Abstract
Understanding antibiotic use in livestock systems is key in combating antimicrobial resistance (AMR) and developing effective interventions. Using a standardised questionnaire, we investigated the patterns and drivers of antibiotic use in 165 cattle farms across the three major cattle production systems in Kenya: intensive, extensive, and semi-intensive systems across in three counties: Machakos, Makueni and Narok in Kenya. We used a causal diagram to inform regression models to explore the drivers of antibiotic use in the study farms. Antibiotic use was reported in 92.7% of farms, primarily for prophylactic purposes. Oxytetracycline, penicillin, and streptomycin were the most used antibiotics to treat and control the most reported diseases including mastitis, diarrhoea and East Coast fever (ECF). Regression analysis indicated a positive association between the frequency of antibiotic use at the farm level and both disease incidence and herd size. Conversely, farms that provided cattle with appropriate housing were less likely to use antibiotics, and there was no difference in antibiotic use between those who consulted with veterinarians or sourced antibiotics directly from animal health providers. Our study highlights the complexities around understanding the interplay between practices and drivers of antibiotic use. It also underscores the necessity to enhance education regarding the appropriate usage of antibiotics among cattle farmers, encourage the adoption of proper herd management practices which may reduce disease burden, and reinforce veterinary services and supportive legislation to promote the prudent use of antimicrobials.
Keywords: Antimicrobial resistance, Livestock, LMIC, Semi-intensive
1. Background
Antimicrobial stewardship (AMS) programs in animal production systems and human health are critical for combating antimicrobial resistance (AMR) [1]. Irrational use of antibiotics in animals is a major driver of AMR globally [2]. In livestock systems, antibiotics are used for therapy, growth promotion, prophylaxis and metaphylaxis [3]. Therapeutic use is controlled by prescription and antibiotic label instructions or through extra-label guidelines by a qualified prescriber e.g., a veterinarian [3]. However, in most low and middle-income countries (LMICs), farmers self-diagnose and administer antibiotics without proper guidance from a veterinarian [4]. Moreover, the use of falsified or sub-standard antimicrobials has been reported due to poor regulatory systems [5], potentially resulting in misuse or overuse which may contribute to the selection and emergence of antimicrobial-resistant pathogens [4]. To mitigate AMR, the World Organization for Animal Health (WOAH), the World Health Organization (WHO), and the Food and Agricultural Organization of the United Nations (FAO) have advocated for a collaborative ‘One Health’ approach [6,7], promoting prudent and optimised use of antimicrobials and the development of AMS programs in both human and animal sectors, given the increased risk of AMR pathogen transmission from livestock farms into the environment and agri-food chain [8]. To inform AMS efforts, data on antimicrobial consumption and the drivers of antimicrobial use (AMU) in food-producing animals is required. One of the aims of Kenya's National Action Plan on AMR is to optimise the use of antimicrobials in animal and human health while considering the needs of the country [6]. It is therefore important to generate local evidence to develop realistic stewardship programmes for the Kenyan agricultural sector without negatively impacting farmers' livelihoods and food security.
Cattle production in Kenya encompasses diverse systems that are structured according to the country's geography, climate, and cultural practices. According to the FAO report of 2018, these production systems can be categorized into traditional extensive, semi-intensive, and intensive or zero-grazing systems [9]. Extensive systems involve free-ranging herding on communal lands or open rangelands, relying on natural grazing resources. Depending on the level of pasture management and interventions, extensive cattle production may be practised as pastoralism, which is traditionally uncontrolled – minimal management interventions, or as ranching, whereby some level of management practices such as rotational grazing or paddocking within a defined tract of land is implemented. Semi-intensive systems combine grazing with supplementary feeds and are commonly found in peri-urban areas or small-scale farms. Intensive systems confine cattle to controlled spaces, such as barns or feedlots. Large-scale intensive systems are characterised by their significant herd sizes and investments in infrastructure, while small-scale intensive systems are operated by individual farmers or small-scale farming enterprises.
Most cattle production systems in Kenya are dual-purpose, encompassing both beef and dairy production. These systems are vital to the country's agricultural sector, contributing significantly to the economy and meeting the demand for both meat and milk products. In particular, the dairy production system contributes approximately 80% to the livestock production sector. Milk consumption is a regular part of the daily diet [10] in Kenya with a significant proportion being supplied by small-holder farmers who either consume the milk domestically or sell it directly at the farm gate [11,12]. These smallholder farmers face various challenges such as poor farm biosecurity practices, disease outbreaks and low milk productivity, resulting in antibiotic overuse [13]. A previous study conducted in Kenyan cattle production systems revealed that 10% of the milk from small-holder dairy farmers contained beta-lactam residue levels that exceeded the maximum allowed limits [14] Similarly, the beef production sector in Kenya also contributes significantly to antibiotic usage. Previous research also shows high levels of antibiotic use in the beef industry [15] mirroring the challenges observed in the dairy sector. Veterinary drug stores which are sometimes operated by unqualified animal health professionals (AHPs) have been previously reported as the main sources of antibiotics (63% usage) for livestock in Kenya [16] A previous study indicated that irrational use of antibiotics in food-producing animals as compensation for poor animal husbandry practices in Kenya is a significant contributor to the rising AMR rates, and reducing such usage was identified as a national priority [17].
Despite the growing availability of data on AMU in LMICs, there is a lack of knowledge about the patterns and factors that drive AMU in Kenyan cattle production systems which will play a key role in informing the development of AMS programmes. This study therefore aimed to investigate patterns of antibiotic use, practices and factors that drive the use of antibiotics in cattle production systems in Kenya. We hypothesized that there could be a link between farm profiles, herd management practices, disease incidence and the use of antibiotics, which could be used to inform measures that could reduce the need for antibiotics for growth promotion and disease control.
