Abstract
The replication region of a 100-kb desulfurization plasmid (pSOX) from Rhodococcus sp. strain X309 was localized to a 4-kb KpnI fragment, and its sequence was determined. The amino acid sequence of one of the predicted open reading frames (ORFs) was related to the putative replication (Rep) protein sequences of the mycobacterial pLR7 family of plasmids. Three of the five predicted ORF products were identified by radiolabelling with the Escherichia coli T7 polymerase/promoter system. In E. coli, the Rep protein of pSOX was apparently synthesized in a shortened form, 21.3 kDa instead of the predicted 41.3 kDa, as a result of an internal initiation. This situation is reminescent of that for some bacterial Rep proteins. A shuttle plasmid was constructed with the pSOX origin, pBluescript II KS−, and the chloramphenicol resistance (Cmr) gene from pRF29. This new shuttle plasmid was used to demonstrate expression of the Bacillus subtilis sacB gene in a strain of Rhodococcus, rendering it sensitive to the presence of sucrose.
Rhodococcus sp. strain X309 was one of the first biodesulfurization strains to be characterized at the molecular level (6–8, 16, 30). Like the prototype Rhodococcus sp. strain IGTS8, recently classified as Rhodococcus erythropolis (25), these bacteria are endowed with the property of specific cleavage of the carbon-sulfur bonds in model organosulfur compounds such as dibenzothiophene (DBT). By not breaking the carbon-carbon backbone, the biodesulfurization process has advantages including conservation of the calorific value of fuels and elimination of noxious emissions of sulfur oxides into the atmosphere when these sulfur-laden compounds are combusted (12, 22, 27).
A long-recognized shortcoming of the biodesulfurization process is its inhibition by the presence of sulfate (22). Sulfur-containing amino acids (methionine and cysteine) also exert a negative effect on desulfurization carried out by the dsz genes (also known as sox [6, 8]) in strain IGTS8 (25); that sulfur acts by repression of the native desulfurization promoter was demonstrated (25).
We are interested in promoter replacement as a possible strategy and an alternative to expression of the sox genes in a heterologous host as a means of alleviating the sulfur repression problem. We reckoned that a native Rhodococcus may contain yet uncharacterized factors or membrane properties necessary for uptake or transport and eventual desulfurization of the sulfur-containing compounds. Toward this goal, the replication region of the sox-containing plasmid, pSOX, indigenous to Rhodococcus sp. strain X309 (6) was delineated. A shuttle plasmid was then constructed, and the utility of the sacB promoter in the expression of the Bacillus subtilis levansucrase-encoding gene (37) was demonstrated. Additional impetus for this study was provided by the general lack of information on the characteristics of plasmid replicons indigenous to members of the gram-positive genus Rhodococcus (4, 18). This information is not only useful in cataloging known replicons and incompatibility groups but also invaluable as a molecular tool for probing potentially new “environmental” plasmids.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Rhodococcus sp. strain X309-11-15 was used as the source of the 100-kb pSOX plasmid (6), and Rhodococcus sp. strain X309-10-2 (henceforth referred to as strain 10-2), a plasmid-free derivative of the parental Rhodococcus sp. strain X309 (6), was used as the rhodococcal host. Growth media and plasmid isolation procedures were as previously described (6, 34). Deletion derivatives of the pSOX plasmid described in this study are represented in Fig. 1. The Escherichia coli host for recombinant plasmids was strain DH10B (Gibco-BRL). Plasmids pUM24 (33) and pRF29 (9, 10) were kindly provided by J. Wall (University of Missouri), and K. Young (University of North Dakota), respectively; pBluescript II KS− vector was purchased from Stratagene, LaJolla, Calif. E. coli K38(pGP1-2) cells were grown by the method of Tabor (39) for expression of the plasmid-encoded gene(s) cloned in the pT7-5 vector.
