Abstract
-
1.
Hunting, trade, and consumption of wildlife present a serious threat to global public health as it places humans in close contact with zoonotic pathogens.
-
2.
We systematically mapped the literature on wild meat handling and zoonotic disease transmission (1996–2022) using the online database Web of Science and Google search engine and identified 6229 articles out of which 253 were finally selected for use in our mapping review; 51 of these provided specific information regarding transmission risks.
-
3.
The reviewed studies reported 43 zoonotic pathogens (17 bacteria, 15 viruses, and 11 parasites) that could pose a potential risk to human health.
-
4.
Sixteen hygienic and sanitary behaviours were described in the reviewed studies. Disease surveillance was the most frequent. Most of the surveillance studies were carried out in Europe and were less common in the tropics.
-
5.
To inform policy and practical actions effectively, it is imperative to broaden our understanding of how various mitigation behaviours can be employed to minimize the risk of transmission.
Keywords: Bushmeat, Disease prevention, Meat handling, Hunting, Food-borne disease
1. Introduction
The emergence of SARS-CoV-2 and resulting COVID-19 pandemic has alerted countries throughout the world of the risks of disease spillover from wildlife used for meat (wild meat) to humans. SARS-CoV-2 has been linked to wildlife trade in “wet markets” in China [1,2,3] and has had enormous consequences on the health and economies of almost every country in the world. While COVID-19 has attracted global attention due to the huge numbers of people affected, other bacterial and viral zoonoses associated with wild meat have caused spillover events in the last few decades, resulting in localized outbreaks (e.g., Marburg virus disease and anthrax), epidemics (e.g., Ebola virus disease and mpox), and occasionally pandemics (e.g., COVID-19 and HIV/AIDs).
In addition to emerging zoonotic diseases (i.e., those that are increasing in incidence or geographic range with origins in wildlife), endemic food-borne diseases (i.e., caused by bacteria, parasites, and viruses infecting animals or contaminating meat) are routinely transmitted to humans. These are an important cause of morbidity and mortality and a significant barrier to socio-economic development worldwide [4,5]. Compared to the known public health significance of endemic zoonoses in domestic meat [6,7], information on risks from wild meat is much more limited.
Transmission of zoonotic diseases from animals to humans can occur through direct physical contact, airborne particles or droplets, biting or mechanical transfer from a vector (arthropod), orally, or through contact with a contaminated environment [8]. The hunting, transport, sale, and consumption of wildlife all create transmission pathways for emerging and food-borne diseases; blocking each of these pathways will require different changes in behaviour. Interventions that may be relevant for reducing one type of risk (e.g., blood-borne pathogen exposure during butchering), will not help to reduce other risks (e.g., oral ingestion of bacteria from poorly preserved meat) and vice versa. It is useful to understand current practices in different regions in order to understand where there is a need to improve practices and to adapt good in places where they are absent.
While other assessments have highlighted the ecological and epidemiological drivers of zoonotic disease risks linked to wild meat [9,10,11,12,13,14], here we systematically review all available information on practices undertaken by human actors along the wild meat supply chain that could facilitate or mitigate disease transmission. The results show what preventive measures are being used to lessen disease transmission from wildlife to humans and identify knowledge gaps.
2. Methods
2.1. Research design
We conducted a systematic search of zoonotic disease risks and detection and mitigation strategies linked to wild meat handling along the wild meat supply chain using the online database Web of Science. We also carried out a Google search engine search (using a reduced number of terms employed in the Web of Science due to Google Search restrictions) in which the first 100 results were treated as articles, given ID numbers, and subjected to our screening process.
2.2. Literature search
We searched the Web of Science database in April 2022 identifying all of the published literature from 1996 until April 15th, 2022. We used three key phrases: wild meat, zoonotic pathogens and human contact (Supplementary Table 2). We also translated the terms into French, Spanish, and Portuguese to ensure adequate representation of the global literature.
2.3. Screening
We determined eligibility on a priori inclusion/exclusion criteria defined over multiple rounds of discussions among co-authors. These are presented in Table 1. Screening of the search results was conducted in three phases. In the first two stages, all authors reviewed the titles and then abstracts of each article. This process was completed in full by the lead author, LT only, but a sub-set of the articles was screened by three co-authors during the kappa analysis to ensure inter-rater reliability. Those articles that were not excluded were subsequently reviewed in full by the lead author to further refine the sample (the screening process flow diagram is shown in Supplementary Fig. 1). Additionally, we carried out snowball sampling in which bibliographies of selected articles were reviewed and relevant publications identified.
Table 1.
Inclusion and exclusion criteria as defined by the research team.
| Inclusion Criteria For inclusion, articles must be/have: |
Exclusion Criteria Studies were excluded if: |
|---|---|
| Been subjected to a process of peer review. | Full text was not available/downloadable. |
| Full text available online (or by request). | Published in a language other than English, Spanish, French or Portuguese. |
| Contain primary data (reviews with secondary data were excluded but cited sources were obtained from bibliographies). | Human health was not a specific focus of the research. Pathogen investigated cannot be transmitted to humans. |
| Available in either English, Spanish, French or Portuguese. | Wider global risks of the Wild Meat trade were explored without specific focus on sanitation at the individual/local scale. |
| Relevance to reducing human disease transmission in wild meat handling at any stage of the value chain, including animal products not intended for human consumption but for use in medicinal or cultural practices. | Wild Meat samples were provided to research team in return for financial reward, as opposed to samples being obtained from a market or from a hunted carcass that was intended for human consumption. |
| Reference to Wild Meat as defined by CITES Bushmeat Working Group (CITES, [82]). | No data was present on hygienic, sanitary, and biosecurity behaviours. |
| Only where human-animal contact is established through hunting OR at a later stage in the Wild Meat commodity chain (e.g., butchery/consumption) of a hunted animal intended for consumption/zootherapeutic use. |
2.4. Kappa analysis
Three co-authors (in addition to the lead author) reviewed the abstracts of a subset of 30 articles from the main sample, as in phase two of the screening process. The results from each reviewer were then compared with those of LT, who screened the entire sample. This allowed us to eliminate any discrepancies between reviewers.
Agreement between reviewers was used to calculate Cohen's Kappa Coefficient [15]. A k = 1 means full agreement, k = 0 is the same as would be expected by chance and k = −1 refers to no agreement. The kappa analysis was repeated three times until a predetermined threshold of k = 0.6 was met between all reviewers. No articles from the first two rounds were included in the final round to ensure reliability of kappa scores. Multiple rounds were used to ensure adaptability of the process as new perspectives were gained by consulting co-authors.
2.5. Data extraction
Following the initial screening process, full texts of each article that passed phase 3 were analysed and underwent a systematic data extraction process by the lead author.
3. Results
Of the initial 6229 articles identified in our search, 341 passed the first two filters. These articles were then reviewed in full, out of which we excluded a further 88 giving a total of 253 reviewed articles (Supplementary Table 1). Reasons for exclusion are given in Table 2 along with their frequencies. From the 253 included articles, 51 provided data on disease transmission risk for specific behaviours (17), or points along the wild meat supply chain (34).
Table 2.
Number of studies excluded at each screening phase and the reason for exclusion.
| Reason for Exclusion | Phase 2 (Frequency) | Phase 2 (%) | Phase 3 (Frequency) | Phase 3 (%) |
|---|---|---|---|---|
| Not about wild meat | 143 | 30.3 | 18 | 20.5 |
| No data on human health | 173 | 36.7 | 39 | 44.3 |
| No primary data | 114 | 24.2 | 10 | 11.4 |
| Language | 6 | 1.3 | 3 | 3.4 |
| Duplicate | 22 | 4.7 | 0 | 0 |
| Full text not available | 14 | 3.0 | 18 | 21.5 |
| Total number of articles excluded | 472 | 88 |
3.1. Kappa analysis
The kappa scores between the lead author and each of the three reviewers were above the agreed threshold, reviewer one: 0.66 (0.46–0.86), reviewer two: 0.84 (0.69–1) and reviewer three: 0.93 (0.80–1) at a 95% CI.
3.2. Geographic and temporal patterns
Of the 253 articles, the majority referred to species and practices in Europe (43%) and Africa (33%), followed by Asia (10%), North America (9%), Latin America (5%), and Australia (0.4%).
We found an increase in the number of articles published since 1996 (Fig. 1) with 37% published since 2020.