2. Materials and methods
2.1. Study sites and sampling
A cross-sectional study targeting different cattle production systems including intensive, semi-intensive and extensive systems, as previously defined (FAO, 2018), was conducted between October and December 2021. A total of 165 farms across the three major production systems were sampled in three counties including Narok, Machakos and Makueni in Kenya. Narok county is characterised by a large-scale, free-range cattle production system with a majority of the farms keeping exclusively indigenous breeds of cattle and a few semi-intensive farms while in Makueni and Machakos counties, there is a mix of farmers practising either exclusively zero-grazing or both zero-grazing and free-range (semi-zero) cattle production systems [18,19]. The study's sample size was estimated based on previous estimates; about 90% of food-producing animals receive medication at some point during their lifespan [9] a precision level of 5%, and a confidence level of 95%, which resulted in a sample size of 139 cattle farms. To account for potential missing data and other factors such as study cost and time constraints, the sample size was increased to 165 (Fig. 1). We obtained a sampling frame comprising all farmers from each of the selected counties with the assistance of county veterinary officers from which we randomly selected 165 farms stratified by the estimated number of livestock in the counties and by the reported production systems according to FAO, 2018. Therefore 38 farms were sampled in Machakos, 23 in Makueni and 104 in Narok Counties to achieve equitable representation of cattle production systems and cattle densities across each county while also accounting for practical considerations in data collection.
Fig. 1.
Sampling sites Machakos, Makueni and Narok Counties in Kenya.
2.2. Data collection
The ‘Antimicrobial use in livestock production systems’ (AMUSE) questionnaire (developed and validated by the International Livestock Research Institute (ILRI) [20] was customised and adopted for this study (Supplementary Material). An Open Data Kit (ODK) version of the questionnaire was created and loaded onto tablets and mobile phones for the field survey. Upon obtaining informed consent from the farm owner or manager, the questionnaire was administered electronically (to the farm owner or manager) to gather information on the socio-demographics and geolocation of the farm, as well as production system characteristics, including housing, nutrition, herd health and management practices, antibiotic use within the last year (frequency, types and reason for usage), and knowledge of antibiotic use and biosecurity practices. The questionnaire was conducted in English with an interpreter in Swahili and the local language where necessary. Data were uploaded onto and stored on a protected cloud server at ILRI.
2.3. Statistical analysis
Data cleaning and statistical analyses were carried out using R software (version 4.2.3). Initial descriptive statistics were prepared for all data including frequencies and percentages for categorical variables (such as production systems, breed types, grazing types, cattle housing, antibiotic outsourcing, consultation, use of water troughs and vaccines) and means, medians, standard deviations (SDs), quartiles, and ranges for quantitative variables such as herd size and diseases incidence) depending on the distribution of the data. To reduce the potential bias that may arise from cattle herd size variations, we categorized farms into quartiles based on their herd size and within each quartile, we analysed the average counts of antibiotic use.
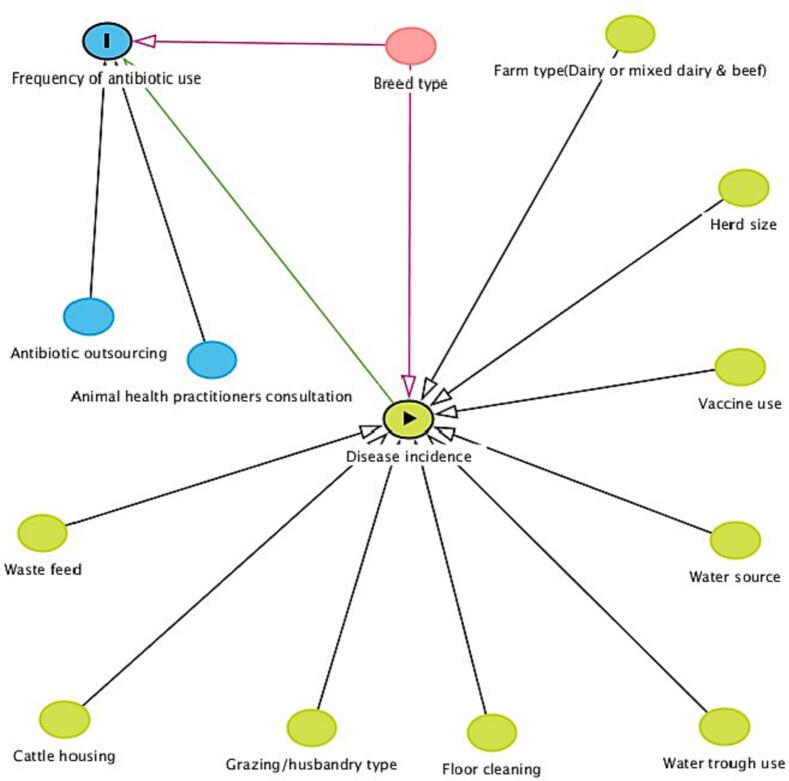
A directed acyclic graph (DAG) was constructed a priori, using DAGgitty software [21] to describe potential relationships between possible predictor variables, confounders, and the outcome of antibiotic use frequency during the year before the study. Each arrow in the DAG denotes a potential causal pathway. A direct effect of a predictor was indicated by an arrow connecting exposure to an outcome, while indirect pathways linked the exposure to the outcome via one or more intervening variables. Fig. 2 illustrates the pathways examined in this study, with disease incidence identified as central in the DAG and thus the primary predictor of antibiotic use. Other possible drivers of antibiotic use included herd size, husbandry/grazing types, biosecurity measures such as floor cleaning and the use of water troughs, and herd management practices such as cattle housing, vaccine use, drug consultation, drug outsourcing, water source, waste feed, and drug expenditure, while breed types were considered potential confounders [22]. (See Fig. 2.)
Fig. 2.
Causal diagram illustrating the potential variables associated with antibiotic use in dairy farms. The green circle with a triangle is the exposure variable (Disease incidence); green circles without the triangle are indirect predictors; the blue circle with “I” is the outcome variable of frequency of antibiotic use; blue circles without “I” are ancestors of the outcome variable (endogenous variables); pink circles are potential confounders (ancestors of the exposure variable and the outcome variable). Green edges represent causal paths and pink edges are biasing/confounding paths. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We then performed univariable analyses to test for the independent associations between the various predictor variables and the frequency of antibiotic use. We used the zero-inflated negative binomial (ZINB) model for both the univariable and multivariable analyses to account for the substantial number of zeros in the outcome variable and overdispersion, as the variance exceeded the mean. Predictor variables with a p-value <0.2 were then included in a multivariable zero-inflated negative binomial model to examine significant associations with the frequency of antibiotic use while controlling for known confounders identified using the DAGs plot and according to the literature [22]. To account for the variability among different households, we used unique household identification numbers as random effects in our model. The analyses were performed in the glmmTMB package and significance was determined using Wald χ2-tests. The top-ranked model was selected using the function ‘dredge’ in the MuMIn package [23] based on the lowest Akaike Information Criterion (AIC). Diagnostic plots of the models were constructed using the DHARMa package to assess goodness of fit and ensure that assumptions were not violated [23].