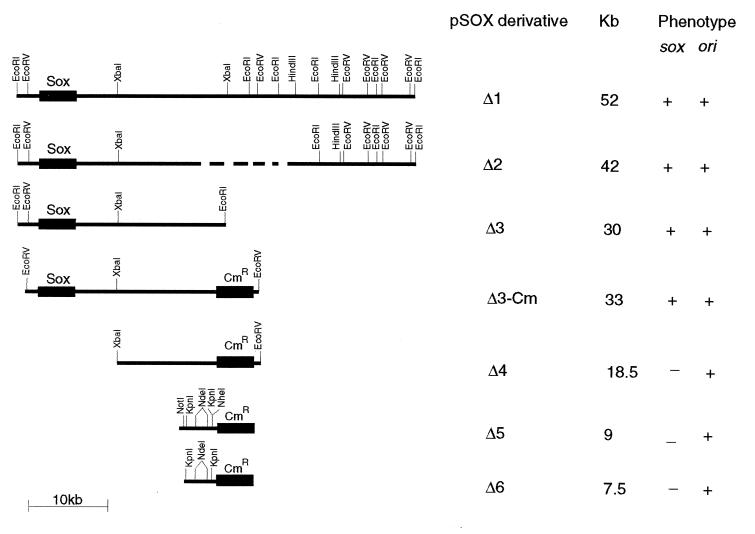
FIG. 1.
Localization of the pSOX plasmid replicon from Rhodococcus sp. strain X309. The ability of each plasmid to replicate (ori) or possess desulfurization activity (sox) is shown at the right by the plus and minus signs. Cmr indicates the chloramphenicol resistance marker gene derived from pRF29 plasmid (10). ---, undefined boundary of deletion.
Localization of the replication region of pSOX plasmid.
Plasmid DNA was introduced into strain 10-2 by electroporation with a Bio-Rad Gene Pulser apparatus. The conditions for electroporation were as described for Rhodococcus sp. strain M5 (24). Plasmid pSOXΔ1, 52 kb in size (Fig. 1), was the first deletion derivative of the pSOX plasmid isolated from the electrotransformed 10-2 strain as a result of repeated selection on DBT-containing plates (6). Digestion of pSOXΔ1 by EcoRI produced five fragments. Additional restriction endonuclease digests provided the map shown in Fig. 1; the largest EcoRI fragment (30 kb) contains the soxABC genes (6–8), since it probed positive with a 4-kb sox-containing DNA fragment in a Southern hybridization experiment (6).
Introduction of pSOXΔ1 into strain 10-2 and further selection on DBT plates resulted in a yet smaller plasmid, pSOXΔ2. Since pSOXΔ2 was still quite large (42 kb), a series of defined manipulations which led to the localization of the pSOX replicon to a 4-kb KpnI fragment were carried out (Fig. 1).
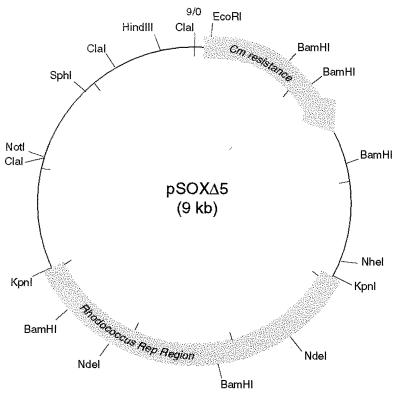
First, self-ligation of the 30-kb sox-containing EcoRI fragment derived from pSOXΔ2 (pSOXΔ3 in Fig. 1) resulted in a transformant that was capable of replication in strain 10-2. On the other hand, similar ligation of the end-filled 12.6-kb EcoRI-XbaI fragment (leftmost sox-containing portion) did not result in any transformants. To test whether the rightmost 15-kb XbaI-EcoRI fragment could replicate, pSOXΔ3-Cm was constructed by addition of a chloramphenicol resistance (Cmr) marker gene from pRF29 (13.2 kb) (9, 10) at the unique EcoRI site of pSOXΔ3. To facilitate cloning, both the linearized vector and the 3.5-kb XbaI-XhoI DNA fragment containing the Cmr gene were blunt ended by filling in with deoxynucleoside triphosphate substrates and Klenow DNA polymerase I (34). Subsequent digestion by EcoRV and XbaI yielded an 18.5-kb fragment which, after end filling, ligation, and transformation in strain 10-2, gave rise to many colonies on chloramphenicol (30 μg/ml) selective media. Surprisingly, when the plasmid content of several transformants was analyzed, two populations of cells were obtained: one harboring the expected 18.5-kb plasmid (designated pSOXΔ4) and the other harboring a 9-kb derivative (designated pSOXΔ5). The pSOXΔ5 plasmid was mapped (Fig. 2) and used as the basis for further constructions.
FIG. 2.
