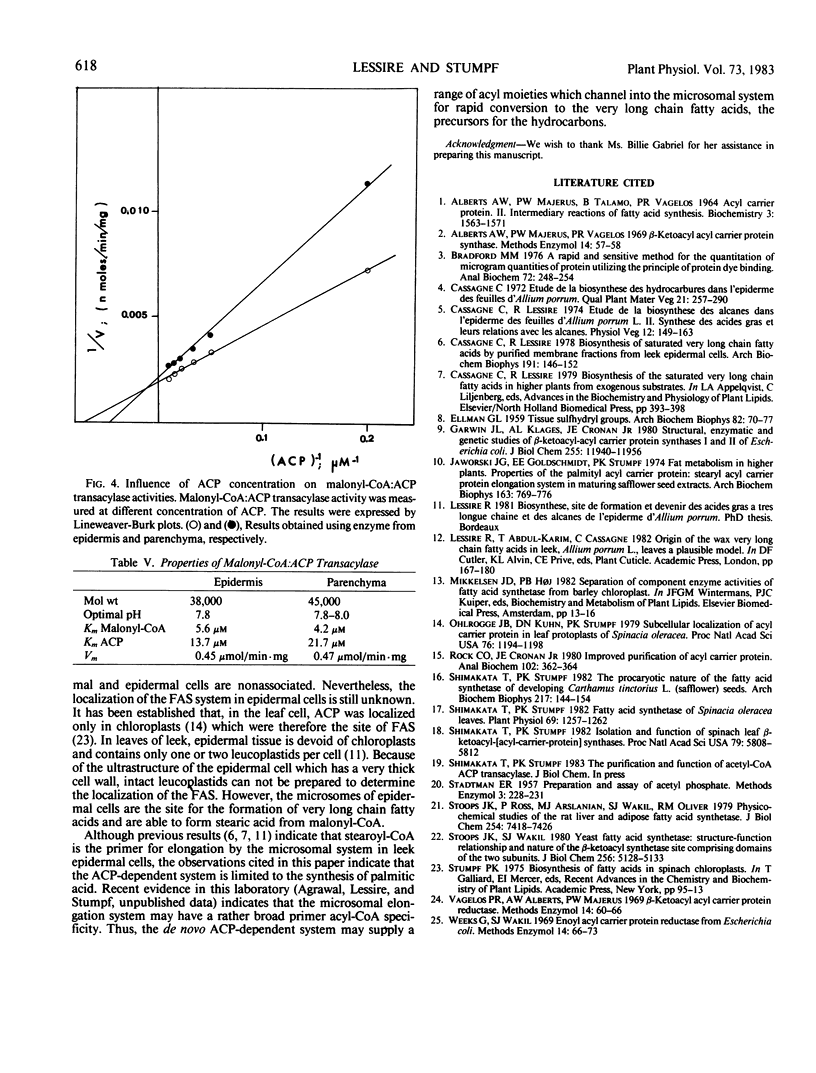
The properties of malonyl-CoA:ACP transacylase were examined from the two different tissues. The molecular weights estimated by Sephadex G-200 chromatography were 38,000 for the epidermal enzyme and 45,000 for parenchymal enzyme. The optimal pH was for both enzymes 7.8 to 8.0 and the maximal velocity 0.4 to 0.5 micromoles per milligram protein per minute. These enzymes had different affinities for malonyl-CoA and ACP. For the malonyl-CoA:ACP transacylase of epidermis, the Km values were 5.6 and 13.7 micromolar for malonyl-CoA and ACP, respectively, and 4.2 and 21.7 micromolar for the parenchymal enzyme. These results suggest that the FAS system in both tissues are nonassociated, that the malonyl-CoA:ACP transacylases are isozymes, and that both in epidermis and in parenchyma tissue two independent FAS system occur. Evidence would suggest that β-ketoacyl-ACP synthase II is present in the parenchymal cells but missing in the epidermal cell.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERTS A. W., MAJERUS P. W., TALAMO B., VAGELOS P. R. ACYL-CARRIER PROTEIN. II. INTERMEDIARY REACTIONS OF FATTY ACID SYNTHESIS. Biochemistry. 1964 Oct;3:1563–1571. doi: 10.1021/bi00898a030. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cassagne C., Lessire R. Biosynthesis of saturated very long chain fatty acids by purified membrane fractions from leek epidermal cells. Arch Biochem Biophys. 1978 Nov;191(1):146–152. doi: 10.1016/0003-9861(78)90076-0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Garwin J. L., Klages A. L., Cronan J. E., Jr Structural, enzymatic, and genetic studies of beta-ketoacyl-acyl carrier protein synthases I and II of Escherichia coli. J Biol Chem. 1980 Dec 25;255(24):11949–11956. [PubMed] [Google Scholar]
- Jaworski J. G., Goldschmidt E. E., Stumpf P. K. Fat metabolism in higher plants. Properties of the palmityl acyl carrier protein: stearyl acyl carrier protein elongation system in maturing safflower seed extracts. Arch Biochem Biophys. 1974 Aug;163(2):769–776. doi: 10.1016/0003-9861(74)90539-6. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Improved purification of acyl carrier protein. Anal Biochem. 1980 Mar 1;102(2):362–364. doi: 10.1016/0003-2697(80)90168-2. [DOI] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Fatty Acid Synthetase of Spinacia oleracea Leaves. Plant Physiol. 1982 Jun;69(6):1257–1262. doi: 10.1104/pp.69.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. Isolation and function of spinach leaf beta-ketoacyl-[acyl-carrier-protein] synthases. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5808–5812. doi: 10.1073/pnas.79.19.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakata T., Stumpf P. K. The procaryotic nature of the fatty acid synthetase of developing Carthamus tinctorius L. (Safflower) seeds. Arch Biochem Biophys. 1982 Aug;217(1):144–154. doi: 10.1016/0003-9861(82)90488-x. [DOI] [PubMed] [Google Scholar]
- Stoops J. K., Ross P., Arslanian M. J., Aune K. C., Wakil S. J., Oliver R. M. Physicochemical studies of the rat liver and adipose fatty acid synthetases. J Biol Chem. 1979 Aug 10;254(15):7418–7426. [PubMed] [Google Scholar]
- Stoops J. K., Wakil S. J. Animal fatty acid synthetase. A novel arrangement of the beta-ketoacyl synthetase sites comprising domains of the two subunits. J Biol Chem. 1981 May 25;256(10):5128–5133. [PubMed] [Google Scholar]


