Summary
Cancer screening has the potential to decrease mortality from several common cancer types. The first cancer screening programme in China was initiated in 1958 and the Cancer High Incidence Fields established in the 1970s have provided an extensive source of information for national cancer screening programmes. From 2012 onwards, four ongoing national cancer screening programmes have targeted eight cancer types: cervical, breast, colorectal, lung, oesophageal, stomach, liver, and nasopharyngeal cancers. By synthesising evidence from pilot screening programmes and population-based studies for various screening tests, China has developed a series of cancer screening guidelines. Nevertheless, challenges remain for the implementation of a fully successful population-based programme. The aim of this Review is to highlight the key milestones and the current status of cancer screening in China, describe what has been achieved to date, and identify the barriers in transitioning from evidence to implementation. We also make a set of implementation recommendations on the basis of the Chinese experience, which might be useful in the establishment of cancer screening programmes in other countries.
Introduction
Cancer is a major global health problem, with an estimated 19·3 million new cases and 10·0 million deaths in 2020.1 The leading six cancers—lung, colorectal, liver, stomach, female breast, and oesophageal—accounted for 55·8% of all cancer deaths in 2020. Cancer is an ageing-related disease. China has the world's largest population of older adults (individuals ≥60 years old) and is experiencing population ageing on an unprecedented scale.2 Nearly 24% of new cancer cases globally and approximately 30% of global cancer deaths in 2020 occurred in China.1 The epidemiological pattern of cancer in China is changing from that of a low-income or middle-income country to that of a high-income country. For example, cancer statistics indicate a decreasing burden of liver, stomach, and oesophageal cancers, but an increasing burden of lung, colorectal, and female breast cancers.3 As such, these demographic shifts and changing cancer epidemiological patterns in China might provide useful information for implementing population-based cancer screening programmes in various other countries.
Screening for specific cancers can detect the disease early, and treatment of cancers that are detected early has the potential to translate into mortality reductions. Screening has contributed to decreased mortality of several cancers over the past decades in the USA and other high-income countries.4, 5 Regular screenings are recommended for several of the most common cancers. Currently, WHO recommends screening programmes for cancers (ie, cervical, breast, and colorectal) that have been shown to be WHO best buys for different groups of countries.6 To date, lung cancer screening with low-dose CT has been implemented in some high-income countries, such as the USA, Canada, and South Korea.7 However, for cancers of the oesophagus, stomach, liver, and nasopharynx, screening programmes have generally been restricted to countries or areas with high incidence rates.8, 9
Cancer screening in China began in 1958 and has rapidly expanded its coverage in the past decade. Currently, China's national cancer screening programmes (NCSPs) include eight targeted cancer types (ie, cervical, breast, colorectal, lung, oesophageal, stomach, liver, and nasopharyngeal).10, 11 In 2019, cervical cancer screening and breast cancer screening were rolled out nationwide across China, whereas screening programmes for the remaining six cancers are restricted to selected pilot areas.10, 11, 12 Other than for cervical and breast cancers, screening programmes in China use a risk-assessment approach to identify individuals for screening, which is different to the approach used to screen asymptomatic individuals in most high-income countries' programmes. As China has a large population and limited resources, there are huge challenges to increasing national coverage, even with screening tests of proven efficacy. Even for cervical cancer, which has the highest coverage among the eight cancer types, China delivered at least 100 million cervical cancer screenings within 3 years but was only able to achieve national coverage of 36·8% in 2018–19.13 Although China has developed a series of cancer screening guidelines,12, 14, 15, 16, 17, 18, 19 there remains a steep road ahead in terms of transitioning from evidence to implementation.
Here, we highlight the key milestones and current status of cancer screening in China, describe what has been achieved, and identify the barriers to moving from evidence to implementation.
Key milestones in cancer screening
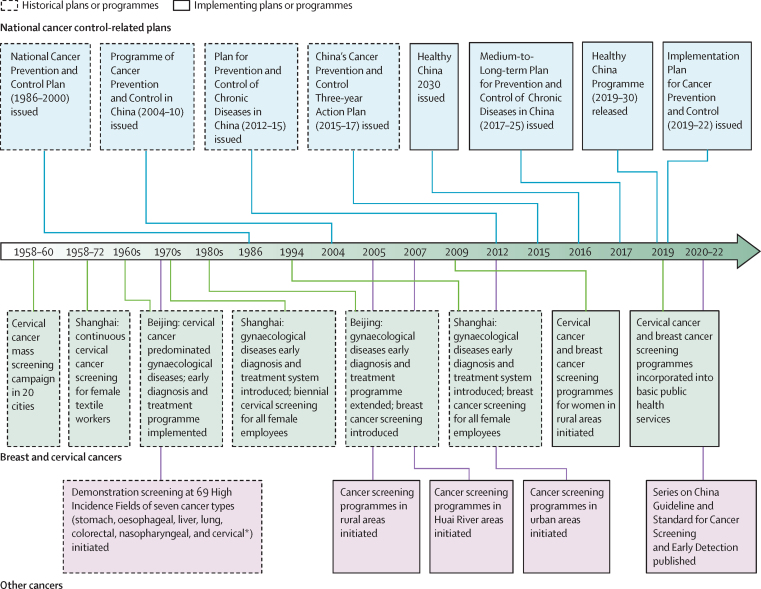
The first coordinated cancer screening programme in China was initiated in 20 cities in 1958 (figure 1), when cervical cytology (Pap smears) were used to screen for cervical cancer in 1·17 million women.20 Although this programme was stopped in most cities in 1960, cervical screening for female textile workers continued in Shanghai until 1972.21 After repeated cervical cancer screening, the observed incidence of cervical cancer in targeted populations fell by 93·1%.21 Shanghai and Beijing pioneered a system of delivering cervical and breast cancer screening through a gynaecological disease early diagnosis and treatment programme. Shanghai's regular cervical and breast cancer screening programme for all female employees was later widely adopted by other Chinese cities.21
Figure 1.
Key milestones in the development of cancer screening programmes in China
*Including 14 High Incidence Fields for cervical cancer prevention.