Fig. 1.
Cumulative curve of publication of studies identified in this investigation over time.
Animal species were mentioned in 241 articles, in which 497 reports of animal taxa included 18 mammalian orders as well as birds and reptiles (Fig. 2). We separated members of the order Artiodactyla into the sub-orders Suina (pigs and peccaries) and Cetruminantia (camels, ungulates, hippopotamuses and sirenians). The Suina were the most commonly reported group (24%) followed by the Cetruminantia (17%). Primates were subjects in 12% of the articles, Rodentia in 10%, and Chiroptera (bats) in 7%.
Fig. 2.
Distribution of case studies based on animal taxa and continent.
In the 253 included articles, we found 627 reports that mentioned actors at different points of the wild meat supply chain. The most frequently cited were consumers (35%) and hunters (32%), followed by butchers (15%), food preparers (9%), vendors (6%), transporters (2%), and zootherapy practitioners (1%). A total of 34 articles linked pathogen exposure data to specific points in the wild meat supply chain to assess zoonotic risk (Table 3); eight of these used surveillance data, while 27 calculated contact rates between actors and wild animals/fresh meat to serve as a proxy for surveillance data.
Table 3.
All articles that provided data on pathogen transmission risk associated with points along the wild meat supply chain.
| ID | Title | Author(s) | Publication Year | Continent | Actor(s) | Pathogen | How is Risk Assessed? |
|---|---|---|---|---|---|---|---|
| 192 | Human T-cell lymphotropic virus type 1 transmission dynamics in rural villages in the Democratic Republic of the Congo with high nonhuman primate exposure | Halbrook et al. [41] | 2021 | Africa | Hunter, Butcher, Preparer | Retroviruses | Pathogen levels measured |
| 403 | Suspected Exposure to Filoviruses Among People Contacting Wildlife in Southwestern Uganda | Smiley-Evans et al. [22] | 2018 | Africa | Hunter, Consumer | Filoviruses | Pathogen levels measured |
| 425 | Knowledge, attitudes, and behavioural risk factors regarding zoonotic infections among bushmeat hunters and traders in Nsukka, southeast Nigeria | Ozioko et al. [42] | 2018 | Africa | Hunter, Vendor, Transporter, Consumer | Infection (general) | Pathogen levels measured |
| 438 | Serologic Evidence of Ebolavirus Infection in a Population With No History of Outbreaks in the Democratic Republic of the Congo | Mulangu et al. [43] | 2018 | Africa | Hunter, Butcher, Preparer, Consumer | Filoviruses | Pathogen levels measured |
| 581 | Seroepidemiological Survey of Q Fever and Brucellosis in Kurdistan Province, Western Iran | Esmaeili et al. [44] | 2014 | Asia | Hunter, Butcher, Consumer | Q Fever and Brucellosis | Pathogen levels measured |
| 621 | Failure to Detect Simian Immunodeficiency Virus Infection in a Large Cameroonian Cohort with High Non-human Primate Exposure | Djoko et al. [45] | 2012 | Africa | Hunter, Butcher | Retroviruses | Pathogen levels measured |
| 678 | Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters | Wolfe et al. [14] | 2005 | Africa | Hunter, Butcher | Retroviruses | Pathogen levels measured |
| 713 | Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013–2014 | Huong et al. [46] | 2020 | Asia | Hunter, Vendor, Butcher, Consumer | Coronaviruses | Pathogen levels measured |
| 14 | Rodent-Human Interface: Behavioral Risk Factors and Leptospirosis in a Province in the Central Region of Thailand | Suwannarong et al. [47] | 2022 | Asia | Hunter, Vendor, Butcher, Preparer, Consumer | Leptospira | Contact rates measured |
| 201 | Market characteristics and zoonotic disease risk perception in Cameroon bushmeat markets | Saylors et al. [48] | 2021 | Africa | Hunter, Transporter, Vendor, Butcher, Preparer, Consumer | NA | Contact rates measured |
| 236 | Attitudes, practices, and zoonoses awareness of community members involved in the bushmeat trade near Murchison Falls National Park, northern Uganda | Dell et al. [30] | 2020 | Africa | Hunter, Butcher, Preparer, Consumer | NA | Contact rates measured |
| 366 | Understanding Ebola virus and other zoonotic transmission risks through human-bat contacts: Exploratory study on knowledge, attitudes and practices in Southern Cameroon | Baudel et al. [49] | 2019 | Africa | Hunter, Preparer, Consumer | Filoviruses | Contact rates measured |
| 408 | Using physical contact heterogeneity and frequency to characterize dynamics of human exposure to nonhuman primate bodily fluids in central Africa | Narat et al. [50] | 2018 | Africa | Hunter, Vendor, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 425 | Knowledge, attitudes, and behavioural risk factors regarding zoonotic infections among bushmeat hunters and traders in Nsukka, southeast Nigeria | Ozioko et al. [42] | 2018 | Africa | Hunter, Vendor, Consumer | NS | Contact rates measured |
| 461 | Human Exposure to Wild Animals in the Sankuru Province of the Democratic Republic of the Congo | Rimoin et al. [51] | 2017 | Africa | Hunter, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 473 | Bushmeat Hunting and Zoonotic Transmission of Simian T-Lymphotropic Virus 1 in Tropical West and Central Africa | Mossoun et al. [52] | 2017 | Africa | Hunter, Butcher, Preparer, Consumer | Retroviruses | Contact rates measured |
| 485 | Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo | Quiner et al. [53] | 2017 | Africa | Hunter, Vendor, Butcher, Consumer | Monkeypox virus | Contact rates measured |
| 491 | Understanding framings and perceptions of spillover Preventing future outbreaks of bat-borne zoonoses | Lawson et al. [54] | 2017 | Africa | Hunter, Vendor, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 509 | High prevalence of IgG antibodies to Ebola virus in the Efe pygmy population in the Watsa region, Democratic Republic of the Congo | Mulangu et al. [55] | 2016 | Africa | Hunter, Consumer | Filoviruses | Contact rates measured |
| 536 | Contact to Non-human Primates and Risk Factors for Zoonotic Disease Emergence in the Taï Region, Côte d'Ivoire | Mossoun et al. [56] | 2015 | Africa | Hunter, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 546 | Hunting, Food Preparation, and Consumption of Rodents in Lao PDR | Suwannarong et al. [57] | 2015 | Asia | Hunter, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 552 | Drivers of Bushmeat Hunting and Perceptions of Zoonoses in Nigerian Hunting Communities | Friant et al. [32] | 2015 | Africa | Hunter, Vendor, Butcher, Consumer, Zootherapy practitioner | NS | Contact rates measured |
| 559 | Characteristics and Risk Perceptions of Ghanaians Potentially Exposed to Bat-Borne Zoonoses through Bushmeat | Kamins et al. [58] | 2015 | Africa | Hunter, Vendor, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 566 | Beyond Bushmeat: Animal Contact, Injury, and Zoonotic Disease Risk in Western Uganda | Paige et al. [59] | 2014 | Africa | Hunter, Butcher | NS | Contact rates measured |
| 571 | Bird harvesting practices and knowledge, risk perceptions, and attitudes regarding avian influenza among Canadian First Nations subsistence hunters: implications for influenza pandemic plans | Charania et al. [60] | 2014 | North America | Hunter, Butcher | Avian influenza | Contact rates measured |
| 604 | Zoonotic Disease Risk and the Bushmeat Trade: Assessing Awareness Among Hunters and Traders in Sierra Leone | Subramanian [61] | 2012 | Africa | Hunter, Vendor, Butcher, Preparer, Consumer | NS | Contact rates measured |
| 621 | Failure to Detect Simian Immunodeficiency Virus Infection in a Large Cameroonian Cohort with High Non-human Primate Exposure | Djoko et al. [45] | 2012 | Africa | Hunter, Butcher | Retroviruses | Contact rates measured |
| 629 | Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa | Kamins et al. [31] | 2011 | Africa | Hunter, Vendor, Consumer | NS | Contact rates measured |
| 661 | Seroprevalence of Toxoplasma gondii Among Nunavik Inuit (Canada) | Messier et al. [62] | 2009 | North America | Preparer, Consumer | Toxoplasmosa gondii | Contact rates measured |
| 672 | Patterns of bushmeat hunting and perceptions of disease risk among central African communities | Le Breton et al. [35] | 2006 | Africa | Hunter, Butcher, Consumer | NS | Contact rates measured |
| 683 | Exposure to nonhuman primates in rural Cameroon | Wolfe et al. [63] | 2004 | Africa | Hunter, Butcher, Consumer | NS | Contact rates measured |
| 685 | Naturally acquired simian retrovirus infections in central African hunters | Wolfe et al. [64] | 2004 | Africa | Hunter, Butcher | Retroviruses | Contact rates measured |
| 701 | Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea | Ter Meulen et al. [65] | 1996 | Africa | Hunter, Consumer | Arenaviruses | Contact rates measured |
| 802 | Knowledge and Practices of Bush Meat Consumption among Indigenous People in Belum Forest, Malaysia: An Analytical Cross-Sectional Study | Maideen et al. [66] | 2022 | Asia | Hunter, Butcher, Preparer, Consumer | NS | Contact rates measured |
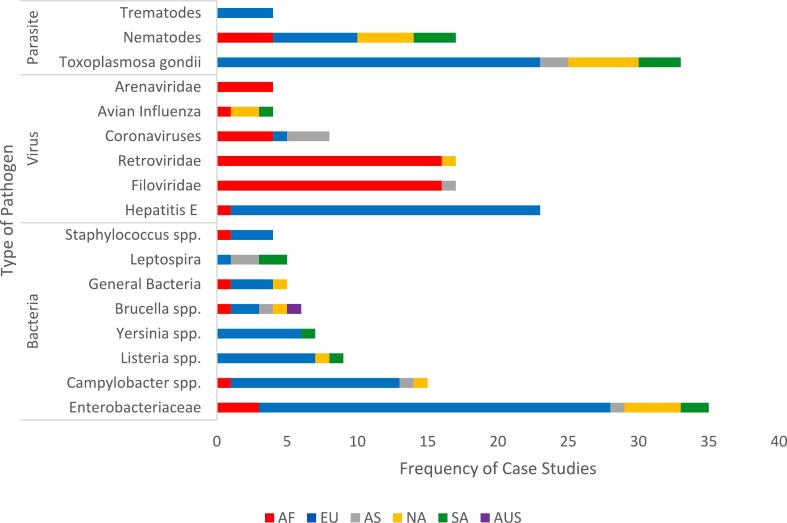
3.3. Pathogen types
We found a total of 256 reports of 43 different pathogens across 204 articles consisting of bacteria (40%), viruses (33%), and parasites (27%). Most pathogens described in the articles were from research undertaken in Europe and Africa (Fig. 3).