3. Results
3.1. Socio-demographics and farm characteristics
Of the 165 farms surveyed, 41.8% were semi-intensive, 40% extensive and only 18.2% were intensive farming systems. Most farmers (77%) were male and kept a mix of both beef and dairy cattle (76.4%), with only 23.6% being exclusively dairy farms. The median herd size was 24 (range 12–81) cows, with Narok County having a larger median herd size (32 cows) compared to Machakos (14 cows) and Makueni (16 cows). Nearly half (49.1%) of the farms practised free-range grazing, while 36.4% used a combination of zero-grazing and free-range. Only 13.9% of farms practised exclusively zero-grazing. (Table 1). The cattle breed types under extensive production systems, practising free-range farming were predominantly indigenous (91.5%) followed by crossbreeds (7.3%) and very few (1.2%) kept exotic breeds while intensive farms (zero-grazing) mostly kept exotic breeds (87%) followed by crossbreeds (8.7%) and very few that kept indigenous breeds (4.3%). Nearly half of the farmers (51.7%), who practised semi-intensive farming with a combination of zero-grazing and free-ranging kept indigenous cattle, 38.3% kept exotic breeds while 10% kept crossbreeds.
Table 1.
Farm sociodemographic characteristics, practices & univariable analyses results.
| Antimicrobial_use predictors | Proportion (%) |
Odds Ratio | Adj. p-value (95% CI) | |
|---|---|---|---|---|
| (n = 165) | ||||
| Farm type | Dairy only | 23.6 | Reference | Reference |
| Both dairy_ and beef | 76.6 | 1.51 | 0.024 (1.06–2.15) | |
| Production system | Intensive | 18.2 | Reference | Reference |
| Semi-intensive | 41.8 | 0.67 | 0.051(0.21–8.95 | |
| Extensive | 40 | 1.21 | 0.343(0.80–2.40) | |
| Breed | Indigenous | 64.8 | Reference | Reference |
| Crossbreed | 8.4 | 1.75 | 0.034(1.04–2.92) | |
| Exotic breed | 26.7 | 0.83 | 0.272(0.59–1.16) | |
| Grazing type | Free-range | 49.7 | Reference | Reference |
| Mixed (zero and free-range) | 60 | 0.81 | 0.219 (0.59–1.12) | |
| Zero-grazing | 13.9 | 0.95 | 0.609 (0.61–1.49) | |
| Cattle housing | No | 40.6 | Reference | Reference |
| Yes | 59.4 | 0.62 | 0.001(0.46–0.83) | |
| Antibiotic_Use consultation | Veterinarians and Para- veterinarians | 61.2 | Reference | Reference |
| No-one | 38.8 | 1.27 | 0.054(0.69–2.35) | |
| Drug source | Vet | 18.8 | Reference | Reference |
| Veterinary drug stores | 77 | 1.53 | 0.006 (1.04–2.24) | |
| Middlemen traders | 1.2 | 2.57 | 0.143 (0.69–9.51) | |
| Waste feed | No | 79.4 | Reference | Reference |
| Yes | 20.6 | 0.64 | 0.020(0.44–0.93) | |
| Water trough use | No | 50.9 | Reference | Reference |
| Yes | 49.1 | 0.83 | 0.220 (0.62–1.12) | |
| Floor cleaning | No | 74.5 | Reference | Reference |
| Yes | 25.5 | 1.05 | 0.770 (0.75–1.48) | |
| Water Source | Borehole | 25.5 | Reference | Reference |
| Municipal water | 2.4 | 0.63 | 0.966 (0.23–1.76) | |
| Rainwater | 2.4 | 0.37 | 0.031 (0.12–1.09) | |
| River | 38.8 | 0.81 | 0.131 (0.55–1.18) | |
| Surface water (dam, pans) | 20 | 0.95 | 0.472 (0.64–1.42) | |
| Vaccine use | No | 30.9 | Reference | Reference |
| Yes | 69.1 | 1.6 | 0.004(1.16–2.21) | |
| Herd size(log) | – | Median = 24 | 1.31 | <0.001(1.19–1.45) |
| Disease incidence | – | Median = 2 | 1.32 | <0.001(1.20–1.44) |
3.2. Antibiotic use patterns and practices
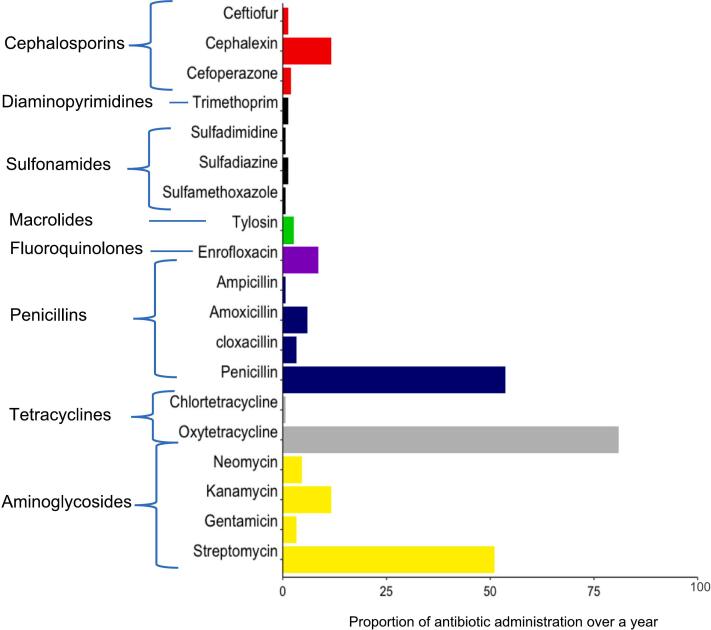
Almost all farms (92.7%) reported administering antibiotics to their cattle at least once in the last year. Reported antibiotic use increased with an increase in herd size and thus was normalized and reported within quartiles. The first 25% (range; 2–12 cattle) quartile reported an average use of 6.2 counts per year, 50% (range; 12–24 cattle) reported 11.1 counts per year, the 75% (range; 24–81 cattle) quartile reported 15 counts per year while the 100% (range; 81–868 cattle) quartile reported an average of 17 counts of use in the past year. The highest reported antibiotic use was 51 counts in the past year in a farm of 75 herd size. Only 7.0% of farmers used antibiotics exclusively for therapeutic purposes, while 43.9% used them for prophylactic purposes only, and 48.8% used them for both prophylactic and therapeutic purposes. Most farmers (88.5%) reported at least three new cases of diseases in the past year across all the herd size quartiles. The most reported diseases were mastitis (30.1%), diarrhoea (38.8%) and East Coast Fever (ECF) (40%). Very few farmers (0.4%) reported using antimicrobials as growth promoters. Oxytetracycline, penicillin, and streptomycin were the three most used antibiotics, with oxytetracycline being the most frequently used (81.0%). Notably, enrofloxacin, a clinically important antibiotic in human medicine, was reported to be used in 8.5% of farms (Fig. 3). Most farmers (77.0%) obtained antibiotics from veterinary drug stores, while a small percentage obtained them from veterinarians (18.8%) or middlemen traders (1.2%). A substantial percentage (38.8%) of the farmers did not consult animal health practitioners (AHPs), (veterinarians nor para-veterinarians), on how to use antibiotics.