In high-risk areas identified by the first nationwide retrospective survey of cancer mortality conducted in 1973–75,22 China established 69 High Incidence Fields (ie, counties or cities that have an extremely high risk for specific cancer types) for prevention of seven major cancers in the 1970s (appendix p 1).23 Mass cancer screening in these areas was associated with a reduction in mortality from oesophageal cancer,24 gastric cancer,25 liver cancer,26 cervical cancer,27 and nasopharyngeal carcinomas.28 As a continuation of this programme of the High Incidence Fields, China launched the first NCSP, the Cancer Screening Programme in Rural Areas, in 2005, because most of the High Incidence Fields were located in rural China. In 2007, China launched the Cancer Screening Programme in the Huai River areas, to respond to the high incidence of upper gastrointestinal and liver cancers in this area. China launched the Cervical Cancer and Breast Cancer Screening Programme for Women in Rural Areas in 2009, aiming to provide 10 million cervical screenings and 1·2 million breast screenings annually, for rural women aged 35–64 years. From 2019 onwards, this programme was incorporated into basic public health services, in which cervical and breast cancer screenings were rolled out nationwide across mainland China. In 2012, China launched the Cancer Screening Programme in Urban Areas (figure 1).
Screening programme governance and coordination
Four NCSPs are funded by the central Chinese Government, and these provide the foundation of cancer screening in China (table 1). Eight cancer types (ie, cervical, breast, colorectal, lung, oesophageal, stomach, liver, and nasopharyngeal) are currently included in China's NCSPs.29 Screening programmes in urban and rural areas are done separately and the targeted cancer types for urban and rural areas were determined according to their cancer mortality patterns. Lung, colorectal, and breast cancer screening is prioritised in urban areas; oesophageal, gastric, liver, and cervical cancer are covered by screening programmes in rural areas. Screening for nasopharyngeal carcinomas is restricted to selected areas in south China, as other areas have a very low mortality rate.
Table 1.
Characteristics of the four national cancer screening programmes
| Initiation year | Target cancer sites | Target population | Coverage | |
|---|---|---|---|---|
| Cancer Screening Programme in Rural Areas | 2005 | Oesophagus, stomach, liver, colorectum, lung, nasopharynx, and cervix* | High-risk individuals identified by questionnaire-based risk assessment | Organised screening in 249 counties or districts across 31 provinces and opportunistic screening in 748 hospitals across 31 provinces |
| Cancer Screening Programme in Huai River Areas | 2007 | Oesophagus, stomach, and liver | High-risk individuals identified by questionnaire-based risk assessment | 38 counties or districts in four provinces |
| Cervical Cancer and Breast Cancer Screening Programme for Women (in Rural Areas)† | 2009 | Cervix and breast | Women aged 35–64 years | All counties or districts in 31 provinces |
| Cancer Screening Programme in Urban Areas | 2012 | Lung, colorectum, breast, oesophagus, stomach, and liver | High-risk individuals identified by questionnaire-based risk assessment or prescreening rapid tests‡ | 75 cities in 30 provinces§ |
Excluded from 2009 onwards to be incorporated in the Cervical Cancer and Breast Cancer Screening Programme for Women.
Not restricted to rural areas from 2019 onwards.
Including faecal immunochemical test, hepatitis B surface antigen test, and Helicobacter pylori antibody test.
Excluding Shanghai.
China's current system for management of the NCSPs consists of three tiers. National-level institutions are responsible for development of technical protocols and for conducting routine management and quality control. Provincial-level cancer hospitals or centres for disease control and prevention are responsible for the guidance and coordination of county-level and city-level screening institutions, which are the implementation units of local hospitals and centres for disease control and prevention. Generally, the local centre for disease control and prevention is responsible for population recruitment and risk assessment, and individuals assessed as high-risk are referred to their local hospital for screening, further investigation as required, and treatment. Each year, the geographical areas undertaking the NCSP provide free cancer screening for local residents until the number of people screened meets the targets assigned by the national screening management office. Generally, local project coordinators are encouraged to prioritise reaching individuals who have not previously been screened—ie, to preferentially focus on the first round of screening rather than repeated screening tests in people previously screened.
Programmes in China are a mix of organised (ie, eligible individuals are invited) and opportunistic screening.29 However, most programmes are organised. The sole exception is a component of the upper gastrointestinal cancer screening initiative in the Cancer Screening Programme in Rural Areas, which is an opportunistic screening programme established in 2014. Although opportunistic screening is considered to be much less effective than organised screening,6 China uses an opportunistic screening strategy when necessary to establish new financing models other than government funding.30, 31 In contrast to organised screening targeted at the whole at-risk population, opportunistic screening is targeted at people who visit clinical care settings for any purpose.31 Those determined to be at high risk for upper gastrointestinal cancer (eg, oesophageal cancer or gastric cancer) are invited to receive an endoscopic examination.31
The increasing coverage of cancer screening
Except for the Cervical Cancer and Breast Cancer Screening Programme for Women, the remaining three programmes are only able to cover a small proportion of the target population.29 Therefore, dozens of cancer screening programmes funded by local governments serve as an important complementary strategy for increasing local coverage (appendix p 2).
Coverage by the four NCSPs is expanding gradually.29 All provinces in mainland China have at least one county participating in the NCSP, with emphasis on counties (or districts) located in the areas of highest population density (figure 2). However, the total number of organised screenings for the top five cancers (ie, lung, liver, stomach, colorectal, and oesophageal) amounts to only 0·5 million people per year, while the target population in China is 534 million.32 It was estimated that the population-level coverage for the top five cancers in China was less than 1%.33, 34, 35 Local government-funded screening programmes and health examinations among employees may increase the proportion of people receiving screenings for the top five cancers, but currently there are no surveillance data or medical records to assess the exact screening rate.36, 37, 38 Population-level screening coverage of cervical cancer and breast cancer for women aged 35–64 years has gradually increased over the last decade (25% in 2010, 27% in 2013, 31% in 2015, and 37% in 2018–19 for cervical cancer; 23% in 2013, 26% in 2015, and 31% in 2018–19 for breast cancer).13, 39 As the Cervical Cancer and Breast Cancer Screening Programmes for Women were rolled out nationwide in 2019, the proportion of women who have received screenings is expected to continue to increase. A national goal is to achieve 70% coverage for cervical cancer screening by 2030.40, 41
Figure 2.