Restriction map of a replicating pSOXΔ5 derivative. The 4-kb KpnI fragment, but not the internal 2.2-kb NdeI fragment, that is sufficient for replication is as outlined. NheI, NotI, SphI, and HindIII are unique restriction sites that are potentially useful for cloning. Although the EcoRI site is also unique, disruption of the plasmid at this site would probably eliminate selection in the presence of chloramphenicol.
The pSOXΔ6 plasmid was derived by ligating the 4-kb KpnI fragment to the 3.5-kb Cmr gene cassette. Because of the incompatible ends of these restriction fragments, repair for blunt-end ligation was carried out accordingly. Similar ligation of the 2.2-kb NdeI fragment to the Cmr cassette did not yield any Cmr transformants.
When screening for the 7.5-kb pSOXΔ6 plasmid, we observed a deletion of ca. 400 bp in one derivative, designated pSOXΔ6-1. This small deleted region was localized to the end of the 4-kb KpnI fragment that has a BamHI site (Fig. 2). Both pSOXΔ6 and pSOXΔ6-1 were subsequently used for the construction of Rhodococcus-E. coli shuttle vectors.
Construction of Rhodococcus-E. coli shuttle vectors.
Either plasmid pSOXΔ6 or plasmid pSOXΔ6-1, linearized by SphI, was ligated to the NaeI site (within the f1 origin) of the pBluescript II KS− plasmid. In each case, it was necessary to end-fill the restriction fragments. The pBluescript vector provides the colE1 replication origin, a multiple cloning site, the lacZα reporter gene for screening inserts in E. coli (blue-white selection), and ampicillin resistance (Ap). Since NaeI digestion disrupts the phage f1 origin, it is anticipated that single-stranded DNA production will be lost in these shuttle plasmids. The resultant plasmids are designated pKSΔ6 and pKSΔ6-1, respectively.
DNA sequencing and analysis.
The 4.6-kb NotI-NheI fragment which contains the 4-kb KpnI fragment was cloned at the equivalent sites of pBluescript KS− vector, and its sequence was determined on both strands by primer walking with the automated fluorescence sequencer (Applied Biosystems model 373A) and the T7 sequencing kit. Sequence analysis was performed with the BLAST programs (1) of the National Center for Biotechnology Information (Bethesda, Md.) and the PC/Gene package (IntelliGenetics Inc., Mountain View, Calif.).
Nucleotide sequence accession number.
The 4,584-kb sequence of the NheI-NotI fragment of pSOX of Rhodococcus sp. strain X309 has been assigned GenBank accession no. AF059700.
RESULTS AND DISCUSSION
Sequence features in the pSOX replication region.
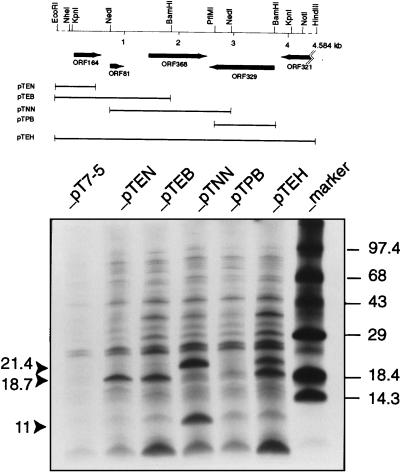
In vivo and specific deletion experiments accompanied by appropriate genetic markers led to the localization of the pSOX replication region to a 4-kb KpnI fragment (Fig. 1). A summary of the predicted open reading frames (ORFs) in this sequenced DNA region and expression of three of the ORF products in E. coli K38 (pGP1-2) cells (Fig. 3) is described below.
FIG. 3.
Predicted ORFs in the pSOX replication region and [35S]methionine identification of proteins. Locations of ORFs and direction of transcription are indicated by arrows. Protein labeling was carried out as described by Tabor (39). Expression plasmids derived from the pT7-5 vector (control) are pTEN (0.72-kb EcoRI-NdeI [Klenow blunted] cloned in the EcoRI-SmaI sites), pTEB (1.86-kb EcoRI-BamHI cloned in the EcoRI-BamHI sites), pTNN (2.2-kb NdeI [Klenow blunted] cloned in the SmaI site), pTPB (1.05-kb PflMI [T4-blunted]-BamHI cloned in the SmaI-BamHI sites), and pTEH (4.6-kb EcoRI-HindIII cloned in the EcoRI-HindIII sites). The calculated molecular sizes indicated by the solid arrowheads are those of the pSOX truncated Rep (ORF368), ORF164, and ORF81 products in descending order. Since low-molecular-weight proteins were being analyzed, the gel recipe (10% T–3% C) of Schagger and von Jagow (35) was used.