Fig. 3.
Distribution of case studies based on pathogen type and continent. Pathogens which appeared fewer than 4 times in the sample were excluded from this figure, for the full dataset refer to Supplementary Table 1.
3.4. Hygienic and sanitary behaviours and practices
A total of 16 hygienic and sanitary behaviours and practices were mentioned in 467 reports in the 253 articles (Fig. 4). Pathogen surveillance was the most common, accounting for 35% of all reports of behaviours. Other behaviours identified include the avoidance of high-risk taxa, sick animals, or undercooked food (13%), the use of personal protective equipment (6%), washing hands (4.5%), and utensils (2%), and the treatment of injuries sustained during animal handling (11%). Preservation of meat by cooking/drying/smoking (16%) and pickling meat (4%) were other practices that help to reduce risk of disease from consuming wild meat. Practices to prevent cross-contamination included the exclusion of children, domestic animals, and pests from butchery areas (3%), the use of storage and waste management systems, including for example, taking the animal to a licensed slaughter facility, or the use of refrigerators/freezers to store fresh meat in a household (5%). Less commonly reported were the use of a dedicated surface for butchery or market display (such as a butcher's block/table/counter) (0.4%), artificial light during harvest/butchery (0.4%), and zoning of wildlife markets to minimize the mixing of different species on sale (0.2%). Behaviours to reduce the risk of disease transmission during hunting included wrapping of carcasses either with plastic or other available materials e.g., banana leaves (0.4%) and the draining of blood from dead animals (0.4%). A total of 17 articles in this review combined behavioural data with pathogen surveillance to provide an assessment of the effectiveness of sanitary and hygienic behaviours (Table 4).
Fig. 4.
Distribution of sanitary and hygienic behaviours investigated.
PERI = Avoidance of carcasses/butchery areas; PRV = Preservation of meat products without heat; UTL = Cleaning of utensils after butchery/harvest; STR = Storage and waste management infrastructure; WASH = Hand washing; PPE = Use of personal Protective Equipment; BIT = Treat injuries sustained from animal handling; HEAT = Preservation or cooking of meat with heat; AV = Avoidance of high-risk taxa, sick animals, undercooked food; LIT = Use of lamp/torch to assist harvest/butchery; WRA = Carcass wrapping/packaging; DRAIN = Draining blood from carcass; TILE = Use of surface for butchery/market display; SEP = Minimize species mixing; ALC = Use of alcohol to disinfect animal parts; VET = Pathogen surveillance.
Table 4.
Selection of articles that provided empirical data on the impact of hygienic and sanitary behaviours on disease risk outcomes. PRV = Preservation of meat products without heat; STR = Storage and waste management infrastructure; WASH = Hand washing; PPE = Use of personal Protective Equipment; HEAT = Preservation or cooking of meat with heat.
| Article ID | Title | Author(s) | Pub Year | Continent | Behaviour | Pathogen | Findings |
|---|---|---|---|---|---|---|---|
| 27 | Hygiene evaluation and microbiological hazards of hunted wild boar carcasses | Peruzy et al. [25] | 2022 | Europe | Using registered premises for evisceration (STR) | Enterobacteriaceae | Carcasses collected from registered premises resulted in more satisfactory mesophilic counts. |
| 86 | Identification of Bacillus anthracis, Brucella spp., and Coxiella burnetii DNA signatures from bushmeat | Katani et al. [67] | 2021 | Africa | Salt curing (PRV) | B. anthracis | Fresh samples had greater relative risk of being positive than processed samples. |
| Sun-drying/Air-drying/Semi-boiling (HEAT) | Brucella spp. | ||||||
| Coxiella burnetii | |||||||
| 277 | Safety and Quality of Fish and Game Meats Prepared by First Nations Communities in British Columbia, Canada | Hingston et al. [68] | 2020 | North America | Canning (PRV) | Enterobacteriaceae | Proccessed samples contained higher microbial loads than unprocessed samples. |
| Smoking/Drying (HEAT) | Listeria spp. | ||||||
| 306 | Microbiota in foods from Inuit traditional hunting | Hauptmann et al. [69] | 2020 | North America | Drying (HEAT) | General Bacteria | This study shpws that traditional drying methods are efficient for limiting microbial growth through desiccation. |
| 322 | Microbial Diversity in Bushmeat Samples Recovered from the Serengeti Ecosystem in Tanzania | Katani et al. [70] | 2019 | Africa | Drying (HEAT) | General Bacteria | Regardless of sample condition (fresh or processed) no significant differences in alpha diversity were found. |
| 374 | Microbial contamination of moose (Alces alces) and white-tailed deer (Odocoileus virginianus) carcasses harvested by hunters | Sauvala et al. [71] | 2019 | Europe | Use of registered slaughter facility (STR) | Enterobacteriaceae | High bacterial counts were associated with smallest facilities having only one room. |
| Campylobacter spp. | |||||||
| Yersinia spp. | |||||||
| Listeria spp. | |||||||
| 434 | Microbiological hazards and potential of spontaneously fermented game meat sausages: A focus on lactic acid bacteria diversity | Zgomba-Maksimovic et al. [72] | 2019 | Europe | Fermentation and ripening (PRV) | General Bacteria | 33.3% of products tested were considered inappropriate for human consumption. |
| Smoking (HEAT) | |||||||
| 457 | A cross-sectional study among Polish hunters: seroprevalence of hepatitis E and the analysis of factors contributing to HEV infections | Baumann-Popczyk et al. [73] | 2017 | Europe | Hand washing after disembowelment (WASH) | Hepatitis E Virus | Washing hands after disembowelment was linked to lower seroprevalence. |
| 507 | The effect of storage conditions on the hygiene and sensory status of wild boar meat | Borilova et al. [74] | 2016 | Europe | Storing meat at various temperatures (STR) | General Bacteria | Storage at low temperatures had good microbiological and hygiene status over 15 days. |
| 538 | Hepatitis E virus antibody prevalence in hunters from a district in Central Germany, 2013: a cross-sectional study providing evidence for the benefit of protective gloves during disemboweling of wild boars | Schielke et al. [75] | 2015 | Europe | Wearing protective gloves during disemboweling (PPE) | Hepatitis E Virus | Lower anti-HEV prevalence when gloves were frequently used during disemboweling of wild boar. |
| 614 | The microbiological conditions of carcasses from large game animals in Italy | Avagnina et al. [28] | 2012 | Europe | Shot placement; Time elapsed between shooting and evisceration (OTHER) | Yersinia spp. | No significant levels of pathogenic bacteria were found using this specific sampling method but low levels suggest potential for amplification at subsequent stage of supply chain. |
| Listeria spp. | |||||||
| Enterobacteriaceae | |||||||
| 635 | Assessment of levels of bacterial contamination of large wild game meat in Europe | Membré et al. [76] | 2011 | Europe | Freezing or chilling meat (STR) | Campylobacter spp. | Levels of contamination were slightly lower when meat was frozen compared with chilled (though not significant). |
| Staphylococcus spp. | |||||||
| Listeria spp. | |||||||
| Enterobacteriaceae | |||||||
| 655 | An investigation of the effects of secondary processing on Mycobacterium spp. in naturally infected game meat and organs | Van der Merwe & Michel [77] | 2010 | Africa | Cooking/Drying (HEAT) | Mycobacterium spp. | Results showed that these processes will kill Mycobacterium bovis however there was unexpected recovery of non-tuberculous mycobacteria. |
| 717 | Effect of Delayed Refrigeration on the Microbial Carcass Contamination of Wild Boars (Sus scrofa) | Cenci-Goga et al. [78] | 2021 | Europe | Delayed refrigeration (STR) | General Bacteria | Results showed a correlation in bacterial population of wild boar carcasses between the time from shot to analysis and from refrigeration to analysis but a lack of correlation between the time from shot to refrigeration. |
| 720 | Tenacity of Alaria alata mesocercariae in homemade German meat products | González-Fuentes et al. [79] | 2014 | Europe | Curing/Fermentation (PRV) | Trematodes | No vital parasites found in final products indicating success of this behaviour as disease mitigation strategy. |