Geographical distribution of four national cancer screening programmes in China
Geographical distribution of the Cervical Cancer and Breast Cancer Screening Programme for Women is not shown, because this programme was rolled out nationwide in mainland China.
Identifying high-risk individuals for cancer screening
With the exception of the Cervical Cancer and Breast Cancer Screening Programme for Women, which provides screening for all women aged 35–64 years, the other three programmes are targeted at high-risk individuals identified by questionnaire-based risk assessment, with or without pre-screening rapid tests (ie, faecal immunochemical test, HBsAg test, Helicobacter pylori antibody test, and Epstein-Barr virus antibody test).12, 29 High-risk criteria incorporate risk exposures, personal history, family history, residential history in high-risk areas, and results of rapid tests (table 2). Lung cancer high-risk criteria include active smoking, passive smoking, presence of chronic obstructive pulmonary disease, occupational exposures, and family history of lung cancer.16 High-risk criteria for oesophageal cancer and gastric cancer include residential history in a high-risk area, personal history of precancerous lesions, family history, and exposure to related risk factors; high-risk criteria for gastric cancer also include H pylori infection.17, 18 Colorectal cancer high-risk criteria are family history of colorectal cancer, personal history of intestinal adenomas or inflammatory bowel diseases, and positive faecal immunochemical test results.14 Liver cancer high-risk criteria are cirrhosis, hepatitis B or hepatitis C chronic infection, and family history of liver cancer.19 Breast cancer high-risk criteria include family history of breast cancer or ovarian cancer, mutation in BRCA genes, atypical menstrual history, history of breast diseases, hormone replacement therapy use, previous atypical mammographic images, not breastfeeding, infertility, and having had one or more abortions.15 For cervical cancer, all women within the target age range (35–64 years) receive a cytology or human papillomavirus (HPV) test to screen for the disease.12 High-risk criteria for nasopharyngeal carcinomas include residential history in a high-risk area, positive Epstein-Barr virus antibody test, positive head and neck physical examination, and family history of nasopharyngeal carcinomas.42, 43
Table 2.
The target populations, high-risk criteria, screening technology, and screening frequency for the cancers currently being screened in China
| Target ages, years | High-risk criteria | Screening test | Frequency | |
|---|---|---|---|---|
| Lung cancer16 | 50–74 | Meet at least one of the following criteria: ≥30 pack-years of smoking and <15 years since quitting; exposed to passive smoking at home or in the workplace for ≥20 years; a diagnosis of chronic obstructive pulmonary disease; >1 year of regular occupational exposure to asbestos, radon, beryllium, chromium, cadmium, nickel, silica, coal smoke, or soot; first degree relative diagnosed with lung cancer | Low-dose CT | Every 2 years |
| Oesophageal cancer17 | 45–74, screening should be stopped for individuals with life expectancy less than 5 years | Meet at least one of the following criteria: permanent resident of area with high oesophageal cancer incidence (counties with age-standardised incidence rate >15 per 100 000); a diagnosis of oesophageal precancerous diseases or precancerous lesions; first degree relative diagnosed with oesophageal cancer; unhealthy lifestyles and dietary habits, such as smoking, drinking, and hot food and beverage thermal exposure | Endoscopy | Every 5 years for high-risk individuals |
| Gastric cancer18 | 45–74, screening should be stopped for individuals with life expectancy less than 5 years | Meet at least one of the following criteria: permanent resident of area with high gastric cancer incidence (counties with age-standardised incidence rate >20 per 100 000); Helicobacter pylori infection; a diagnosis of gastric precancerous conditions, such as chronic atrophic gastritis, gastric ulcer, gastric polyp, residual stomach after gastrectomy, hypertrophic gastritis, or pernicious anaemia; first degree relative diagnosed with gastric cancer; unhealthy lifestyles and dietary habits, such as smoking, drinking, and salted or pickled food consumption | Endoscopy | Every 1 to 3 years, as determined by the gastric cancer screening scoring system |
| Colorectal cancer14 | 50–74 for non-high-risk individuals, 40–74 for high-risk individuals | Meet at least one of the following criteria: first degree relative diagnosed with colorectal cancer; a diagnosis of intestinal adenomas; non-curative inflammatory bowel disease with duration of 8–10 years; positive faecal immunochemical test | Faecal immunochemical test followed by colonoscopy | Every 5–10 years for colonoscopy, annually for faecal immunochemical test |
| Liver cancer19 | 40–74, any age for patients with liver cirrhosis; screening should be stopped for individuals with life expectancy less than 5 years | Meet at least one of the following criteria: cirrhosis induced by any causes (no age restriction); hepatitis B or hepatitis C chronic infection; first degree relative diagnosed with liver cancer | Ultrasound and α-fetoprotein test | Annually |
| Breast cancer*15 | 45–64 for average risk, 40–64 for high risk | Meet at least one of the following criteria: first degree relative diagnosed with breast cancer or ovarian cancer; two or more second degree relatives diagnosed with breast cancer or ovarian cancer before the age of 50 years; oneself or one first degree relative carry pathogenic mutations in the BRCA1 and BRCA2 genes; age of menarche ≤12 years; age of menopause ≥55 years; history of breast biopsy or breast benign disease surgery, or history of atypical hyperplasia of breast (lobule or duct) confirmed by pathology; hormone replacement therapy with oestrogen and progestin combination >0·5 year; mammographic density asymmetry or dense breast tissue after age of 45 years. Meet any two of the following criteria: no breastfeeding or breastfeeding duration <4 months; no livebirth (including never giving birth, miscarriage, or stillbirth) or age above 30 years at first livebirth; hormone replacement therapy with oestrogen >0·5 year; abortion (including spontaneous abortion and induced abortion) ≥2 times | Average risk and non-dense breast: mammogram or ultrasound (if dense breast on mammogram); high-risk or dense breast: mammogram and ultrasound | Annually for high-risk; every 1–2 years for average risk |
| Breast cancer†12 | 35–64 | All women in the age group | Ultrasound with mammogram triage | Every 2–3 years |
| Cervical cancer12 | 35–64 | All women in the age group | Cytology or HPV test | Every 3 years for cytology; every 5 years for HPV test |
| Nasopharyngeal carcinoma42, 43 | 30–69 | Meet at least one of the following criteria: permanent resident of area with high nasopharyngeal carcinoma mortality (counties with age standardised mortality rate >3 per 100 000); positive or high-risk EBV antibody test; head and neck physical examination identifying a suspected nasopharyngeal carcinoma; first degree relative diagnosed with nasopharyngeal carcinoma | EBV serology test followed by nasopharyngoscopy | Every 5 years for negative EBV test; every 2 years for weakly positive EBV test; annually for strongly positive EBV test |
HPV=human papillomavirus. EBV=Epstein-Barr virus.