ORF164.
ORF164 (nucleotides 77 to 571, encoding a product of 164 amino acids) encodes a product whose amino acid sequence is 31.4% identical to TrbA (103 amino acids) of the broad-host-range IncPα plasmid RK2 (21). Protein expression of ORF164 was evidenced by the 18.7-kDa labelled band (cf. the predicted Mr of 18,117) derived from three independent clones (pTEN, pTEB, and pTEH) based on the pT7 system (Fig. 3). The ORF164-related TrbA protein functions as a repressor that controls both vegetative replication and conjugative transfer of the RK2 plasmid (21, 28). Like TrbA, ORF164 has a predicted high isoelectric point (pI = 10.6). Among the conserved amino acid sequence is a putative helix-turn-helix motif near the N terminus and a leucine zipper motif at amino acids 68 to 82 (LKQIAQELDVSISVL). The latter motif provides the possible basis for protein dimerization, as noted previously for the TrbA-related proteins (21). There is no evidence yet for a repressor role of ORF164, but its involvement in plasmid replication and/or maintenance is supported by the fact that the 4-kb KpnI fragment of pSOX, but not the internal NdeI fragment, is sufficient for replication (Fig. 3).
ORF81.
ORF81 (nucleotides 721 to 966, encoding a product of 81 amino acids with a predicted pI of 4.7) encodes a product that is unusual in not having cysteine, tryptophan, or tyrosine. The amino acid sequence of this polypeptide has no apparent counterpart in the available protein databases; expression of ORF81 as an 11-kDa protein (predicted Mr, 8,787) appeared only in the pTNN clone when the putative initiator codon of ORF81 was placed adjacent to the T7 promoter. Unlike the pTEB and pTEH plasmids, the pTNN DNA is devoid of the ORF164-ORF81 intergenic sequence which can potentially form several secondary structures that may prevent or attenuate the expression of ORF81 (results not shown).
ORF368.
ORF368 (nucleotides 1403 to 2509) encodes a product which is most probably a replication (Rep) protein (Fig. 4). Homology to the putative Rep proteins encoded by the mycobacterial plasmids pLR7 of Mycobacterium avium (2), pJAZ38 of M. fortuitum (14), and pMSC262 of M. scrofulaceum (14, 31) is most extensive at the N-terminal portions. Although the putative Rep protein of pMSC262 is shorter (see reference 14 for a discussion), all four proteins have a high arginine, tryptophan, and tyrosine content. The pSOX Rep protein is predicted to be basic (the calculated pI is 11.2).
FIG. 4.
Amino acid sequence alignment of the predicted replication proteins from plasmids pSOX (this study), pLR7 (M. avium [2]), pJAZ38 (M. fortuitum [14]), and pMSC262 (M. scrofulaceum [31; see reference 14 for a discussion]). The CLUSTAL program of PC/Gene was used. Asterisks indicate invariant residues. Dots indicate chemically similar residues. Pairwise alignment of either the pLR7 or the pJAZ38 Rep sequence with that of ORF368 gave 34.5% identity and 47% overall similarity. Note that the relatively short sequence of the putative Rep protein of the M. scrofulaceum pMSC262 plasmid skews the sequence similarity and identity at the C termini of the first three proteins.
The predicted Mr of pSOX Rep is 41,328, but from two independent clones a protein band of only 21.4 kDa was observed (Fig. 3). Analysis of the nucleotide sequence preceding the presumptive initiator codon of pSOX Rep showed a weak consensus ribosome-binding site (RBS) sequence (gcggtactgccagATG; potential RBS underlined). Instead, initiation at an internal GTG codon (positions 1949 to 1951), preceded by a strong consensus RBS (GAGG), would produce a 186-amino-acid residue protein of approximately 21.4 kDa. Such internal initiation or in-frame translation has been noted in the broad-host-range plasmid RSF1010, in which RepB was found to exist as either a 70-kDa or a 38-kDa (RepB*) form (36), and in the RK2 plasmid of Pseudomonas aeruginosa, where a 44-kDa form of TrfA is required for RK2 replication in P. aeruginosa but a 33-kDa form of TrfA is sufficient for replication in many other bacterial species, including E. coli (11).