Fig. 3.
Antibiotic use patterns by single antibiotics and their classes.
3.2.1. Drivers of antibiotic use
Our univariable analysis revealed significant associations between antibiotic use and various factors, including the type of farm, breed types, cattle housing, animal health practitioners' consultation, the source of antibiotics, waste feed, vaccine use, herd size and disease incidence (Table 1).
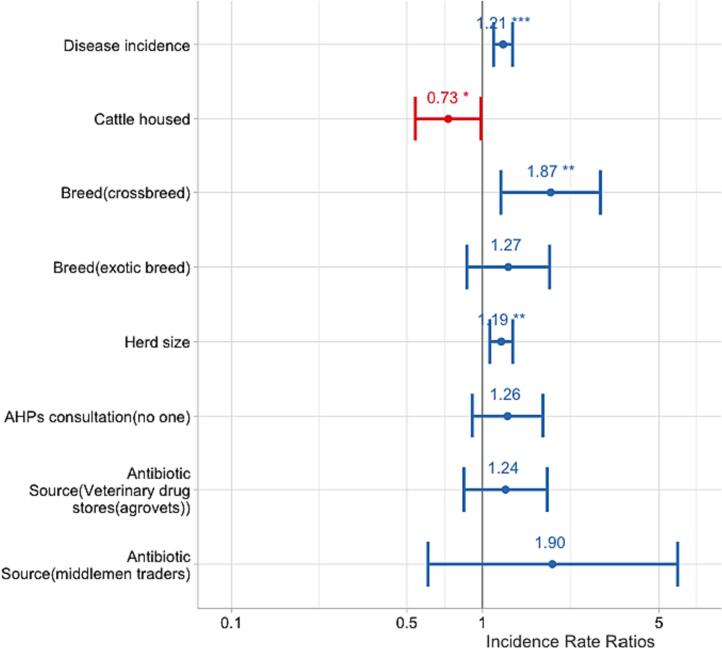
The final multivariable analysis revealed that disease incidence and herd size were significantly associated with a higher frequency of antibiotic use (OR = 1.21, p < 0.001, 95%CI [1.11–1.32]; OR = 1.19, p = 0.001, 95%CI [1.07–1.32], respectively). Additionally, the use of antibiotics was significantly higher in farms keeping crossbreeds (OR = 1.87, p = 0.007, 95%CI [1.19–3.10]) compared to those keeping indigenous breeds, while cattle housing reduced the likelihood of antibiotic use (OR = 0.73, p = 0.041, 95%CI [0.54–0.99]) (Table 2 and Fig. 4).
Table 2.
Multivariable zero-inflated negative binomial regression model results showing predictors of antibiotic use.
| Predictors | Odds Ratios | 95% CI | P-values |
|---|---|---|---|
| Disease incidence | 1.21 | 1.11–1.32 | <0.001 |
| Cattle housing | |||
| No (Reference) | |||
| Yes | 0.73 | 0.54–0.99 | 0.041 |
| Breed | |||
| Indigenous cattle (Reference) | |||
| Crossbreeds | 1.87 | 1.18–2.95 | 0.007 |
| Exotic breed | 1.27 | 0.87–1.85 | 0.222 |
| Herd size | 1.19 | 1.07–1.32 | 0.001 |
| AHPs⁎ consultation | |||
| Veterinarians and Paraveterinerians (Reference) | |||
| No one | 1.26 | 0.91–1.74 | 0.164 |
| Antibiotic source | |||
| Veterinarians (Reference) | |||
| Veterinary drug stores | 1.24 | 0.84–1.81 | 0.277 |
| Middlemen traders | 1.90 | 0.61–5.94 | 0.270 |
AHPs – Animal Health Practitioners.
Fig. 4.
Multivariable zero-inflated negative binomial regression model results showing predictors of antibiotic use.