Used in the Cancer Screening Programme in Urban Areas.
Used in the Cervical Cancer and Breast Cancer Screening Programme for Women.
Tests and procedures adopted by cancer screening programme
Screening target age, frequency, and tests adopted by each NCSP are tailored to the Chinese population (table 2). Screening recommendations are based on age, geographical risk patterns, exposures, and feasibility. Some special recommendations are summarised in this section. For screening age, China has a lower target age than the US Preventive Services Task Force (USPSTF) recommendation (ie, 45–64 years vs 50–74 years) for breast cancer in those at average risk,44 whereas the starting age for cervical cancer screening is higher in China than the USPSTF recommendation (ie, 35 years vs 21 years).45 For screening frequency, although the guidelines recommend regular screening, currently one-time screening is provided by most areas of the NCSPs. Providing one-time screening is a pragmatic strategy, because it would be difficult to provide repeated screening for such a large population, and wider coverage provides more benefit than a focus on repeated screening of the same individuals.46 For screening tests, China uses combined screening by ultrasound and α-fetoprotein testing for liver cancer, and by ultrasound and mammogram for breast cancer among high-risk women or those with dense breasts.12, 15 WHO recommends using HPV DNA detection as the primary screening test in cervical cancer screening. However, only 8·6% of cervical screenings in 2018 in China used HPV testing.47, 48 WHO suggests that HPV mRNA detection can be used as a primary screening test, but it is currently rarely used in China.49 China's programmes might also use tests differently to programmes in other countries; for example, the faecal immunochemical test is used as a pre-screening test in China, rather than as a primary screening test as in many other countries.
NCSPs usually adopt a procedure of risk assessment, clinical screening, diagnostic investigation, treatment, and follow-up for each cancer type. Specifically, the Cancer Screening Programme in Urban Areas carries out multiple screening tests at the same screening visit. Using the risk criteria listed in table 2, local residents aged 45–74 years are assessed by a questionnaire interview, rapid tests for HBsAg and H pylori, and the faecal immunochemical test. Individuals assessed as high-risk for one or more cancers are invited to receive clinical screening tailored to their individual risk profile during the same visit. If all six cancers are assessed as not high-risk, no further screening would be done other than the breast cancer screen in average-risk women. Positive clinical screening results are referred for diagnostic investigation. Standard treatment is recommended for clinically confirmed diagnoses. Pre-cancerous lesions that do not meet treatment criteria and post-treatment patients with cancer will receive follow-up surveillance. Costs of treatment are not covered by NCSP; however, medical assistance programmes give priority to people with screening-detected cancer and low socioeconomic status.
Translating evidence into cancer screening policy
China developed cancer screening guidelines in 2020–22 on the basis of studies within the Chinese population, or other countries when necessary.12, 14, 15, 16, 17, 18, 19 These studies include large-scale cancer screening studies in China, including in the High Incidence Field, multicentre randomised controlled trials, single-arm population-based cohort studies, and NCSP-based real-world studies in the eight targeted cancer types (appendix p 3).
Informed by evidence from a multicentre randomised controlled trial and a real-world pilot study,47, 49, 51 high-risk HPV testing was officially incorporated into China's Cervical Cancer and Breast Cancer Screening Programme for Women in 2022.12 Real-world studies based on the Cancer Screening Programme in Urban and in Rural Areas pointed to the promise of one-time screening for lung cancer and upper gastrointestinal cancer.46, 52 Although Chinese real-world studies were large, their results should be interpreted with caution because they were prone to important confounding factors and study biases inherent to their design. However, these studies provide evidence in Asian people, a population that is under-represented in the majority of randomised controlled trials done in high-income countries.
Computer-aided detection and artificial intelligence in clinical imaging might improve the screening performance of clinical endoscopies.53, 54, 55 Blood-based biomarkers for liquid biopsy and risk prediction might have a role in refining the selection criteria of screenings for lung cancer,56, 57 gastric cancer,58 and nasopharyngeal carcinomas.59 Incorporating artificial intelligence and biomarkers could be promising in China because of the broad use of risk assessment and limitations in screening resources. However, these technologies are far from being used in population-based cancer screening programmes, as it is presently unclear how they can be appropriately applied to the screening pathway, there are implementation issues, and their cost-effectiveness is unclear in a population setting.
Challenges ahead for cancer screening
Although China is accelerating its efforts towards nationwide cancer screening, major challenges to the implementation of a successful population-based programme remain. Remaining hurdles include acceptance to undergo screening by those in the population who will derive benefit and health inequalities on the basis of socioeconomic status. Identification and implementation of measures to ensure high quality are also challenges to any screening programme.6 Only when the people most likely to benefit agree to have effective cancer screening tests and disease management can China realise the full potential of national screening programmes. Finally, there should be regular systematic formal assessment of outcomes and any new data that would suggest modification, intensification, or discontinuation of programmatic screening.
Affordability and large eligible population
In the yearly plenary meetings of the National People's Congress and the Chinese People's Political Consultative Conference, which are China's top legislature and top political advisory bodies, respectively, proposals and motions have been made to include cancer screening as a service to be covered by health insurance.11 However, regular cancer screening for the entire eligible population in China can be a daunting task, with 534 million adults aged 45–74 years as of 2020.32 Incorporating screening for another five cancer types (lung, liver, stomach, colorectal, and oesophageal cancer) in addition to cervical cancer and breast cancer screening for women into the basic public health services will lead to a huge investment gap, as the current annual investment of the Chinese government for five-cancer screening is estimated to be less than US$0·1 billion. A balanced strategy could be for the government to pay for basic one-time screening for high-risk populations, with subsequent screenings paid for by the individuals themselves.