During the preparation of the manuscript, the DNA sequences of three replicons derived from cryptic plasmids of R. erythropolis, R. equi, and R. rhodochrous were determined (5, 23, 40). The pFAJ2600 plasmid of R. erythropolis was found to contain two potential Rep proteins; RepA (310 amino acids) and RepB (93 amino acids), both of which are predicted to be basic (5). The replication region of the pKA22 plasmid of R. rhodochrous (23) was also predicted to contain two ORFs (encoding the ORF1 protein, of 242 amino acids, and the ORF2 protein, of 296 amino acids). In R. equi, a putative protein of 240 amino acids, with no apparent counterpart in the available database, was assumed to be a Rep protein of the pTOS plasmid (40).
Interestingly, RepA of pFAJ2600 and ORF2 of pKA22 are related to one of the two Rep proteins (ORF1 or RepA; 307 amino acids) of Mycobacterium fortuitum plasmid pAL5000 (38). A family of pAL5000-like replicons hence emerged (5). By the same token, the Rep protein of pSOX from strain X309 is believed to belong to the family of the pLR7 replicon (2), which now has four members (Fig. 4). In all these cases, further characterization of the Rep proteins, together with their possible binding site(s), is required. There is an 11-bp tandem repeat, 5′-GTCCGCGGGCA, which is 76 bp upstream of the potential start codon of ORF368. It is likely that this tandem repeat will be essential for binding to initiate replication (4, 18). Alternatively, inverted-repeat sequences found in the noncoding sequence of ORF368 are potential binding sites (4, 18).
ORF329.
ORF329 (nucleotides 2520 to 3509) encodes a protein whose predicted amino acid sequence in the complementary strand was found to have 39.2% overall similarity to a protein of an equivalent size (345 amino acids) in the Mycobacterium tuberculosis cosmid sequence (GenBank accession no. Z95436; PID: e316540). The functions of these proteins, which are predicted to be acidic, are unknown. The C terminus of the ORF329 product has a segment (positions 229 to 245) that is sufficiently hydrophobic to qualify it as a transmembrane segment. The molecular size of this protein has not been verified, but the complementary strand (clone pTPB, Fig. 3) appeared not to encode any protein.
ORF321.
ORF321 (nucleotide 3619 to the NotI site) encodes a protein whose sequence is homologous to the hemoprotein domains of various bacterial cytochrome P-450 systems (17). The highest score in the BLAST search (43.3% identity and 58.9% overall similarity) came from the hemoprotein domain of the cytochrome P-450 of Bacillus megaterium (32). This is an interesting finding since it suggests a possible cytochrome P-450 function in the pSOX plasmid.
Sucrose sensitivity as a positive selection marker in Rhodococcus sp. strain X309.
The production of levansucrase, encoded by sacB of Bacillus subtilis (37), is lethal in the presence of sucrose in numerous bacteria (15, 19, 20, 29). By screening for sacB inactivation, this positive selection system has been found useful in the isolation of insertion sequence elements from numerous bacteria including R. fascians (15, 19, 20, 29).
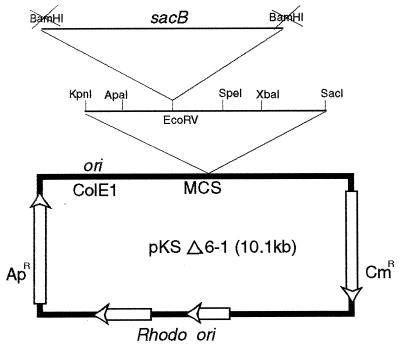
Since sucrose sensitivity was demonstrated only once in the genus Rhodococcus, we wished to confirm this in strain X309 as a prelude to exploring the possibility of using the promoter elements of sacB to drive sox gene expression. To test this, Rhodococcus-E. coli shuttle vectors (pKSΔ6-1 and pKSΔ6 [10.1 and 10.5 kb, respectively]) were constructed (see Materials and Methods), and both plasmids were found to be capable of replication in E. coli and Rhodococcus by selection on Luria-Bertani (LB) plates containing ampicillin (50 μg/ml) or chloramphenicol (25 to 40 μg/ml). Figure 5 outlines the cloning of the sacB gene in plasmid pKSΔ6-1. Selection in E. coli for Apr and Kmr produced many white colonies, two of which were electrotransformed into strain 10-2 and plated on selective media (containing chloramphenicol at 30 μg/ml). Subsequently, plasmids were isolated and their size was determined by restriction endonuclease digestion. No deletion was apparent in these plasmids. The orientation of the sacB gene was from left to right, the same direction as that of Apr (Fig. 5).