4. Discussion
This study revealed that the use of antibiotics is common in all cattle production systems in Kenya. Increased use of antibiotics observed among intensive and semi-intensive production systems may be necessitated by the need for disease prevention and treatment due to their controlled and confined environments, and preference for exotic cattle breeds [24]. Moreover, the increased use of antibiotics was also observed in extensive cattle production systems, where cattle are reared under free-ranging pastoral conditions often involving significant mobility of the animals over open grazing areas in search of pastures and watering grounds. A previous study conducted in Ethiopia [25] shows that the use of antibiotics in the extensive pastoral systems in some low- and middle-income settings may not be what is considered rational. The high use of antibiotics by pastoralists may potentially be attributed to a combination of factors such as harsh climates, and inadequate housing that can increase their vulnerability to diseases. The nomadic nature of free-ranging pastoralist communities can pose challenges to the implementation of proper biosecurity measures [25]. With these communities often moving with their large herds over long distances in search of grazing lands, which can increase the risk of exposure to different pathogens and transmission may be increased [24]. Additionally, the increased ease of access to antibiotics by farmers over the counter [26], combined with limited access to veterinary services in remote areas [27] may pose difficulties for pastoralists to seek professional guidance when their animals fall sick and as a result, they may rely on antibiotics as a quick and easily accessible solution for treatment and disease prevention.
Our analyses found that antibiotic use was attributable to high disease incidence with mastitis, ECF and diarrhoea being the most reported diseases. Lack of veterinary consultation before antibiotic use, large herd size, and poor housing were also associated with antibiotic use. These findings are in line with a previous study conducted in Tanzania, which reported that 83% of cattle farmers often used antibiotics without a prescription or veterinary advice [28]. Additionally, another study in Argentina suggests that in dairy farming, antibiotics are extensively used to treat mastitis. It showed that clinical mastitis and dry cow therapy represented 85.4% of total drug usage in lactating cows [27].
Globally, antibiotics are primarily used in animals for treatment and prevention purposes [29]. However, despite the ban on antibiotic use for growth promotion in most high-income countries [30], some farmers are still using antibiotics for the same purpose, especially for animal production systems related to meat in LMICs [31] This study found that most farmers used antibiotics for preventive purposes, either prophylactic or metaphylactic, with only a small percentage using them exclusively for therapeutic purposes and very few used for growth promotion. Previous studies in Kenya [32,33] found that farmers mostly used antibiotics for disease prevention and control confirming our findings. Similar to other studies [34,35], we found oxytetracycline to be the most widely used antibiotic, followed by penicillin and streptomycin. According to the WHO's recent classification of antibiotics, AWaRe (Access, Watch, Reserve) criteria [36], enrofloxacin is considered a ‘watch’ antibiotic of clinical importance effective against a wide range of bacterial infections which should be used where other antibiotics have failed [37]. Our findings show that enrofloxacin is still being used (8.5%) in Kenyan cattle production systems. Despite enrofloxacin being banned in food-producing animals in some countries [38,39], its use has not been banned in Kenya [37]. Similar patterns of antibiotic use have been reported by other studies in Kenya [40] and surrounding countries, such as Tanzania [41] and Ethiopia [42] and are reflective of the local disease epidemiology.
Our analysis found that farmers with larger herds reported a higher frequency of antibiotic use, which is partly attributable to the higher risk of disease incidence and transmission within larger herds [43]. These findings align with previous studies in Kenya and Ethiopia that identified disease incidence, especially mastitis as a risk factor for antibiotic use in dairy cattle [40,44]. On the other hand, we found that appropriate cattle housing, such as using cattle sheds, was protective against antibiotic use, possibly due to the reduced risks of diseases as a result of protection from harsh weather conditions or exposure to hazards in the open fields [45]. However, these findings differ from some previous studies [46,47] that found that cattle housing systems where animals are confined in a small, enclosed structure with concrete floors were associated with higher use of antibiotics. This discrepancy may be due to the increased chance of foot-rot infections in housed cattle, which may necessitate antibiotic use and therefore, cattle housing may not fully eliminate reliance on antibiotics [48].
Akin to previous findings in Kenya [4,49], Ethiopia [25], Uganda [50] and Ghana [49] most farmers in this study reported obtaining antibiotics from veterinary drug stores, while a significant number also obtained them from middlemen traders without consulting veterinary professionals before their use. Our analysis indicated that there was no difference in antibiotic use between farmers who obtained antibiotics directly from veterinary drug stores or traders without consulting veterinarians and those who consulted with veterinarians before obtaining antibiotics. This contrasts previous findings that have indicated that acquiring antibiotics exclusively from veterinary professionals may promote responsible antibiotic use [51,52]. These findings underscore the need for sustained antibiotic stewardship targeting all stakeholders.
In the current study, counts of antibiotic treatments were used as a metric to measure antibiotic use. Count metric provides a quick estimate of the number of antimicrobial doses administered and is relatively easy to collect but does not consider the differences in potency and duration of antimicrobial drugs [53]. However, counts can still provide valuable information when used in conjunction with other metrics, such as dose or duration [54]. Future research should consider the development of standardised methodologies for collecting and reporting data on quantifying AMU that considers drug potency and duration [55], while also addressing the challenges of comparability across different production systems and regions. Results from this study provide an important baseline and benchmark for future studies that aim to compare relative antimicrobial use in animal production. Our cross-sectional study did not establish causality between the factors examined and the frequency of antibiotic use [55]. To obtain accurate estimates of antibiotic consumption at the farm level and understand its associated drivers, longitudinal studies collecting disaggregated data on animal demographics such as species, age, weight, specific disease incidence and antibiotic classes used are necessary [56]. Furthermore, self-reported data from farmers may have introduced bias in the results due to potential over or under-reporting of practices and knowledge [57]. Additionally, our study's generalizability is limited to the three counties in Kenya and the production systems investigated and thus further studies covering a larger study area and other production systems like poultry, pig in Kenya are recommended.
5. Conclusion
In conclusion, this study reveals that the use of antibiotics in cattle production systems in Kenya is mainly for disease prevention and control, likely due to high disease incidence, improper herd management practices, and limited access to veterinary services. These findings provide important data on antibiotic use patterns and drivers in Kenyan dairy farms, supporting the development of appropriate AMS programmes and serving as a baseline for future studies and interventions to reduce irrational antibiotic use. Lastly, the study highlights the importance of educating cattle farmers on appropriate antibiotic usage, promoting proper herd management practices, and reinforcing veterinary services especially for marginalised farmers to ensure responsible antimicrobial use. These measures are crucial for minimising antimicrobial resistance, reducing disease burden, and preserving the effectiveness of antibiotics.