Accessibility and limited health resources
There is geographical disparity in access to health resources in China.60 Geographical accessibility plays a key role in determining the effectiveness of cancer screening and care, an issue that is not limited to cancer because it affects a broad spectrum of health care. Local hospitals in resource-limited areas without up-to-date facilities cannot provide effective screening programmes, and addressing these situations would minimise missed diagnoses or misdiagnosis. Central government-facilitated health resource allocation, creating a balance between supply and demand, should be one of the key solutions to reduce inequalities between regions, instead of relying on locally fragmented decision making. A screening programme should be designed to ensure affordability, sustainability, and benefits to all segments of society. It is particularly important to address accessibility barriers for the most disadvantaged individuals and groups, such as highly vulnerable migrant populations61 and rural older people,62 who usually have particularly high cancer morbidity and mortality rates. Emergencies such as the COVID-19 pandemic can cause delays in cancer screenings,63 which highlights the need to develop more effective strategies to enhance the accessibility of screening. For instance, relying more on self-collection of samples at home for HPV detection and faecal immunochemical tests, and leveraging the investments made to build molecular testing capacities during the pandemic for HPV detection tests.64
Low uptake rates
Diagnostic yield and effectiveness of some cancer screening programmes in China are impeded by a relatively low uptake rate, particularly endoscopic screening.65 The reasons for the low uptake of freely available cancer screening tests and non-compliance with further evaluation might include low health literacy, fear of discomfort from invasive examinations, stigma, and anxiety. Participants might decline a colonoscopy due to its invasive nature and requirement for bowel preparation. Stigma and anxiety about the process of screening might deter some people, especially from participating in breast screening, cervical screening, and colonoscopy. Activities aimed to improve cancer-related health literacy for underserved populations, including public awareness campaigns that present what is known about the benefits and harms of each test, could increase public trust in NCSPs. New technologies, such as liquid biopsy, and more accurate risk prediction models also have the potential to improve the screening uptake rate, an important area for future study.
Insufficient screening quality
The key performance indicators for evaluating cancer screening quality in China include precancer and cancer detection rates, the proportion of cancers detected at an early stage, and the proportion of screen-detected cancers that receive treatment.66 A low detection rate has often been reported in routine quality assessments of the NCSPs.48, 67, 68, 69, 70, 71 However, it would also be important to determine whether screening has led to a reduction in late-stage and metastatic disease.46, 48, 68 Reliance solely on incidence of early-stage disease might obscure the problem of overdiagnosis. Performance measurements with key performance indicators rely on good-quality data collected through an effective information system. Quality assurance systems should be enhanced for China's cancer screening programmes. Further qualitative information on the quality of services can be collected through visits to facilities, self-assessment questionnaires, patient surveys, and audits of screening processes and cancer cases. This information must be linked with quality-improvement activities, and a dedicated team could be responsible for carrying out all such activities.
Screening-related harms and overdiagnosis
The primary goal of cancer screening is to reduce mortality from a specific cancer. However, most people who are screened do not have cancer, so many people can be exposed to the harms of screening without benefiting from it. Lead-time bias (when survival duration appears longer because diagnosis was made earlier) and length bias (when a relative excess of asymptomatic slowly progressing cases leads to an overestimation of survival duration) are the most common biases that can lead to the overestimation of screening benefits.72 Overdiagnosis is an extreme form of length bias and is considered one of the most serious harms of screening, alongside screening-related complications and false-positive screen results.72 Screening-related harms and overdiagnosis could be reduced by identifying high-risk individuals accurately and improving understanding of the possible consequences of a cancer screening test result. Investing in ongoing trials is key to mitigating harm while preserving any existing benefits. For example, identification of biomarkers of overdiagnosis might help further refine management of screen-detected lesions.
Learning from experience
The effort of transitioning from partial coverage to full coverage in cervical and breast cancer screenings might provide useful insights for other screening programmes. Mass screenings are based on screening techniques that are suitable for the Chinese setting, which not only have high diagnostic accuracy, but also are easy to promote.6, 50, 73 New screening technologies imported or domestically developed need to undergo population-based research in China.74, 75, 76 It will be important that reviews of synthesised evidence support the introduction of newer screening approaches, including features of efficacy, harms, acceptability, and cost-effectiveness.6 Gradually obtaining evidence from High Incidence Fields to pilot programmes can provide practical evidence for nationwide implementation and continuous improvement of screening strategies.51, 75 A successful universal cancer screening programme is built on a highly coordinated network of primary health-care institutions, such as a nationwide network of maternal and child health-care hospitals.77
China's experience might be a useful for other countries in developing national cancer screening programmes. First, a pilot project of cancer screening in high-incidence areas could help in generating evidence needed to better implement screening in other countries. Second, risk-stratified approaches incorporate high-risk areas and individual risk, such as urban–rural tailored programmes and risk assessment, which might improve screening efficiency. Third, pragmatic strategies including multiple screening tests at the same visit and one-time screening might enhance screening feasibility in a low-resource setting. Fourth, evidence, as it continues to build, on screening effectiveness for cancers of the cervix, breast, colorectum, lung, oesophagus, stomach, liver, and nasopharynx might support decision making in countries with similar settings. However, it is worth noting that at the current time, not all of China's target cancers are included in the WHO-recommended screening approaches.6
Conclusion
With the increasing burden of cancer, China has launched four NCSPs to provide screenings for six cancers (colorectal, lung, oesophageal, stomach, liver, and nasopharyngeal) in selected areas, and for cervical cancer and breast cancer in women nationwide. Although coverage for cancer screening has been expanded over the past decade, the majority of people who could benefit are still unscreened and underserved in China. Risk assessments might help to improve screening coverage and make NCSPs more effective and efficient, focusing on individuals at high risk. Although dozens of key studies and a series of guidelines on cancer screening have been published, China still has a steep road from evidence to national implementation. The challenges ahead to fully unlock the potential of cancer screening are manifold, including mobilising investment and health resources, increasing participation rates, and ensuring quality to reduce screening-related harms and overdiagnosis.