FIG. 5.
Construction of the pKSΔ6-1 Rhodococcus-E. coli shuttle vector for sacB expression in Rhodococcus strain 10-2. MCS, multiple-cloning site (expanded region). Blunt-end cloning of the end-filled 3.8-kb BamHI fragment of pUM28 plasmid containing sacB (33) at the EcoRV site is indicated.
The effect of 10% sucrose on the growth of two clones and a control strain containing the shuttle vector alone in LB selective medium is presented in Table 1. This result demonstrated that sucrose sensitivity is a screenable phenotype in strain X309. Like the previously described sacB-sensitive systems, the basis for toxicity of levansucrase action on sucrose is unknown. The fact that not all gram-positive bacteria are sensitive to sucrose (20) suggests that perhaps a different “sucrose-sensing” pathway or device exists in these organisms. Sucrose was recently shown to act as a signal molecule in the control of resource allocation between plant tissues (3).
TABLE 1.
Effect of sucrose on growth of Rhodococcus sp. strain X309-10-2 transformed with pKSΔ6-1 or pKSΔ6-1 containing B. subtilis sacB
| Strain | ×309 growth (CFU) in:
|
||
|---|---|---|---|
| LB | Selective mediuma
|
||
| Without sucrose | Plus 10% sucrose | ||
| 10-2 (pKSΔ6-1) | >500 | >500 | >500 |
| 10-2 (pKSΔ6-1sac) | 326 | 305 | 0 |
| 10-2 (pKSΔ6-1sac)b | 232 | 236 | 0 |
a LB medium with chloramphenicol (30 μg/ml). (cfu) are from 10 μl of a 10−4 dilution.
b An independent clone.
Preliminary experiments in our laboratory, using the sacB promoter to drive soxABC expression in cells of Rhodococcus sp. strain 10-2 grown in LB broth and added DBT, have produced some of the expected products of DBT metabolism (26). Further experiments are needed to show the possible advantages of the present approach. The feasibility of sox gene expression alleviating the sulfate inhibition problem was recently demonstrated in Pseudomonas (13).
Finally, the relatedness of at least one of the putative Rep proteins of the rhodococcal replicons to those of the Mycobacterium plasmids appears to be an emerging feature in these two genera. Like pLR7 and pJAZ38 (2, 14), the pSOX replicon may expand the transformation spectrum of mycobacteria, an important genus in medicine. Thus far, the majority of vectors available for use in mycobacteria are based on the pAL5000 system (5, 38). Isolation of a new rhodococcal replicon also offers new potentials for complementation studies.
ACKNOWLEDGMENTS
In an unrelated project, J. Wall (University of Missouri) alerted us to using the sacB system. We thank V. Pelicic (Institut Pasteur) for useful references.
Funding from Imperial Oil Ltd. is gratefully acknowledged.