Ethical clearance
The collection of data adhered to the legal requirements of the Government of Kenya. Ethical approval for data collection was obtained from the ILRI Institutional Research Ethics Committee (Ref. ILRI-IREC2021–31). This study was approved by the National Commission for Science and Technology and Innovation (NACOSTI) (Ref. 803,721), and permits were obtained from the Directorate of Veterinary Services (DVS). Written informed consent was obtained from all participants.
Funding
This study was funded by the Wellcome Trust – International Masters’ Fellowship in collaboration with NIHR award (Ref. 221483/Z/20/Z), granted to Lydiah Kisoo and the Royal Society under the Future Leaders Africa Independent Research (FLAIR) fellowship award reference FLR/RI/201709 granted to Lillian Wambua. The study also received support from the CGIAR One Health initiative “Protecting Human Health Through a One Health Approach”, which was supported by contributors to the CGIAR Trust Fund (https://www.cgiar.org/funders/) and CGIAR Research Program on Agriculture for Nutrition and Health (A4NH).
Author Ccontributions
Lydiah Kisoo: Conceptualization, Data curation, Formal analysis, Visualization, Writing - original draft preparation, funding acquisition. Dishon Muloi: Supervision, Conceptualization, Data curation, Formal analysis, Writing - Reviewing and Editing. Walter Oguta: Methodology. Daisy Ronoh: Methodology. Lynn Kirwa: Methodology. James Akoko: Methodology. Eric Fèvre: Supervision, Conceptualization, Writing- Reviewing and Editing. Arshnee Moodley: Supervision, Conceptualization, Writing- Reviewing and Editing. Lillian Wambua: Conceptualization, Methodology Resources, Writing - Reviewing and Editing.
Declaration of Competing Interest
The authors disclose no conflicts of interest.
Acknowledgements
We extend our gratitude to the enumerators, and respondents, for their willing cooperation and generosity in providing us with their valuable time and information during the interview process.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2023.100646.
Contributor Information
Lydiah Kisoo, Email: L.Kisoo@cgiar.org.
Lillian Wambua, Email: wambua.lillian@gmail.com.
Appendix A. Supplementary data
Supplementary tables and figures, R-script, and questionnaire used.
Data availability
The data referenced in this study is retrievable upon request from the corresponding author and supplementary material is submitted herein. R scripts used to perform analyses can be found under the supplementary material.
References
- 1.WHO Antimicrobial resistance. Global report on surveillance. World health. Organization. 2014 doi: 10.1007/s13312-014-0374-3. [DOI] [Google Scholar]
- 2.Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report. 2022. https://www.who.int/initiatives/glass [Google Scholar]
- 3.Caneschi A., Bardhi A., Barbarossa A., Zaghini A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics. 2023;12:487. doi: 10.3390/ANTIBIOTICS12030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caudell M.A., Dorado-Garcia A., Eckford S., Creese C., Byarugaba D.K., Afakye K., et al. Towards a bottom-up understanding of antimicrobial use and resistance on the farm: a knowledge, attitudes, and practices survey across livestock systems in five African countries. PLoS One. 2020:15. doi: 10.1371/JOURNAL.PONE.0220274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antimicrobial Resistance Multi-Partner Trust Fund Annual Report. 2022. https://www.fao.org/documents/card/en/c/cc0271en [Google Scholar]
- 6.Kenya national Action Plan on Antimicrobial Resistance Review of Progress in the Human Health Sector. Antimicrobial Resistance Policy Information and Action Brief Series. 2022. https://www.who.int/publications/i/item/9789240062689 [Google Scholar]
- 7.Antimicrobial Resistance and The United Nations Sustainable Development Cooperation Framework. 2021. https://www.who.int/publications/i/item/9789240036024 [Google Scholar]
- 8.Pokharel S., Shrestha P., Adhikari B. Antimicrobial use in food animals and human health: time to implement ‘one health’ approach. Antimicrob. Resist. Infect. Control. 2020;9:1–5. doi: 10.1186/S13756-020-00847-X/METRICS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenya . 2018. Livestock Production Systems Spotlight Cattle and Poultry Sectors Livestock Production Systems Spotlight Cattle and Poultry Sectors in Kenya.https://www.fao.org/documents/card/en/c/I8270EN [Google Scholar]
- 10.Behnke R., Muthami D. The Contribution of Livestock to the Kenyan Economy IGAD Livestock Policy Initiative. 2011;2 [Google Scholar]
- 11.Mutura J.K., Nyairo N., Mwangi M., Wambugu S.K. 2016. Analysis of Determinants of Market Channel Choice among Smallholder Dairy Farmers in Lower Central Kenya. 4. [Google Scholar]
- 12.Omore A., Muriuki H., Kenyanjui M., Owango M., Staal S. The Kenyan Dairy Sub-Sector. A Rapid Appraisal MOA/KARI/ILRI Smallholder Dairy (Research & Development) Project Report. https://hdl.handle.net/10568/2054
- 13.Hosain M.Z., Lutful Kabir S.M., Kamal M.M. Antimicrobial uses for livestock production in developing countries. Vet. World. 2021;14:210. doi: 10.14202/VETWORLD.2021.210-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aduda O.T. Nakuru, and Nyeri Counties; 2022. Assessment of Antibiotics Residues in Milk Supplied by Smallholder Farmers to Processors in Kenya: A Case of Bomet. [Google Scholar]