| Smoking/Drying (HEAT) | |||||||
| 743 | Factors affecting the microbiological load of Italian hunted wild boar meat (Sus scrofa) | Orsoni et al. [80] | 2020 | Europe | Carcasses cleaned with potable water (WASH) | Enterobacteriaceae | When handling practices reported in European and National regulations are met, the wild meat supply chain can be a safe process. |
| Time elapsed between shooting and evisceration (OTHER) | |||||||
| 744 | Assessment of microbial carcass contamination of hunted wild boars | Mirceta et al. [81] | 2017 | Europe | Use of adequate hygiene practices in handling and dressing carcasses (or lack thereof) (OTHER) | Enterobacteriaceae | Evisceration in laying position on the ground and washing of skin and interior carcass after evisceration were found to have the most significant influence on microbial conditions. Need for implementation and strict adherence to good hygiene practice. |
4. Discussion
Our study takes a systematic approach to understanding the risks of zoonotic spillover associated with wild meat consumption, beyond specific disease systems. By identifying research that focuses on the intersection of wild meat, zoonotic pathogens, and hygiene practices, our pathogen-agnostic approach sheds light on this important pathway for zoonotic disease transmission and identifies potential intervention points to prevent such transmission.
We found that the number of articles on the subject of wild meat handling and zoonotic disease has increased since 1996. These studies were largely undertaken in Europe and Africa and involved a large diversity of wild animal species and associated zoonotic risks. Information on stakeholders within the wild meat supply chain involved mostly consumers, hunters, butchers, but also, vendors, preparers, transporters of wild meat and zootherapy practitioners. Data on sanitary behaviours were also described but were rarely the main focus of studies, representing a large gap in the literature and suggesting the need for targeted studies which address this.
Most studies were undertaken in European countries and focused on species commonly hunted throughout this region, particularly wild boar. At the wildlife-livestock interface, where closely related domestic pigs live in proximity to wild boar populations, understanding disease transmission dynamics is crucial to protect consumers of wild meat, as well as the wider domestic meat market [16]. In Europe and other countries in the global north, surveillance systems have been effectively integrated into wild meat hunting and commodity chains, which could be adapted to other contexts.
Primates, rodents, and bats were also commonly researched groups of animals. These taxa represent a significant risk for pathogen spillover as they are known carriers of many zoonotic viruses and are widely consumed in Africa, Asia, and Latin America [12,17]. Despite this and the increase in human populations, habitat loss, and the increasing demand for wild meat, these regions were underrepresented in our sample [18,19]. Most research on the spread of viruses from primates, rodents, and bats was conducted in African countries (largely in the forested West and Central regions). Significant research efforts have been directed to understanding risks from filo- and retroviruses which are endemic to Africa, and cause diseases including Marburg Fever, Lassa Fever, Ebola Haemorrhagic Fever, and HIV/AIDS, all of which pose significant risks for regional and international spread [20,21,22,23]. Global health priorities seem to direct resources toward emerging threats that can affect the global north as seen in research efforts in Africa. However, other endemic pathogens, including hepatitis and bacterial and parasitic infections were more commonly studied in the global north, despite also being highly prevalent in the global south. The governance and infrastructural capacity of nations in the global north are, in most instances, more conducive to the implementation and monitoring of biosecurity practices [24].
Of the 16 hygienic and sanitary behaviours identified in this study, pathogen surveillance was the most common. Most of these studies were done in Europe where integrated disease surveillance programmes are incorporated into hunting practices. In Italy, for example, samples of freshly harvested wild meat are sent to control centres or game-handling establishments where they are examined by trained staff or veterinarians to be approved for sale [25]. Systems are in place throughout Europe for the screening of bacteria e.g. Enterobacteriaceae [25], as well as viruses e.g. Hepatitis E [26], and parasitic cysts [27]. Data regarding the prevalence of these pathogens may be measured against other factors such as shot placement, the time between shooting and evisceration, or fermentation time in traditional preservation processes [28,29]. However, such analyses made up only a small portion of the articles identified in this review.
In several articles, practices reported by participants were used to calculate contact frequency rates between humans and animals, or their meat products, which were then used (often alongside data on perceptions) to model risk. Dell et al. [30] used contact frequency for both hunters and cooks alongside responses to questions on attitudes and awareness to zoonotic diseases to conduct a principal components analysis to estimate the risks faced by a hunting community in northern Uganda. In recognition of the complexity within the bat wild meat chain, Kamins et al. [31] surveyed subsistence and commercial hunters separately, and categorised customer types to make risk assessments that can be linked to these pathways. These kinds of investigations draw on a rich knowledge base to make meaningful assessments even in the absence of surveillance equipment. They also allow for the design of horizontal interventions that target a major transmission pathway – i.e., wild meat/commercial hunters/local trade – as opposed to interventions which target a particular pathogen. Horizontal interventions are public health measures that aim to address multiple infectious diseases simultaneously by targeting common risk factors or modes of transmission. In the context of zoonotic diseases, such as those associated with wild meat consumption or wildlife trade, horizontal interventions can be designed to target major transmission pathways, such as commercial hunting or local trade, rather than focusing on individual pathogens.
Other commonly observed behaviours included in our review included the preservation of meat through thorough cooking, smoking, drying and curing; the avoidance of sick animals, high-risk taxa or tainted meat; and treating (or avoiding) injuries sustained from animal/meat handling. In contexts where clean water, soap, and personal protective equipment are scarce and alternative sources of protein and income are less available, integrating these practices may allow for individuals to balance the trade-off between hygiene and food security [32,33].
In some cases, we found that avoidance is not practiced due to the perception that the threat of zoonotic disease is non-existent [34,35], or a reluctance to waste food in areas historically affected by shortages [21].
4.1. Limitations
The terms used in our search strategy were chosen to capture studies with a combined focus on wild meat, pathogens, and sanitation. Many of the studies in our sample provide useful but implicit data on sanitary precautions, for example by stating the number of participants who reported avoiding eating undercooked meat. It is possible that this kind of information may also be present in studies that do not explicitly reference one of the three components we chose to address and thus may not have been captured. An alternative approach could be to create search strings for all known biosecurity practices, both active (hand washing) and passive (avoidance behaviours); however this was beyond the scope of the present study. This would also limit our findings to known practices, preventing the possibility of identifying unknown, innovative practices, particularly those which may be applied in low-resource settings.