Search strategy and selection criteria
We searched PubMed, the China Academic Journals full-text database (otherwise known as the CNKI), and Wanfang Data, using the terms “screening” and “China”, “early detection” and “China”, “early diagnosis and treatment” and “China”, and “surveillance” and “China”, in combination with the terms “cervical cancer”, “breast cancer”, “colorectal cancer”, “lung cancer”, “esophageal cancer”, “gastric cancer”, “liver cancer”, “hepatocellular carcinoma”, and “nasopharyngeal carcinoma”, in the title or abstract, with no date restrictions (last search for all three databases was done on Jan 31, 2023). We searched Chinese governmental websites and identified articles through searches of the authors' own files. We reviewed English and Chinese language papers only. References of initially identified articles were retrieved to identify additional relevant papers. We generated the final reference list on the basis of the originality and relevance to the broad scope of this Review.
Declaration of interests
KC is co-Principal Investigator of an investigator-initiated trial of cervical screening, Compass, run by the Australian Centre for Prevention of Cervical Cancer (ACPCC), a government-funded not-for-profit charity. Compass receives infrastructural support from the Australian government and the ACPCC has received equipment and a funding contribution from Roche Molecular Diagnostics, USA. KC is also co-Principal Investigator on a major implementation programme, Elimination of Cervical Cancer in the Western Pacific, which has received support from the Minderoo Foundation, and equipment donations from Cepheid. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge funding from the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (number 2021-I2M-1-033), the National Natural Science Foundation of China (no 82273721), and the Sanming Project of the Medicine in Shenzhen (no SZSM201911015). The authors alone are responsible for the views expressed in this Review and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated. Where authors are identified as personnel of the International Agency for Research on Cancer/WHO, the authors alone are responsible for the views expressed in this Review and they do not necessarily represent the decisions, policy, or views of the International Agency for Research on Cancer/WHO.
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations with respect to territorial claims in published figures.
Contributors
CX and WC contributed to the conception and design of the study. CX and HL did the literature search and constructed the tables and figures. CX and WC contributed to the literature review. CX drafted and finalised the Review with input from PB, BSK, CQ, XQY, KC, YQ, and BKA. All authors contributed to data interpretation and approved the final manuscript for submission.
Supplementary Material
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Giles J, Yao Y, et al. The path to healthy ageing in China: a Peking University–Lancet commission. Lancet. 2022;400:1967–2006. doi: 10.1016/S0140-6736(22)01546-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia C, Dong X, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J. 2022;135:584–590. doi: 10.1097/CM9.0000000000002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 5.Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22:1002–1013. doi: 10.1016/S1470-2045(21)00199-6. [DOI] [PubMed] [Google Scholar]
- 6.WHO Regional Office for Europe A short guide to cancer screening: increase effectiveness, maximize benefits and minimize harm. 2022. https://apps.who.int/iris/handle/10665/351396
- 7.Adams SJ, Stone E, Baldwin DR, Vliegenthart R, Lee P, Fintelmann FJ. Lung cancer screening. Lancet. 2023;401:390–408. doi: 10.1016/S0140-6736(22)01694-4. [DOI] [PubMed] [Google Scholar]
- 8.Leung WK, Wu MS, Kakugawa Y, et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 9.Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 10.National Health Commission Reply to “Suggestions on strengthening major cancer screening”. 2021. http://www.nhc.gov.cn/wjw/jiany/202201/bb5e90b5773c45a7a8eb34c72b958a33.shtml
- 11.National Health Commission Reply to “Suggestions on national health insurance includes the nationwide screening for common cancers”. 2022. http://www.nhc.gov.cn/wjw/jiany/202201/7e05490d44d04ceebc11a2803eefd19e.shtml
- 12.National Health Commission Cervical cancer and breast cancer screening work programme. 2022. http://www.nhc.gov.cn/fys/s3581/202201/cad44d88acca4ae49e12dab9176ae21c.shtml
- 13.Zhang M, Zhong Y, Wang L, et al. Cervical cancer screening coverage—China, 2018-2019. China CDC Wkly. 2022;4:1077–1082. doi: 10.46234/ccdcw2022.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Center, China. Expert Group of the Development of China Guideline for the Screening. Early Detection and Early Treatment of Colorectal Cancer China guideline for the screening, early detection and early treatment of colorectal cancer (2020, Beijing) Chin J Oncol. 2021;43:16–38. doi: 10.3760/cma.j.cn112152-20210105-00010. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 15.He J, Chen WQ, Li N, et al. China guideline for the screening and early detection of female breast cancer (2021, Beijing) Chin J Oncol. 2021;43:357–382. doi: 10.3760/cma.j.cn112152-20210119-00061. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 16.He J, Li N, Chen WQ, et al. China guideline for the screening and early detection of lung cancer (2021, Beijing) Chin J Oncol. 2021;43:243–268. doi: 10.3760/cma.j.cn112152-20210119-00060. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 17.He J, Chen WQ, Li Z, et al. China guideline for the screening, early detection and early treatment of esophageal cancer (2022, Beijing) Chin J Oncol. 2022;44:491–522. doi: 10.3760/cma.j.cn112152-20220517-00348. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 18.He J, Chen W, Li ZS, et al. China guideline for the screening, early detection and early treatment of gastric cancer (2022, Beijing) Chin J Oncol. 2022;44:634–666. doi: 10.3760/cma.j.cn112152-20220617-00430. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 19.He J, Chen WQ, Shen H, et al. China guideline for liver cancer screening (2022, Beijing) Chin J Oncol. 2022;44:779–814. (in Chinese). [Google Scholar]
- 20.Lim KT, Kao JC, Chang CF, Ch'En PC. Mass survey for cancer of cervix uteri in China. Preliminary report. Chin Med J. 1962;81:705–712. [PubMed] [Google Scholar]