Footnotes
NRCC publication 41774.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Beggs M L, Crawford J T, Eisenach K D. Isolation and sequencing of the replication region of Mycobacterium avium plasmid pLR7. J Bacteriol. 1995;177:4836–4840. doi: 10.1128/jb.177.17.4836-4840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiou T-J, Bush D R. Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA. 1998;95:4784–4788. doi: 10.1073/pnas.95.8.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Solar G, Giraldo R, Ruiz-Echevarria M J, Espinosa M, Diaz-Orejas R. Replication and control of circular bacterial plasmids. Microbiol Mol Biol Rev. 1998;62:434–464. doi: 10.1128/mmbr.62.2.434-464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Mot Ren, Nagy I, de Schrijver A, Pattanapipitpaisal P, Schoofs G, Vanderleyden J. Structural analysis of the 6 kb cryptic plasmid pFAJ2600 from Rhodococcus erythropolis NI86/21 and construction of Escherichia coli-Rhodococcus shuttle vectors. Microbiology. 1997;143:3137–3147. doi: 10.1099/00221287-143-10-3137. [DOI] [PubMed] [Google Scholar]
- 6.Denis-Larose C, Labbé D, Bergeron H, Alison A M, Greer C W, Al-Hawari J, Grossman M J, Sankey B M, Lau P C K. Conservation of plasmid-encoded dibenzothiophene desulfurization genes in several rhodococci. Appl Environ Microbiol. 1997;63:2915–2919. doi: 10.1128/aem.63.7.2915-2919.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denome S A, Oldfield C, Nash L J, Young K D. Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. J Bacteriol. 1994;176:6707–6717. doi: 10.1128/jb.176.21.6707-6716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denome S A, Olson E S, Young K D. Identification and cloning of genes involved in specific desulfurization of dibenzothiophene by Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1993;59:2837–2843. doi: 10.1128/aem.59.9.2837-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desomer J, Dhaese P, van Montagu M. Transformation of Rhodococcus fascians by high-voltage electroporation and development of R. fascians cloning vectors. Appl Environ Microbiol. 1990;56:2818–2825. doi: 10.1128/aem.56.9.2818-2825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desomer J, Vereecke D, Crespi M, van Montegu M. The plasmid-encoded chloramphenicol-resistance protein of Rhodococcus fascians is homologous to the transmembrane tetracycline efflux proteins. Mol Microbiol. 1992;6:2377–2385. doi: 10.1111/j.1365-2958.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 11.Filutowicz M, Dellis S, Levchenko I, Urh M, Wu F, York D. Regulation of replication of an iteron-containing DNA molecule. Prog Nucleic Acid Res Mol Biol. 1994;48:239–273. doi: 10.1016/s0079-6603(08)60857-0. [DOI] [PubMed] [Google Scholar]
- 12.Finnerty W R. Fossil resource biotechnology: challenges and prospects. Curr Opin Biotechnol. 1992;3:277–282. [Google Scholar]
- 13.Gallardo M E, Ferrandez A, de Lorenzo V, Garcia J L, Diaz E. Designing recombinant Pseudomonas strains to enhance biodesulfurization. J Bacteriol. 1997;179:7156–7160. doi: 10.1128/jb.179.22.7156-7160.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavigan J-A, Ainsa J A, Perez E, Otal I, Martin C. Isolation by genetic labeling of a new mycobacterial plasmid, pJAZ38, from Mycobacterium fortuitum. J Bacteriol. 1997;179:4115–4122. doi: 10.1128/jb.179.13.4115-4122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gay P, de Coq D, Steinmetz M, Berkelman T, Kado C I. Positive selection procedure for entrapment of insertion sequence elements in gram-negative bacteria. J Bacteriol. 1985;164:918–921. doi: 10.1128/jb.164.2.918-921.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray K A, Pogrebinsky O S, Mrachko G T, Xi L, Monticello D J, Squires C H. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat Biotechnol. 1996;14:1705–1709. doi: 10.1038/nbt1296-1705. [DOI] [PubMed] [Google Scholar]
- 17.Hasemann C A, Kurumbail R G, Boddupalli S S, Peterson J A, Deisenhofer J. Structure and function of cytochrome P450: a comparative analysis of three crystal structures. Structure. 1995;2:41–62. doi: 10.1016/s0969-2126(01)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Helinski D R, Toukdarian A E, Novick R P. Replication control and other stable maintenance mechanisms of plasmids. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology 2nd ed. II. Washington, D.C: American Society for Microbiology; 1996. pp. 2295–2324. [Google Scholar]
- 19.Jager W, Schafer A, Kalinowski J, Puhler A. Isolation of insertion elements from Gram-positive Brevibacterium, Corynebacterium and Rhodococcus strains using the Bacillus subtilis sacB gene as a positive selection marker. FEMS Microbiol Lett. 1995;126:1–6. doi: 10.1111/j.1574-6968.1995.tb07381.x. [DOI] [PubMed] [Google Scholar]