5. Conclusions
Preventing the transmission of zoonotic diseases requires a multidisciplinary approach that involves cooperation between public health officials, wildlife conservationists, and local communities. This may involve implementing measures to reduce the demand for wildlife products, promoting safe food handling practices, improving sanitation and hygiene in communities, and supporting sustainable wildlife management practices.
It is also important to invest in research to better understand the ecology and transmission dynamics of zoonotic diseases to develop effective prevention and control strategies. Preventing the transmission of zoonotic diseases requires a coordinated and sustained effort across multiple sectors and stakeholders, and a long-term commitment to promoting behaviour change and sustainable practices. Understanding the safety of wild meat from production through to consumption is an important prerequisite to effective control [36].
Two articles in this review address the importation of wild meat specimens from Africa to Europe [37,38], and one from Africa to the USA [39]. We found that studies focused on European taxa hunted in Europe, dominate the literature with most of these focusing on pathogen surveillance as the primary disease risk reduction strategy. Asia and the Americas were underrepresented in the literature. Most research carried out in Europe concentrate on endemic food-borne pathogens, while the focus of African research has been on emerging zoonotic diseases [40]. In Europe, many of the common food-borne diseases such as Salmonella, Campylobacter, and Listeria are endemic and have been extensively studied. This research has led to the development of effective prevention and control measures, such as food safety regulations and guidelines for food handling and preparation. In contrast, in Africa, there is a higher burden of emerging zoonotic diseases, such as Ebola, Rift Valley fever, and Lassa fever, which are less well understood and have been the focus of more recent research efforts. This is partly since many of these diseases have only emerged or re-emerged in recent decades, and their epidemiology and transmission dynamics are still being studied. However, it is important to note that both food-borne and zoonotic diseases are important public health issues that require continued research and attention. The emergence of new food-borne pathogens and the threat of antimicrobial resistance highlight the need for ongoing surveillance and research globally. Similarly, the impact of zoonotic diseases on human health and the potential for spillover into global pandemics underscores the importance of studying these diseases in Africa and other regions of the world.
Some surveillance studies (n = 17) try to link surveillance with research on behaviours. We recommend coupling surveillance and behavioural research at various points along the wild meat supply chain to improve understanding of the effectiveness of specific behaviours for reducing disease risk. By also focusing on the actors within the wild meat supply chain, entry points for effective intervention can be identified; for example, by determining where high pathogen prevalence is coupled with risky handling behaviours.
Practices most frequently described in studies carried out in Africa were the heating of foods, treating animal bites, and avoiding high risk taxa and sick animals. These individual practices are important but to reduce the risk of endemic food-borne and emerging zoonotic disease transmission these actions should be coupled with adequate surveillance and regulatory approaches. In most cases, investment in basic infrastructure such as clean, accessible water for hand and surface washing and electricity for refrigeration, will likely have even larger marginal impacts.
Several studies found that people were not aware of zoonotic diseases or did not believe that they posed a risk to them suggesting a very important role for education and community engagement to convince people that these risks are real. This is a necessary condition for any risk-reducing behaviours to be adopted.
Though unevenly distributed geographically, the research we report in this paper demonstrates that there is an important cadre of studies on the different behaviours and practices used to reduce risk of disease transmission from wild meat handling across the globe. There is a need for more research, particularly in Asia and the Americas though overall, we highlight the need for more analytical research to evaluate the causal impacts of different behaviours and practices on disease transmission. Future international development efforts should, therefore, address structural inequalities and focus on capacity building, such that countries in the global south may share the benefits of advancements made in food safety.
Author contributions
Luke Tumelty, Julia Fa, Amy Ickowitz and Lauren Coad conceived the ideas and designed methodology; Luke Tumelty collected and analysed the data; Luke Tumelty and Julia Fa led the writing of the manuscript with substantial contributions from Amy Ickowitz, Sagan Friant, and Lauren Coad.Luke Tumelty, Amy Ickowitz, Sagan Friant, and Lauren Coad reviewed the abstracts and a sub-set of the articles for the Kappa Analysis. All authors edited the drafts and gave final approval for publication.
Funding information
This work was funded by the “Mitigating risks of disease transmission in the wild meat food chain from forest to fork in Cameroon” project (Agreement number: 81279235; Project processing number: 20.2256.4-002.00), funded by the German Agency for International Cooperation GmbH, Deutsche fur Internationale Zusammenarbeit (GIZ) as support to the International Alliance against Health Risks in Wildlife Trade. We acknowledge additional funding from United States Agency for International Development 's Forestry and Biodiversity Office, the UK Research and Innovation's Global Challenges Research Fund (UKRI GCRF) through the Trade, Development and the Environment Hub project (project number ES/S008160/1).
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100637.
Appendix A. Supplementary data
Supplementary fig. 1
Supplementary table 2
Details of all extracted data
Data availability
Data will be made available on request.
References
- 1.Pekar J.E., Magee A., Parker E., Moshiri N., Izhikevich K., Havens J.L., Gangavarapu K., Malpica Serrano L.M., Crits-Christoph A., Matteson N.L., Zeller M., Levy J.I., Wang J.C., Hughes S., Lee J., Park H., Park M.-S., Ching Zi Yan K., Lin R.T.P., Mat Isa M.N., Noor Y.M., Vasylyeva T.I., Garry R.F., Holmes E.C., Rambaut A., Suchard M.A., Andersen K.G., Worobey M., Wertheim J.O. The molecular epidemiology of multiple zoonotic origins of SARS-CoV-2. Science. 2022;377:960–966. doi: 10.1126/science.abp8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang X., Wang R. Wildlife trade is likely the source of SARS-CoV-2. Science. 2022;377:925–926. doi: 10.1126/science.add8384. [DOI] [PubMed] [Google Scholar]
- 3.Worobey M., Levy J.I., Malpica Serrano L., Crits-Christoph A., Pekar J.E., Goldstein S.A., Rasmussen A.L., Kraemer M.U.G., Newman C., Koopmans M.P.G., Suchard M.A., Wertheim J.O., Lemey P., Robertson D.L., Garry R.F., Holmes E.C., Rambaut A., Andersen K.G. The Huanan seafood wholesale market in Wuhan was the early epicenter of the COVID-19 pandemic. Science. 2022;377:951–959. doi: 10.1126/science.abp8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newell D.G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H., Opsteegh M., Langelaar M., Threfall J., Scheutz F., van der Giessen J., Kruse H. Food-borne diseases -– the challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010;139(Suppl. 1):S3–15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . World Health Organization; 2015. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007-2015. [Google Scholar]