- 21.Zheng Y. Seventy years of cancer prevention and control in Shanghai. Chinese Health Resources. 2019;22:269–273. (in Chinese). [Google Scholar]
- 22.Wei W, Zeng H, Zheng R, et al. Cancer registration in China and its role in cancer prevention and control. Lancet Oncol. 2020;21:e342–e349. doi: 10.1016/S1470-2045(20)30073-5. [DOI] [PubMed] [Google Scholar]
- 23.Dong Z, Qiao Y, Li L, et al. A report of cancer high incidence scene in china. China Cancer. 2009;18:4–9. (in Chinese). [Google Scholar]
- 24.He Y, Yang L, Hou J, et al. The prevalence trend of esophageal cancer in Cixian of Hebei Province and Linxian of Henan Province. Cancer Res Prev Treat. 2001;28:485–486. (in Chinese). [Google Scholar]
- 25.Ma J, Liu W, Zhang L, Feng G, Zhao H, You W. Analysis of mortality trend of cancer from 1980 to 2002 in Linqu County Shandong Province. Chin J Prev Med. 2006;40:405–408. (in Chinese). [PubMed] [Google Scholar]
- 26.Chen J, Zhu J, Zhang Y, et al. Trends in the mortality of liver cancer in Qidong, China: an analysis of fifty years. Chin J Oncol. 2012;34:532–537. doi: 10.3760/cma.j.issn.0253-3766.2012.07.012. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 27.Li L, Li L. Analysis of cervical cancer prevention and control in rural areas of the Jiangxi province. Chin J Clin Oncol. 2014;41:947–950. (in Chinese). [Google Scholar]
- 28.Wei K, Liang Z, Liu X, Lin M, Liu Q. Effect of Epstein-Barr virus serological screening on mortality of nasopharyngeal carcinoma. Chin J Epidemiol. 2003;24:171. (in Chinese). [Google Scholar]
- 29.National Health Commission Reply to “Suggestions on the implementation of nationwide cancer screening programme for high incidence cancers”. 2021. http://www.nhc.gov.cn/wjw/jiany/202102/9057fd5dfecc49c5a05e7a1c3cd9adcb.shtml
- 30.Xu Z. Significance of the opportunistic cancer screening and medical examination for cancer in the cancer control system. Chin J Health Manage. 2019;13:369–375. (in Chinese). [Google Scholar]
- 31.Wang G, Wei W. A new transition of the screening, early diagnosis and early treatment project of the upper gastrointestinal cancer: opportunistic screening. Chin J Prev Med. 2019;53:1084–1087. doi: 10.3760/cma.j.issn.0253-9624.2019.11.002. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 32.Office of the Leading Group of the State Council for the Seventh National Population Census China Population Census Yearbook 2020. 2022. http://www.stats.gov.cn/sj/pcsj/rkpc/7rp/indexch.htm
- 33.Zeng H, Ran X, An L, et al. Disparities in stage at diagnosis for five common cancers in China: a multicentre, hospital-based, observational study. Lancet Public Health. 2021;6:e877–e887. doi: 10.1016/S2468-2667(21)00157-2. [DOI] [PubMed] [Google Scholar]
- 34.An L, Zheng R, Zeng H, et al. The survival of esophageal cancer by subtype in China with comparison to the United States. Int J Cancer. 2023;152:151–161. doi: 10.1002/ijc.34232. [DOI] [PubMed] [Google Scholar]
- 35.Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 36.Zhu C, Gong W, Zhong J, et al. Zhejiang colorectal cancer screening program: overview and study design. China Cancer. 2020;29:899–903. (in Chinese). [Google Scholar]
- 37.Gong Y, Peng P, Bao P, et al. The implementation and first-round results of a community-based colorectal cancer screening program in Shanghai, China. Oncologist. 2018;23:928–935. doi: 10.1634/theoncologist.2017-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong W, Cheng KK. Challenges in screening and general health checks in China. Lancet Public Health. 2022;7:e989–e990. doi: 10.1016/S2468-2667(22)00207-9. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Bao H, Zhang X, et al. Breast cancer screening coverage—China, 2018–2019. China CDC Wkly. 2023;5:321–326. doi: 10.46234/ccdcw2023.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.State Council of China China national program for women's development (2021–2030) Sept 27, 2021. http://www.gov.cn/zhengce/content/2021-09/27/content_5639412.htm
- 41.State Council of China Action plan for accelerating the elimination of cervical cancer (2023–2030) Jan 5, 2023. http://www.gov.cn/zhengce/zhengceku/2023-01/21/content_5738364.htm
- 42.Huang T, Wang H, Li J, et al. Screening program for nasopharyngeal carcinoma (NPC)—early detection for NPC. Chin J Cancer. 1997;16:245–247. (in Chinese). [Google Scholar]
- 43.Chinese Center for Disease Control and Prevention Technical proposal for early diagnosis and treatment of nasopharyngeal carcinoma. Feb 8, 2007. https://www.chinacdc.cn/jkzt/mxfcrjbhsh/zl/fzcl/200702/t20070208_42652.html
- 44.Siu AL. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164:279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- 45.Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA. 2018;320:674–686. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- 46.Li N, Tan F, Chen W, et al. One-off low-dose CT for lung cancer screening in China: a multicentre, population-based, prospective cohort study. Lancet Respir Med. 2022;10:378–391. doi: 10.1016/S2213-2600(21)00560-9. [DOI] [PubMed] [Google Scholar]
- 47.WHO WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, 2nd ed. July 6, 2021. https://www.who.int/publications/i/item/9789240030824 [PubMed]
- 48.Zhao YX, Ma L, Ren WH, et al. Analysis of the reported data of national cervical cancer screening program in rural areas in china from 2009 to 2018. Natl Med J China. 2021;101:1863–1868. doi: 10.3760/cma.j.cn112137-20210111-00075. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 49.WHO WHO guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention, second edition: use of mRNA tests for human papillomavirus (HPV) Dec 21, 2021. https://www.who.int/publications/i/item/9789240040434 [PubMed]
- 50.Zhang J, Zhao Y, Dai Y, et al. Effectiveness of high-risk human papillomavirus testing for cervical cancer screening in China: a multicenter, open-label, randomized clinical trial. JAMA Oncol. 2021;7:263–270. doi: 10.1001/jamaoncol.2020.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Y, Bao H, Ma L, et al. Real-world effectiveness of primary screening with high-risk human papillomavirus testing in the cervical cancer screening programme in China: a nationwide, population-based study. BMC Med. 2021;19:164. doi: 10.1186/s12916-021-02026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen R, Liu Y, Song G, et al. Effectiveness of one-time endoscopic screening programme in prevention of upper gastrointestinal cancer in China: a multicentre population-based cohort study. Gut. 2021;70:251–260. doi: 10.1136/gutjnl-2019-320200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang P, Berzin TM, Glissen Brown JR, et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813–1819. doi: 10.1136/gutjnl-2018-317500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P, Liu X, Berzin TM, et al. Effect of a deep-learning computer-aided detection system on adenoma detection during colonoscopy (CADe-DB trial): a double-blind randomised study. Lancet Gastroenterol Hepatol. 2020;5:343–351. doi: 10.1016/S2468-1253(19)30411-X. [DOI] [PubMed] [Google Scholar]