- 20.Jager W, Schafer A, Puhler A, Labes G, Wohlleben W. Expression of the Bacillus subtilis sacB gene leads to sucrose sensitivity in the gram-positive bacterium Corynebacterium glutamicum but not in Streptomyces lividans. J Bacteriol. 1992;174:5462–5465. doi: 10.1128/jb.174.16.5462-5465.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jagura-Burdzy G, Khanim F, Smith C A, Thomas C M. Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res. 1992;20:3939–3944. doi: 10.1093/nar/20.15.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilbane J J. Desulfurization of coal: the microbial solution. Trends Biotechnol. 1989;7:97–101. [Google Scholar]
- 23.Kulakov L A, Larkin M J, Kulakova A N. Cryptic plasmid pKA22 isolated from the naphthalene degrading derivative of Rhodococcus rhodochrous NCIMB 13064. Plasmid. 1997;38:61–69. doi: 10.1006/plas.1997.1293. [DOI] [PubMed] [Google Scholar]
- 24.Labbé D, Garnon J, Lau P C K. Characterization of the genes encoding a receptor-like histidine kinase and a cognate response regulator from a biphenyl/polychlorobiphenyl-degrading bacterium, Rhodococcus sp. strain M5. J Bacteriol. 1997;179:2772–2776. doi: 10.1128/jb.179.8.2772-2776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li M Z, Squires C H, Monticello D J, Childs J D. Genetic analysis of the dsz promoter and associated regulatory regions of Rhodococcus erythropolis IGTS8. J Bacteriol. 1996;178:6409–6418. doi: 10.1128/jb.178.22.6409-6418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacPherson T, Greer C W, Zhou E, Jones A M, Wisse G, Lau P C K, Sankey B, Grossman M J, Hawari J. Application of SPME/GC-MS to characterize metabolites in the biodesulfurization of organosulfur model compounds in bitumen. Environ Sci Technol. 1998;32:421–426. [Google Scholar]
- 27.Monticello D J. Biocatalytic desulfurization of petroleum and middle distillates. Environ Prog. 1993;12:1–4. [Google Scholar]
- 28.Pansegrau W, Lanka E, Barth P T, Figurski D H, Guiney D G, Haas D, Helinski D R, Schwab H, Stanish V A, Thomas C M. Complete nucleotide sequence of Birmingham IncPα plasmids: compilation and comparative analysis. J Mol Biol. 1994;239:623–663. doi: 10.1006/jmbi.1994.1404. [DOI] [PubMed] [Google Scholar]
- 29.Pelilic V, Reyrat J-M, Gicquel B. Expression of the Bacillus subtilis sacB gene confers sucrose sensitivity on mycobacteria. J Bacteriol. 1996;178:1197–1199. doi: 10.1128/jb.178.4.1197-1199.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piddington C S, Kovacevich B R, Rambosek J. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1995;61:468–475. doi: 10.1128/aem.61.2.468-475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin M, Taniguchi H, Mizuguchi Y. Analysis of the replication region of a mycobacterial plasmid, pMSC262. J Bacteriol. 1994;176:419–425. doi: 10.1128/jb.176.2.419-425.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ravichandran K G, Boddupalli S S, Hasemann C A, Petersen J A, Deisenhofer J. Crystal structure of hemoprotein domain of P-450 BM-3, a prototype for microsomal P-450s. Science. 1993;261:731–736. doi: 10.1126/science.8342039. [DOI] [PubMed] [Google Scholar]
- 33.Reid J L, Collmer A. An nptII-sacB-sacR cartridge for constructing directed, unmarked mutations in Gram-negative bacteria by marker exchange-eviction mutagenesis. Gene. 1987;57:239–246. doi: 10.1016/0378-1119(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 36.Scholz P, Haring V, Wittmann-Liebold B, Ashman K, Bagdasarian M, Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989;75:271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- 37.Steinmetz M, Le Coq D, Aymerich S, Gonzy-Treboul G, Gay P. The DNA sequence of the gene for the secreted Bacillus subtilis enzyme levansucrase and its genetic control sites. Mol Gen Genet. 1985;200:220–228. doi: 10.1007/BF00425427. [DOI] [PubMed] [Google Scholar]
- 38.Stolt P, Stoker N G. Functional definition of regions necessary for replication and incompatibility in the Mycobacterium fortuitum plasmid pAL5000. Microbiology. 1996;142:2795–2802. doi: 10.1099/13500872-142-10-2795. [DOI] [PubMed] [Google Scholar]
- 39.Tabor S. Expression using the T7 RNA polymerase/promoter system. In: Ausubel F A, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley-Interscience; 1990. pp. 16.2.1–16.2.11. [Google Scholar]
- 40.Zheng H, Tkachuk-Saad O, Prescott J F. Development of a Rhodococcus equi-Escherichia coli plasmid shuttle vector. Plasmid. 1997;38:180–187. doi: 10.1006/plas.1997.1311. [DOI] [PubMed] [Google Scholar]