- 6.Fegan N., Jenson I. The role of meat in foodborne disease: is there a coming revolution in risk assessment and management? Meat Sci. 2018;144:22–29. doi: 10.1016/j.meatsci.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Jay-Russell M., Doyle M.P., editors. Food Safety Risks from Wildlife: Challenges in Agriculture, Conservation, and Public Health. Springer International Publishing; Cham: 2016. [DOI] [Google Scholar]
- 8.Murray K.A., Allen T., Loh E., Machalaba C., Daszak P. Emerging viral Zoonoses from wildlife associated with animal-based food systems: risks and opportunities. Food Safety Risks Wildl. 2015;31–57 doi: 10.1007/978-3-319-24442-6_2. [DOI] [Google Scholar]
- 9.Van Vliet N., Moreno Calderón J.L., Gómez J., Zhou W., Fa J.E., Golden C., Nóbrega Alves R.R., Nasi R. 2017. Bushmeat and Human Health: Assessing the Evidence in Tropical and Sub-Tropical Forests. [DOI] [Google Scholar]
- 10.Kurpiers L.A., Schulte-Herbrüggen B., Ejotre I., Reeder D.M. Bushmeat and emerging infectious diseases: lessons from Africa. Problematic Wildl. 2015;507–551 doi: 10.1007/978-3-319-22246-2_24. [DOI] [Google Scholar]
- 11.Karesh W.B., Noble E. THE Bushmeat trade: increased opportunities for transmission of zoonotic disease: THE BUSHMEAT TRADE. Mt Sinai J. Med. 2009;76:429–434. doi: 10.1002/msj.20139. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe N.D., Escalante A.A., Karesh W.B., Kilbourn A., Spielman A., Lal A.A. Wild primate populations in emerging infectious disease research: the missing link? Emerg. Infect. Dis. 1998;4:149–158. doi: 10.3201/eid0402.980202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe N.D., Eitel M.N., Gockowski J., Muchaal P.K., Nolte C., Tassy Prosser A., Ndongo Torimiro J., Weise S.F., Burke D.S. Deforestation, hunting and the ecology of microbial emergence. Global Change Human Health. 2000;1:10–25. doi: 10.1023/A:1011519513354. [DOI] [Google Scholar]
- 14.Wolfe N.D., Heneine W., Carr J.K., Garcia A.D., Shanmugam V., Tamoufe U., Torimiro J.N., Prosser A.T., LeBreton M., Mpoudi-Ngole E., McCutchan F.E., Birx D.L., Folks T.M., Burke D.S., Switzer W.M. Emergence of unique primate T-lymphotropic viruses among central African bushmeat hunters. Proc. Natl. Acad. Sci. 2005;102:7994–7999. doi: 10.1073/pnas.0501734102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards P., Clarke M., DiGuiseppi C., Pratap S., Roberts I., Wentz R. Identification of randomized controlled trials in systematic reviews: accuracy and reliability of screening records. Stat. Med. 2002;21:1635–1640. doi: 10.1002/sim.1190. [DOI] [PubMed] [Google Scholar]
- 16.Jori F., Hernandez-Jover M., Magouras I., Dürr S., Brookes V.J. Wildlife–livestock interactions in animal production systems: what are the biosecurity and health implications? Anim. Front. 2021;11:8–19. doi: 10.1093/af/vfab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luis A.D., Hayman D.T.S., O’Shea T.J., Cryan P.M., Gilbert A.T., Pulliam J.R.C., Mills J.N., Timonin M.E., Willis C.K.R., Cunningham A.A., Fooks A.R., Rupprecht C.E., Wood J.L.N., Webb C.T. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Biol. Sci. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Engel R., Siegler S. WWF; Berlin: 2020. Pandora’s Box: A Report on the Human Zoonotic Disease Risk in South-East-Asia with a Focus on Wildlife Markets. [Google Scholar]
- 19.Allen T., Murray K.A., Zambrana-Torrelio C., Morse S.S., Rondinini C., Di Marco M., Breit N., Olival K.J., Daszak P. Global hotspots and correlates of emerging zoonotic diseases. Nat. Commun. 2017;8:1124. doi: 10.1038/s41467-017-00923-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aghokeng A.F., Ayouba A., Mpoudi-Ngole E., Loul S., Liegeois F., Delaporte E., Peeters M. Extensive survey on the prevalence and genetic diversity of SIVs in primate bushmeat provides insights into risks for potential new cross-species transmissions. Infect. Genet. Evol. 2010;10:386–396. doi: 10.1016/j.meegid.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonwitt J., Kelly A.H., Ansumana R., Agbla S., Sahr F., Saez A.M., Borchert M., Kock R., Fichet-Calvet E. Rat-atouille: A mixed method study to characterize rodent hunting and consumption in the context of Lassa fever. Ecohealth. 2016;13:234–247. doi: 10.1007/s10393-016-1098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smiley Evans T., Tutaryebwa L., Gilardi K.V., Barry P.A., Marzi A., Eberhardt M., Ssebide B., Cranfield M.R., Mugisha O., Mugisha E., Kellermann S., Mazet J.A.K., Johnson C.K. Suspected exposure to filoviruses among people contacting wildlife in southwestern Uganda. J. Infect. Dis. 2018;218:S277–S286. doi: 10.1093/infdis/jiy251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wirsiy F.S., Boock A.U., Akoachere J.-F.T.K. Assessing the determinants of Ebola virus disease transmission in Baka Community of the Tropical Rainforest of Cameroon. BMC Infect. Dis. 2021;21:324. doi: 10.1186/s12879-021-06011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebreyes W.A., Dupouy-Camet J., Newport M.J., Oliveira C.J.B., Schlesinger L.S., Saif Y.M., Kariuki S., Saif L.J., Saville W., Wittum T., Hoet A., Quessy S., Kazwala R., Tekola B., Shryock T., Bisesi M., Patchanee P., Boonmar S., King L.J. The global one health paradigm: challenges and opportunities for tackling infectious diseases at the human, animal, and environment Interface in low-resource settings. PLoS Negl. Trop. Dis. 2014;8 doi: 10.1371/journal.pntd.0003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peruzy M.F., Murru N., Smaldone G., Proroga Y.T.R., Cristiano D., Fioretti A., Anastasio A. Hygiene evaluation and microbiological hazards of hunted wild boar carcasses. Food Control. 2022;135 doi: 10.1016/j.foodcont.2021.108782. [DOI] [Google Scholar]
- 26.Forzan M., Pacini M.I., Periccioli M., Mazzei M. Hepatitis E virus RNA presence in wild boar carcasses at slaughterhouses in Italy. Animals. 2021;11:1624. doi: 10.3390/ani11061624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivastri A., Paoletti B., Lauteri C., Pennisi L., Paludi D., Festino A.R., Vergara A. Parasitic cysts in wild boars hunted in Central Italy: the sanitary controls in the wild game meats chain. Ital. J. Food Saf. 2021;10:9383. doi: 10.4081/ijfs.2021.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avagnina A., Nucera D., Grassi M.A., Ferroglio E., Dalmasso A., Civera T. The microbiological conditions of carcasses from large game animals in Italy. Meat Sci. 2012;91:266–271. doi: 10.1016/j.meatsci.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Mrkonjic Fuka M., Tanuwidjaja I., Zgomba Maksimovic A., Zunabovic-Pichler M., Kublik S., Hulak N., Domig K.J., Schloter M. Bacterial diversity of naturally fermented game meat sausages: sources of new starter cultures. LWT. 2020;118 doi: 10.1016/j.lwt.2019.108782. [DOI] [Google Scholar]
- 30.Dell B.M., Souza M.J., Willcox A.S. Attitudes, practices, and zoonoses awareness of community members involved in the bushmeat trade near Murchison falls National Park, northern Uganda. PLoS One. 2020;15 doi: 10.1371/journal.pone.0239599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamins A.O., Restif O., Ntiamoa-Baidu Y., Suu-Ire R., Hayman D.T.S., Cunningham A.A., Wood J.L.N., Rowcliffe J.M. Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa. Biol. Conserv. 2011;144:3000–3008. doi: 10.1016/j.biocon.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friant S., Paige S.B., Goldberg T.L. Drivers of Bushmeat hunting and perceptions of Zoonoses in Nigerian hunting communities. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friant S., Ayambem W.A., Alobi A.O., Ifebueme N.M., Otukpa O.M., Ogar D.A., Alawa C.B.I., Goldberg T.L., Jacka J.K., Rothman J.M. Eating Bushmeat Improves Food Security in a Biodiversity and Infectious Disease “Hotspot”. EcoHealth. 2020;17:125–138. doi: 10.1007/s10393-020-01473-0. [DOI] [PubMed] [Google Scholar]