- 15.Diana A., Lorenzi V., Penasa M., Magni E., Alborali G.L., Bertocchi L., et al. Effect of welfare standards and biosecurity practices on antimicrobial use in beef cattle. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-77838-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muloi D., Fèvre E.M., Bettridge J., Rono R., Ong’are D., Hassell J.M., et al. A cross-sectional survey of practices and knowledge among antibiotic retailers in Nairobi, Kenya. J. Glob. Health. 2019:9. doi: 10.7189/JOGH.09.020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iskandar K., Molinier L., Hallit S., Sartelli M., Catena F., Coccolini F., et al. Drivers of Antibiotic Resistance Transmission in Low- and Middle-Income Countries from a “One Health” Perspective—A Review. Antibiotics. 2020;9:372. doi: 10.3390/ANTIBIOTICS9070372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosire C.K., Rao J., Muchenje V., Van Wijk M., Ogutu J.O., Mekonnen M.M., et al. Adaptation opportunities for smallholder dairy farmers facing resource scarcity: integrated livestock, water and land management. Agric. Ecosyst. Environ. 2019;284 doi: 10.1016/J.AGEE.2019.106592. [DOI] [Google Scholar]
- 19.Wambua J.M. Factors Influencing Dairy Productivity in Machakos County: A Case of Wamunyu Dairy Farmers co-Operative. Society. 2014 http://hdl.handle.net/11295/74285 [Google Scholar]
- 20.Wieland B., Dione M., Alemu Gemeda B., Fevre E., Grace D., Omoya L., et al. 2019. AMUSE Livestock, Version 2—Antimicrobial Use in Livestock Production: A Tool to Harmonise Data Collection on Knowledge, Attitude and Practices.https://hdl.handle.net/10568/107443 [Google Scholar]
- 21.DAGitty v3.0. 2014. http://www.dagitty.net/dags.html#
- 22.Malijan G.M., Howteerakul N., Ali N., Siri S., Kengganpanich M., Nascimento R., et al. A scoping review of antibiotic use practices and drivers of inappropriate antibiotic use in animal farms in WHO Southeast Asia region. One Health. 2022;15 doi: 10.1016/J.ONEHLT.2022.100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(PDF) glmmTMB: Generalized linear mixed models using Template Model Builder. 2017. https://www.researchgate.net/publication/323384790_glmmTMB_Generalized_linear_mixed_models_using_Template_Model_Builder
- 24.Seid M.A., Kuhn N.J., Fikre T.Z. The role of pastoralism in regulating ecosystem services. Rev. Sci. Et Techn. De L Off. Int. Des Epizooties. 2016;35:435–444. doi: 10.20506/RST.35.2.2534. [DOI] [PubMed] [Google Scholar]
- 25.Gemeda B.A., Amenu K., Magnusson U., Dohoo I., Hallenberg G.S., Alemayehu G., et al. Antimicrobial use in extensive smallholder livestock farming Systems in Ethiopia: knowledge, attitudes, and practices of livestock keepers. Front. Vet. Sci. 2020;7 doi: 10.3389/FVETS.2020.00055/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Map showing the study area. The Narok County adjoins the Serengeti NP... | Download Scientific Diagram. 2019. https://www.researchgate.net/figure/Map-showing-the-study-area-The-Narok-County-adjoins-the-Serengeti-NP-to-the-south_fig1_332355288
- 27.Osadolor O.O., Osadolor A.J., Osadolor O.O., Enabulele E., Akaji E.A., Odiowaya D.E. Access to health services and health inequalities in remote and rural areas. Janaki Med. Coll. J. Med. Sci. 2022;10:70–74. doi: 10.3126/JMCJMS.V10I2.47868. [DOI] [Google Scholar]
- 28.Azabo R., Mshana S., Matee M., Kimera S.I. Antimicrobial usage in cattle and poultry production in Dar Es Salaam, Tanzania: pattern and quantity. BMC Vet. Res. 2022;18:1–12. doi: 10.1186/S12917-021-03056-9/FIGURES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meek R.W., Vyas H., Piddock L.J.V. Nonmedical uses of antibiotics: time to restrict their use? PLoS Biol. 2015;13 doi: 10.1371/JOURNAL.PBIO.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cogliani C., Goossens H., Greko C. Restricting Antimicrobial Use in Food Animals: Lessons from Europe Banning Nonessential Antibiotic Uses in Food Animals Is Intended to Reduce Pools of Resistance Genes. Microbe Magazine. 2019;6:274–279. [Google Scholar]
- 31.Robinson T.P., Bu D.P., Carrique-Mas J., Fèvre E.M., Gilbert M., Grace D., et al. Antibiotic resistance: mitigation opportunities in livestock sector development. Animal. 2017;11:1–3. doi: 10.1017/S1751731116001828. [DOI] [PubMed] [Google Scholar]
- 32.Mitema E., Kikuvi G., Wegener H.C., Stohr K. An assessment of antimicrobial consumption in food producing animals in Kenya. J. Vet. Pharmacol. Ther. 2001;24:385–390. doi: 10.1046/J.1365-2885.2001.00360.X. [DOI] [PubMed] [Google Scholar]
- 33.Laxminarayan R., Duse A., Wattal C., Zaidi A.K.M., Wertheim H.F.L., Sumpradit N., et al. Antibiotic resistance—the need for global solutions. Lancet Infect. Dis. 2013;13:1057–1098. doi: 10.1016/S1473-3099(13)70318-9. [DOI] [PubMed] [Google Scholar]
- 34.Cameron A., McAllister T.A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016;20(16):1–22. doi: 10.1186/S40104-016-0127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ziv G., Wanner M., Nicolet J. Distribution of penicillin G, dihydrostreptomycin, oxytetracycline, and chloramphenicol in serum and subcutaneous chamber fluid. J. Vet. Pharmacol. Ther. 1982;5:59–69. doi: 10.1111/J.1365-2885.1982.TB00498.X. [DOI] [PubMed] [Google Scholar]
- 36.Sulis G., Sayood S., Katukoori S., Bollam N., George I., Yaeger L.H., et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multidrug resistant bacteria: a systematic review and meta-analysis. Clin. Microbiol. Infect. 2022;28:1193–1202. doi: 10.1016/J.CMI.2022.03.014. [DOI] [PubMed] [Google Scholar]
- 37.Peng M., Salaheen S., Biswas D. Animal health: global antibiotic issues. Encycloped. Agricult Food Syst. 2014:346–357. doi: 10.1016/B978-0-444-52512-3.00187-X. [DOI] [Google Scholar]
- 38.Cox L.A., Popken D.A. Quantifying potential human health impacts of animal antibiotic use: Enrofloxacin and macrolides in chickens. Risk Anal. 2006;26:135–146. doi: 10.1111/J.1539-6924.2006.00723.X. [DOI] [PubMed] [Google Scholar]