- 55.Luo H, Xu G, Li C, et al. Real-time artificial intelligence for detection of upper gastrointestinal cancer by endoscopy: a multicentre, case-control, diagnostic study. Lancet Oncol. 2019;20:1645–1654. doi: 10.1016/S1470-2045(19)30637-0. [DOI] [PubMed] [Google Scholar]
- 56.Dai J, Lv J, Zhu M, et al. Identification of risk loci and a polygenic risk score for lung cancer: a large-scale prospective cohort study in Chinese populations. Lancet Respir Med. 2019;7:881–891. doi: 10.1016/S2213-2600(19)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin N, Wang C, Chen C, et al. Association of the interaction between mosaic chromosomal alterations and polygenic risk score with the risk of lung cancer: an array-based case-control association and prospective cohort study. Lancet Oncol. 2022;23:1465–1474. doi: 10.1016/S1470-2045(22)00600-3. [DOI] [PubMed] [Google Scholar]
- 58.Jin G, Lv J, Yang M, et al. Genetic risk, incident gastric cancer, and healthy lifestyle: a meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020;21:1378–1386. doi: 10.1016/S1470-2045(20)30460-5. [DOI] [PubMed] [Google Scholar]
- 59.He YQ, Wang TM, Ji M, et al. A polygenic risk score for nasopharyngeal carcinoma shows potential for risk stratification and personalized screening. Nat Commun. 2022;13 doi: 10.1038/s41467-022-29570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Q, Jiao J. Health disparity and cancer health disparity in China. Asia Pac J Oncol Nurs. 2016;3:335–343. doi: 10.4103/2347-5625.195899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo Y, Guo C, Wang Y, Zheng X. Trends and challenges for population health and migration—China, 2015–2050. China CDC Wkly. 2020;2:520–524. doi: 10.46234/ccdcw2020.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang N, Xu J, Ma M, et al. Targeting vulnerable groups of health poverty alleviation in rural China—what is the role of the new rural cooperative medical scheme for the middle age and elderly population? Int J Equity Health. 2020;19:161. doi: 10.1186/s12939-020-01236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Villain P, Carvalho AL, Lucas E, et al. Cross-sectional survey of the impact of the COVID-19 pandemic on cancer screening programs in selected low- and middle-income countries: study from the IARC COVID-19 impact study group. Int J Cancer. 2021;149:97–107. doi: 10.1002/ijc.33500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Basu P, Lucas E, Zhang L, Muwonge R, Murillo R, Nessa A. Leveraging vertical COVID-19 investments to improve monitoring of cancer screening programme—a case study from Bangladesh. Prev Med. 2021;151 doi: 10.1016/j.ypmed.2021.106624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen H, Li N, Ren J, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut. 2019;68:1450–1457. doi: 10.1136/gutjnl-2018-317124. [DOI] [PubMed] [Google Scholar]
- 66.Dong Z, Qiao Y, Wang G, et al. The exploration of evaluating indicators for early detection and treatment of cancers in China. China Cancer. 2010;19:633–638. (in Chinese). [Google Scholar]
- 67.Zhang X, Chen W, Zhu X, et al. Multiple center research on relationship between screening quality and detection of cervical cancer—six provinces, China, June–December 2021. China CDC Wkly. 2023;5:301–305. doi: 10.46234/ccdcw2023.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang SX, Wu JL, Zheng RM, et al. A preliminary cervical cancer screening cascade for eight provinces rural Chinese women: a descriptive analysis of cervical cancer screening cases in a 3-stage framework. Chin Med J. 2019;132:1773–1779. doi: 10.1097/CM9.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen W, Li N, Cao M, et al. Preliminary analysis of cancer screening program in urban China from 2013 to 2017. China Cancer. 2020;29:1–6. (in Chinese). [Google Scholar]
- 70.Li J, Li H, Zeng H, et al. Trends in high-risk rates and screening rates for the population-based cancer screening program on esophageal, stomach and liver cancer in China, 2010–2016. J Natl Cancer Cent. 2021;1:101–107. doi: 10.1016/j.jncc.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cancer Foundation of China Annual Report of Cancer Foundation of China, 2022. April 18, 2023. http://www.cfchina.org.cn/show.php?contentid=2725
- 72.Ripping TM, Ten Haaf K, Verbeek ALM, van Ravesteyn NT, Broeders MJM. Quantifying overdiagnosis in cancer screening: a systematic review to evaluate the methodology. J Natl Cancer Inst. 2017;109 doi: 10.1093/jnci/djx060. [DOI] [PubMed] [Google Scholar]
- 73.Shen S, Zhou Y, Xu Y, et al. A multi-centre randomised trial comparing ultrasound vs mammography for screening breast cancer in high-risk Chinese women. Br J Cancer. 2015;112:998–1004. doi: 10.1038/bjc.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qiao YL, Sellors JW, Eder PS, et al. A new HPV-DNA test for cervical-cancer screening in developing regions: a cross-sectional study of clinical accuracy in rural China. Lancet Oncol. 2008;9:929–936. doi: 10.1016/S1470-2045(08)70210-9. [DOI] [PubMed] [Google Scholar]
- 75.Zhao FH, Lin MJ, Chen F, et al. Performance of high-risk human papillomavirus DNA testing as a primary screen for cervical cancer: a pooled analysis of individual patient data from 17 population-based studies from China. Lancet Oncol. 2010;11:1160–1171. doi: 10.1016/S1470-2045(10)70256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan QJ, Hu SY, Zhang X, et al. Pooled analysis of the performance of liquid-based cytology in population-based cervical cancer screening studies in China. Cancer Cytopathol. 2013;121:473–482. doi: 10.1002/cncy.21297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qiao J, Wang Y, Li X, et al. A Lancet Commission on 70 years of women's reproductive, maternal, newborn, child, and adolescent health in China. Lancet. 2021;397:2497–2536. doi: 10.1016/S0140-6736(20)32708-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.