- 34.Forgie E.M.E., Masumbuko Claude K., Hawkes M.T. Perceptions of ebola virus disease among the bambuti hunter group: a mixed-methods study. Pathog. Glob. Health. 2022;116:244–253. doi: 10.1080/20477724.2021.1970909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Breton M., Prosser A.T., Tamoufe U., Sateren W., Mpoudi-Ngole E., Diffo J.L.D., Burke D.S., Wolfe N.D. Patterns of bushmeat hunting and perceptions of disease risk among central African communities. Anim. Conserv. 2006;9:357–363. doi: 10.1111/j.1469-1795.2006.00030.x. [DOI] [Google Scholar]
- 36.Brashears M.M., Chaves B.D. The diversity of beef safety: A global reason to strengthen our current systems. Meat Sci. 2017;132:59–71. doi: 10.1016/j.meatsci.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 37.Chaber A.-L., Cunningham A. Public health risks from illegally imported African Bushmeat and smoked fish. EcoHealth. 2016;13:135–138. doi: 10.1007/s10393-015-1065-9. [DOI] [PubMed] [Google Scholar]
- 38.Temmam S., Davoust B., Chaber A.-L., Lignereux Y., Michelle C., Monteil-Bouchard S., Raoult D., Desnues C. Screening for viral pathogens in African simian Bushmeat seized at A French airport. Transbound. Emerg. Dis. 2017;64:1159–1167. doi: 10.1111/tbed.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith K.M., Anthony S.J., Switzer W.M., Epstein J.H., Seimon T., Jia H., Sanchez M.D., Huynh T.T., Galland G.G., Shapiro S.E., Sleeman J.M., McAloose D., Stuchin M., Amato G., Kolokotronis S.-O., Lipkin W.I., Karesh W.B., Daszak P., Marano N. Zoonotic viruses associated with illegally imported wildlife products. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute for Health Metrics and Evaluation, Human Development Network, The World Bank . Regional edition. 2013. The Global Burden of Disease: Generating Evidence, Guiding Policy — Sub-Saharan Africa. Seattle, WA. [Google Scholar]
- 41.Halbrook M., Gadoth A., Shankar A., Zheng H., Campbell E.M., Hoff N.A., Muyembe J.-J., Wemakoy E.O., Rimoin A.W., Switzer W.M. Human T-cell lymphotropic virus type 1 transmission dynamics in rural villages in the Democratic Republic of the Congo with high nonhuman primate exposure. PLoS Negl. Trop. Dis. 2021;15 doi: 10.1371/journal.pntd.0008923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ozioko K.U., Okoye C.I., Obiezue R.N., Agbu R.A. Knowledge, attitudes, and behavioural risk factors regarding zoonotic infections among bushmeat hunters and traders in Nsukka, Southeast Nigeria. Epidemiol. Health. 2018;40 doi: 10.4178/epih.e2018025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mulangu S., Alfonso V.H., Hoff N.A., Doshi R.H., Mulembakani P., Kisalu N.K., Okitolonda-Wemakoy E., Kebela B.I., Marcus H., Shiloach J., Phue J.-N., Wright L.L., Muyembe-Tamfum J.-J., Sullivan N.J., Rimoin A.W. Serologic evidence of ebolavirus infection in a population with no history of outbreaks in the Democratic Republic of the Congo. J. Infect. Dis. 2018;217:529–537. doi: 10.1093/infdis/jix619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esmaeili S., Pourhossein B., Gouya M.M., Amiri F.B., Mostafavi E. Seroepidemiological survey of Q fever and brucellosis in Kurdistan Province, Western Iran. Vector Borne Zoonotic Dis. 2014;14:41–45. doi: 10.1089/vbz.2013.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Djoko C.F., Wolfe N.D., Aghokeng A.F., Lebreton M., Liegeois F., Tamoufe U., Schneider B.S., Ortiz N., Mbacham W.F., Carr J.K., Rimoin A.W., Fair J.N., Pike B.L., Mpoudi-Ngole E., Delaporte E., Burke D.S., Peeters M. Failure to detect simian immunodeficiency virus infection in a large Cameroonian cohort with high non-human primate exposure. Ecohealth. 2012;9:17–23. doi: 10.1007/s10393-012-0751-0. [DOI] [PubMed] [Google Scholar]
- 46.Huong N.Q., Nga N.T.T., Long N.V., Luu B.D., Latinne A., Pruvot M., Phuong N.T., Quang L.T.V., Hung V.V., Lan N.T., Hoa N.T., Minh P.Q., Diep N.T., Tung N., Ky V.D., Roberton S.I., Thuy H.B., Long N.V., Gilbert M., Wicker L., Mazet J.A.K., Johnson C.K., Goldstein T., Tremeau-Bravard A., Ontiveros V., Joly D.O., Walzer C., Fine A.E., Olson S.H. Coronavirus testing indicates transmission risk increases along wildlife supply chains for human consumption in Viet Nam, 2013-2014. PLoS One. 2020;15 doi: 10.1371/journal.pone.0237129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suwannarong K., Soonthornworasiri N., Maneekan P., Yimsamran S., Balthip K., Maneewatchararangsri S., Saisongkorh W., Saengkul C., Sangmukdanun S., Phunta N., Singhasivanon P. Rodent-human Interface: behavioral risk factors and leptospirosis in a province in the central region of Thailand. Vet. Sci. 2022;9:85. doi: 10.3390/vetsci9020085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saylors K.E., Mouiche M.M., Lucas A., McIver D.J., Matsida A., Clary C., Maptue V.T., Euren J.D., LeBreton M., Tamoufe U. Market characteristics and zoonotic disease risk perception in Cameroon bushmeat markets. Soc. Sci. Med. 2021;268 doi: 10.1016/j.socscimed.2020.113358. [DOI] [PubMed] [Google Scholar]
- 49.Baudel H., De Nys H., Mpoudi Ngole E., Peeters M., Desclaux A. Understanding Ebola virus and other zoonotic transmission risks through human-bat contacts: exploratory study on knowledge, attitudes and practices in southern Cameroon. Zoonoses Public Health. 2019;66:288–295. doi: 10.1111/zph.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Narat V., Kampo M., Heyer T., Rupp S., Ambata P., Njouom R., Giles-Vernick T. Using physical contact heterogeneity and frequency to characterize dynamics of human exposure to nonhuman primate bodily fluids in Central Africa. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rimoin A.W., Alfonso V.H., Hoff N.A., Doshi R.H., Mulembakani P., Kisalu N.K., Muyembe J.-J., Okitolonda E.W., Wright L.L. Human exposure to wild animals in the Sankuru Province of the Democratic Republic of the Congo. Ecohealth. 2017;14:552–563. doi: 10.1007/s10393-017-1262-9. [DOI] [PubMed] [Google Scholar]
- 52.Mossoun A., Calvignac-Spencer S., Anoh A.E., Pauly M.S., Driscoll D.A., Michel A.O., Nazaire L.G., Pfister S., Sabwe P., Thiesen U., Vogler B.R., Wiersma L., Muyembe-Tamfum J.-J., Karhemere S., Akoua-Koffi C., Couacy-Hymann E., Fruth B., Wittig R.M., Leendertz F.H., Schubert G. Bushmeat hunting and zoonotic transmission of simian T-Lymphotropic virus 1 in tropical west and Central Africa. J. Virol. 2017;91 doi: 10.1128/JVI.02479-16. e02479–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quiner C.A., Moses C., Monroe B.P., Nakazawa Y., Doty J.B., Hughes C.M., McCollum A.M., Ibata S., Malekani J., Okitolonda E., Carroll D.S., Reynolds M.G. Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lawson E.T., Ohemeng F., Ayivor J., Leach M., Waldman L., Ntiamoa-Baidu Y. Understanding framings and perceptions of spillover: preventing future outbreaks of bat-borne zoonoses. Disaster Prev. Manag.: Int. J. 2017;26:396–411. doi: 10.1108/DPM-04-2016-0082. [DOI] [Google Scholar]
- 55.Mulangu S., Borchert M., Paweska J., Tshomba A., Afounde A., Kulidri A., Swanepoel R., Muyembe-Tamfum J.-J., Van der Stuyft P. High prevalence of IgG antibodies to Ebola virus in the Efé pygmy population in the Watsa region, Democratic Republic of the Congo. BMC Infect. Dis. 2016;16:263. doi: 10.1186/s12879-016-1607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mossoun A., Pauly M., Akoua-Koffi C., Couacy-Hymann E., Leendertz S.A.J., Anoh A.E., Gnoukpoho A.H., Leendertz F.H., Schubert G. Contact to non-human Primates and risk factors for zoonotic disease emergence in the Taï region, Côte d’Ivoire. Ecohealth. 2015;12:580–591. doi: 10.1007/s10393-015-1056-x. [DOI] [PubMed] [Google Scholar]