- 39.Trouchon T., Lefebvre S. A review of Enrofloxacin for veterinary use. Open J. Vet. Med. 2016;6:40–58. doi: 10.4236/OJVM.2016.62006. [DOI] [Google Scholar]
- 40.Dione M., Amia W.C., Wieland B. Assessment of Antimicrobial Use and Management in Livestock Systems Tool for Assessing the Knowledge, Attitudes and Practices (KAP) of Veterinary Drug Inputs Suppliers about Antimicrobial Use in Livestock Production Systems. 2020. https://hdl.handle.net/10568/110870 [Google Scholar]
- 41.Mdegela R.H., Mwakapeje E.R., Rubegwa B., Gebeyehu D.T., Niyigena S., Msambichaka V., et al. Antimicrobial use, residues, resistance and governance in the food and agriculture sectors, tanzania. Antibiotics. 2021;10:454. doi: 10.3390/ANTIBIOTICS10040454/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rana E.A., Fazal M.A., Alim M.A. Frequently used therapeutic antimicrobials and their resistance patterns on Staphylococcus aureus and Escherichia coli in mastitis affected lactating cows. 2022;10:1–10. doi: 10.1080/23144599.2022.2038494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barkema H.W., von Keyserlingk M.A.G., Kastelic J.P., Lam T.J.G.M., Luby C., Roy J.P., et al. Invited review: changes in the dairy industry affecting dairy cattle health and welfare. J. Dairy Sci. 2015;98:7426–7445. doi: 10.3168/JDS.2015-9377. [DOI] [PubMed] [Google Scholar]
- 44.Bett B., Jost C., Allport R., Mariner J. Using participatory epidemiological techniques to estimate the relative incidence and impact on livelihoods of livestock diseases amongst nomadic pastoralists in Turkana South District, Kenya. Prev. Vet. Med. 2009;90:194–203. doi: 10.1016/J.PREVETMED.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 45.van Aken A., Hoop D., Friedli K., Mann S. Udder health, veterinary costs, and antibiotic usage in free stall compared with tie stall dairy housing systems: an optimized matching approach in Switzerland. Res. Vet. Sci. 2022;152:333–353. doi: 10.1016/J.RVSC.2022.08.021. [DOI] [PubMed] [Google Scholar]
- 46.Mastitis occurrence and Constraints to Mastitis Control in Smallholder Dairy Farming Systems in Uganda. 1997. http://www.lrrd.cipav.org.co/lrrd20/1/byar20005.html
- 47.Stanford K., Gibb D.J., Schwartzkopf-Genswein K.S., van Herk F., McAllister T.A. Feeding subtherapeutic antimicrobials to low-risk cattle does not confer consistent performance benefits. Can. J. Anim. Sci. 2015;95:589–597. doi: 10.4141/CJAS-2015-008/ASSET/IMAGES/CJAS-2015-008TAB6.GIF. [DOI] [Google Scholar]
- 48.Cramer G., Lissemore K.D., Guard C.L., Leslie K.E., Kelton D.F. The association between foot lesions and culling risk in Ontario Holstein cows. J. Dairy Sci. 2009;92:2572–2579. doi: 10.3168/JDS.2008-1532. [DOI] [PubMed] [Google Scholar]
- 49.Afakye K., Kiambi S., Koka E., Kabali E., Dorado-Garcia A., Amoah A., et al. The Impacts of Animal Health Service Providers on Antimicrobial Use Attitudes and Practices: An Examination of Poultry Layer Farmers in Ghana and Kenya. Antibiotics. 2020;9:554. doi: 10.3390/ANTIBIOTICS9090554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dione M.M., Amia W.C., Ejobi F., Ouma E.A., Wieland B. Supply chain and delivery of antimicrobial drugs in smallholder livestock production Systems in Uganda. Front. Vet. Sci. 2021;8:954. doi: 10.3389/FVETS.2021.611076/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ogwuche A., Ekiri A.B., Endacott I., Maikai B.V., Idoga E.S., Alafiatayo R., et al. Antibiotic use practices of veterinarians and Para-veterinarians and the implications for antibiotic stewardship in Nigeria. J. S. Afr. Vet. Assoc. 2021;92:1–14. doi: 10.4102/JSAVA.V92I0.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adekanye U.O., Ekiri A.B., Galipó E., Muhammad A.B., Mateus A., La Ragione R.M., et al. Knowledge, Attitudes and Practices of Veterinarians Towards Antimicrobial Resistance and Stewardship in Nigeria. Antibiotics. 2020;9:453. doi: 10.3390/ANTIBIOTICS9080453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Umair M., Abdullah R.M., Aslam B., Nawaz M.H., Ali Q., Fatima F., et al. First case report on quantification of antimicrobial use in corporate dairy farms in Pakistan. Front. Vet. Sci. 2020;7:967. doi: 10.3389/FVETS.2020.575848/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Redding L.E., Bender J., Baker L. Quantification of antibiotic use on dairy farms in Pennsylvania. J. Dairy Sci. 2019;102:1494–1507. doi: 10.3168/JDS.2018-15224. [DOI] [PubMed] [Google Scholar]
- 55.Van der Stede W.A. A manipulationist view of causality in cross-sectional survey research. Acc. Organ. Soc. 2014;39:567–574. doi: 10.1016/J.AOS.2013.12.001. [DOI] [Google Scholar]
- 56.Introduction to Longitudinal Research - Elisabetta Ruspini - Google Books. 2002. https://books.google.co.ke/books?hl=en&lr=&id=-6wiDVhPPmAC&oi=fnd&pg=PR9&dq=longitudinal+studies+collecting+disaggregated+data+on+animal+demographics+such+as+&ots=JHvrM5HtBz&sig=sLhupaCOQLDTuz3QbsZ3ZKYs3P8&redir_esc=y#v=onepage&q&f=false
- 57.Spector P.E. Method variance as an artifact in self-reported affect and perceptions at work: myth or significant problem? J. Appl. Psychol. 1987;72:438–443. doi: 10.1037/0021-9010.72.3.438. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables and figures, R-script, and questionnaire used.
Data Availability Statement
The data referenced in this study is retrievable upon request from the corresponding author and supplementary material is submitted herein. R scripts used to perform analyses can be found under the supplementary material.