- 57.Suwannarong K., Chapman R.S., Lantican C., Michaelides T., Zimicki S. Hunting, food preparation, and consumption of rodents in Lao PDR. PLoS One. 2015;10 doi: 10.1371/journal.pone.0133150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kamins A.O., Rowcliffe J.M., Ntiamoa-Baidu Y., Cunningham A.A., Wood J.L.N., Restif O. Characteristics and risk perceptions of Ghanaians potentially exposed to bat-borne Zoonoses through Bushmeat. EcoHealth. 2015;12:104–120. doi: 10.1007/s10393-014-0977-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paige S.B., Frost S.D.W., Gibson M.A., Jones J.H., Shankar A., Switzer W.M., Ting N., Goldberg T.L. Beyond bushmeat: animal contact, injury, and zoonotic disease risk in Western Uganda. Ecohealth. 2014;11:534–543. doi: 10.1007/s10393-014-0942-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charania N.A., Martin I.D., Liberda E.N., Meldrum R., Tsuji L.J. Bird harvesting practices and knowledge, risk perceptions, and attitudes regarding avian influenza among Canadian first nations subsistence hunters: implications for influenza pandemic plans. BMC Public Health. 2014;14:1113. doi: 10.1186/1471-2458-14-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Subramanian M. Zoonotic disease risk and the bushmeat trade: assessing awareness among hunters and traders in Sierra Leone. Ecohealth. 2012;9:471–482. doi: 10.1007/s10393-012-0807-1. [DOI] [PubMed] [Google Scholar]
- 62.Messier V., Lévesque B., Proulx J.-F., Rochette L., Libman M.D., Ward B.J., Serhir B., Couillard M., Ogden N.H., Dewailly É., Hubert B., Déry S., Barthe C., Murphy D., Dixon B. Seroprevalence of toxoplasma gondii among Nunavik Inuit (Canada) Zoonoses Public Health. 2009;56:188–197. doi: 10.1111/j.1863-2378.2008.01177.x. [DOI] [PubMed] [Google Scholar]
- 63.Wolfe N.D., Switzer W.M., Carr J.K., Bhullar V.B., Shanmugam V., Tamoufe U., Prosser A.T., Torimiro J.N., Wright A., Mpoudi-Ngole E., McCutchan F.E., Birx D.L., Folks T.M., Burke D.S., Heneine W. Naturally acquired simian retrovirus infections in central African hunters. Lancet. 2004;363:932–937. doi: 10.1016/S0140-6736(04)15787-5. [DOI] [PubMed] [Google Scholar]
- 64.Wolfe N.D., Prosser A.T., Carr J.K., Tamoufe U., Mpoudi-Ngole E., Torimiro J.N., LeBreton M., McCutchan F.E., Birx D.L., Burke D.S. Exposure to nonhuman Primates in rural Cameroon. Emerg. Infect. Dis. 2004;10:2094. doi: 10.3201/eid1012.040062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ter Meulen J., Lukashevich I., Sidibe K., Inapogui A., Marx M., Dorlemann A., Yansane M.L., Koulemou K., Chang-Claude J., Schmitz H. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am. J. Trop. Med. Hyg. 1996;55:661–666. doi: 10.4269/ajtmh.1996.55.661. [DOI] [PubMed] [Google Scholar]
- 66.Maideen S.F.K., Lau S.F., Rashid A., Hod R., Shafie I.N.F. Knowledge and practices of bush meat consumption among indigenous people in Belum Forest, Malaysia: an analytical cross-sectional study. J. Clin. Health Sci. 2022;7:25–36. doi: 10.24191/jchs.v7i1.12988. [DOI] [Google Scholar]
- 67.Katani R., Schilling M.A., Lyimo B., Eblate E., Martin A., Tonui T., Cattadori I.M., Francesconi S.C., Estes A.B., Rentsch D., Srinivasan S., Lyimo S., Munuo C.K. Tiambo, Stomeo F., Gwakisa P., Mosha F., Hudson P.J., Buza J.J., Kapur V. Identification of Bacillus anthracis, Brucella spp., and Coxiella burnetii DNA signatures from bushmeat. Sci. Rep. 2021;11:14876. doi: 10.1038/s41598-021-94112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hingston P., Johnson K., Kitts D., Wang S. Safety and quality of fish and game meats prepared by first nations communities in British Columbia, Canada. J. Food Prot. 2020;83:896–901. doi: 10.4315/JFP-19-492. [DOI] [PubMed] [Google Scholar]
- 69.Hauptmann A.L., Paulová P., Hansen L.H., Sicheritz-Pontén T., Mulvad G., Nielsen D.S. Microbiota in foods from Inuit traditional hunting. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Katani R., Schilling M.A., Lyimo B., Tonui T., Cattadori I.M., Eblate E., Martin A., Estes A.B., Buza T., Rentsch D., Davenport K.W., Hovde B.T., Lyimo S., Munuo L., Stomeo F., Tiambo C.K., Radzio-Basu J., Mosha F., Hudson P.J., Buza J.J., Kapur V. Microbial diversity in bushmeat samples recovered from the Serengeti ecosystem in Tanzania. Sci. Rep. 2019 doi: 10.1038/s41598-019-53969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sauvala M., Laaksonen S., Laukkanen-Ninios R., Jalava K., Stephan R., Fredriksson-Ahomaa M. Microbial contamination of moose (Alces alces) and white-tailed deer (Odocoileus virginianus) carcasses harvested by hunters. Food Microbiol. 2019;78:82–88. doi: 10.1016/j.fm.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Zgomba Maksimovic A., Zunabovic-Pichler M., Kos I., Mayrhofer S., Hulak N., Domig K.J., Mrkonjic Fuka M. Microbiological hazards and potential of spontaneously fermented game meat sausages: A focus on lactic acid bacteria diversity. LWT. 2018;89:418–426. doi: 10.1016/j.lwt.2017.11.017. [DOI] [Google Scholar]
- 73.Baumann-Popczyk A., Popczyk B., Gołąb E., Rożej-Bielicka W., Sadkowska-Todys M. A cross-sectional study among polish hunters: seroprevalence of hepatitis E and the analysis of factors contributing to HEV infections. Med. Microbiol. Immunol. 2017;206:367–378. doi: 10.1007/s00430-017-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borilova G., Hulankova R., Svobodova I., Jezek F., Hutarova Z., Vecerek V., Steinhauserova I. The effect of storage conditions on the hygiene and sensory status of wild boar meat. Meat Sci. 2016;118:71–77. doi: 10.1016/j.meatsci.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Schielke A., Ibrahim V., Czogiel I., Faber M., Schrader C., Dremsek P., Ulrich R.G., Johne R. Hepatitis E virus antibody prevalence in hunters from a district in Central Germany, 2013: a cross-sectional study providing evidence for the benefit of protective gloves during disembowelling of wild boars. BMC Infect. Dis. 2015;15:440. doi: 10.1186/s12879-015-1199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Membré J.-M., Laroche M., Magras C. Assessment of levels of bacterial contamination of large wild game meat in Europe. Food Microbiol. 2011;28:1072–1079. doi: 10.1016/j.fm.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 77.Van der Merwe M., Michel A.L. An investigation of the effects of secondary processing on Mycobacterium spp. in naturally infected game meat and organs. J. S. Afr. Vet. Assoc. 2010;81:166–169. doi: 10.4102/jsava.v81i3.141. [DOI] [PubMed] [Google Scholar]
- 78.Cenci-Goga B., Amicabile A., Karama M., El-Ashram S., Saraiva C., García-Díez J., Finotti S., Genna V., Moretti G., Murari R., Muliari R., Bonizzato S., Lugoboni E., Cassini S., Dal-Ben C., Grispoldi L. Effect of delayed refrigeration on the microbial carcass contamination of wild boars (Sus scrofa) Animals. 2021;11:1434. doi: 10.3390/ani11051434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.González-Fuentes H., Hamedy A., von Borell E., Luecker E., Riehn K. Tenacity of Alaria alata mesocercariae in homemade German meat products. Int. J. Food Microbiol. 2014;176:9–14. doi: 10.1016/j.ijfoodmicro.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 80.Orsoni F., Romeo C., Ferrari N., Bardasi L., Merialdi G., Barbani R. Factors affecting the microbiological load of Italian hunted wild boar meat (Sus scrofa) Meat Sci. 2020;160 doi: 10.1016/j.meatsci.2019.107967. [DOI] [PubMed] [Google Scholar]
- 81.Mirceta J., Petrovic J., Malesevic M., Blagojevic B., Antic D. Assessment of microbial carcass contamination of hunted wild boars. Eur. J. Wildl. Res. 2017;63:37. doi: 10.1007/s10344-017-1096-3. [DOI] [Google Scholar]
- 82.CITES . Eleventh Meeting of the Conference of the Parties. Gigiri, Kenya. 2000. Bushmeat as a trade and wildlife management issue.https://cites.org/sites/default/files/eng/cop/11/doc/44.pdf Doc 11.4. Available: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary fig. 1
Supplementary table 2
Details of all extracted data
Data Availability Statement
Data will be made available on request.