Abstract
Recently, boron neutron capture therapy (BNCT) has been attracting attention as a minimally invasive cancer treatment. In 2020, the accelerator-based BNCT with L-BPA (Borofalan) as its D-sorbitol complex (Steboronine®) for head and neck cancers was approved by Pharmaceutical and Medical Devices Agency for the first time in the world. As accelerator-based neutron generation techniques are being developed in various countries, the development of novel tumor-selective boron agents is becoming increasingly important and desired. The Japanese Society of Neutron Capture Therapy believes it is necessary to propose standard evaluation protocols at each stage in the development of boron agents for BNCT. This review summarizes recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for BNCT based on our experience with L-BPA approval.
Keywords: boron neutron capture therapy (BNCT), boron agents, evaluation protocols, in vitro, in vivo
INTRODUCTION
Patient-friendly and minimally invasive therapies have recently gained popularity, allowing patients to return to their daily lives quickly. This is anticipated to lower healthcare costs while also assisting patients in realizing a healthy and fulfilling quality of life. In this regard, boron neutron capture therapy (BNCT) is attracting attention as a minimally invasive cancer treatment. In BNCT, the patients are given a boron agent and the target area is exposed to a neutron beam for several tens of minutes. In addition, compared with conventional radiotherapy, posttreatment skin damages are relatively small [1–3]. The thermal and epithermal neutrons used in BNCT have a very low energy of 0.025–10 keV, whereas the particles such as 7Li and 4He (α ray) nuclei produced by neutron capture reactions with boron (10B) have a high linear energy transfer with 210 and 163 keV/μm, respectively. Furthermore, their ranges inside tissue are 4–9 μm, so they come to a full stop inside the tissue where the reaction took place. There are other nuclei in the human body that can capture a thermal neutron. Hydrogen and nitrogen, in particular, are present in high concentrations and thus affect significantly on the neutron irradiation dose. Although the probability of a thermal neutron being captured by 10B atom is over 2000 times higher than the above-mentioned atoms, it is calculated that 109 10B atoms per cancer cell are required to achieve cytocidal effects while minimizing the side effects. Thus, for BNCT, a cancer cell-selective radiotherapy, selective delivery of 10B to cancer cells is essential (Fig. 1). BNCT is effective for invasive cancers that are difficult to treat with conventional radiotherapy because thermal neutron irradiation can ideally generate α rays selectively to cancer cells.
Fig. 1.

Neutron capture reaction of 10B (left) and a conceptual diagram of cancer cell-selective BNCT (right). Reproduced with permission from JSNCT website (http://www.jsnct.jp/e/about_nct/index.html).
In 2009, Japan succeeded in developing the world’s first accelerator to generate neutrons for BNCT. Until then, a nuclear reactor was the only source of neutrons for BNCT. The advent of accelerator neutron sources has made BNCT possible in hospitals, paving the way for general treatment [4, 5]. Finally, in 2020, the accelerator-based BNCT with L-BPA (Borofalan) as its D-sorbitol complex (Steboronine®) for head and neck cancers was approved by Pharmaceutical and Medical Devices Agency [6, 7].
Furthermore, neutron generation technology using accelerators is being developed in various countries [8–12]. Therefore, the development of novel tumor-selective boron agents is becoming increasingly important and desired. The Japanese Society of Neutron Capture Therapy (JSNCT) believes it is necessary to propose standard evaluation protocols at each stage in the development of boron agents for BNCT in the future. In this review, we summarize recommended experimental protocols for in vitro and in vivo evaluation methods of boron agents for BNCT based on our experience with L-BPA approval.
PROPERTIES REQUIRED FOR BNCT BORON AGENTS
Conventional anticancer drugs are effective at concentrations in the order of 10−6–10−9 M in cancer cells because they often target cell growth signals or genes that are active in cancer cells. However, the drugs themselves do not always selectively accumulate and act on cancer cells. In BNCT, on the other hand, cells containing 10B are killed by thermal neutron irradiation, so the key point is how to accumulate 10B compounds in cancer cells. As stated beforehand, ~109 10B atoms are required in the cancer cells to obtain sufficient therapeutic effect, which means that a boron concentration of about 10−3 M is required. As L-BPA is administered at 500 mg/kg in clinical practice [6,7], the development of boron agents used in BNCT requires a completely different approach from that of anticancer agents, which are usually effective at low doses. According to the biodistribution study in tumor bearing hamsters, the blood half-life of L-BPA is short, and boron concentrations in tumors are related to the blood concentration [13]. Therefore, in clinical practice, intravenous administration is continued during neutron irradiation to maintain the 10B concentrations in the blood [14]. It should be noted that, not all cancers can be treated with L-BPA, as some cancers do not take up L-BPA.
Successful treatment with BNCT is highly dependent on the ability of 10B to selectively accumulate in tumor cells and intracellular biodistribution. The following three characteristics are thought to be the ideal circumstances required for boron agents:
[1] The 10B concentration in tumor tissue should be kept constant throughout the neutron irradiation to maintain the expected antitumor effect (ideally 25~30 μg10B/g). The probability of a nucleus capturing a thermal neutron is expressed as a ‘thermal neutron capture cross section’ (barn = 10−24 cm−2). Elements in living organisms also capture thermal neutrons (e.g. H = 0.332 barns, n = 1.75 barns), but their thermal neutron capture cross sections are several orders of magnitude smaller than 10B (3838 barns). However, since these elements are present in high concentrations in the body, it cannot be ignored. It is calculated that, approximately 85% of the radiation dose is generated from 10B neutron capture reactions when the 10B concentration exceeds 25 μg/g [15].
[2] To ensure safety, the ratios of ‘concentration in tumor tissue/normal tissue’ and ‘concentration in tumor tissue/blood’ should be high (ideally >3) [16], and the systemic toxicity should be low.
[3] Boron agents should be rapidly excreted from normal tissues and blood after neutron irradiation. In other words, boron agents used for BNCT must have tumor selectivity to minimize effects on normal tissues and 10B concentration in tumor tissue to ensure a therapeutic dose to the tumor. Furthermore, boron agents should be considered for compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines for ‘neoplastic agents’ in addition to national guidelines in each country. Needless to say, the criteria described here are based on the L-BPA approval.
STANDARDIZATION OF EVALUATION METHODS FOR BORON AGENTS
Boron concentration measurement methods
Inductively coupled plasma (ICP) or prompt gamma-ray analysis (PGA) is commonly used as a method to measure the boron concentration in blood, tissue, or in vitro samples. In this section, we briefly introduce these quantification methods. One point to note when using these methods to measure boron is that pretreatment should be performed without using laboratory equipment (e.g. borosilicate glass) that contains boron. For sample preparation, it is desirable to use boron-free plastic containers instead of glassware.
ICP-related analytical methods
ICP is the gold standard for boron concentration determination. The main advantage of ICP is that it does not require neutron irradiation for measurement. ICP can be divided into two main categories: inductively coupled plasma atomic emission spectroscopy (ICP-AES) and inductively coupled plasma mass spectroscopy (ICP-MS). ICP-AES is sufficient for standard boron concentration measurement, while ICP-MS has excellent sensitivity and can discriminate between 10B and 11B. The measurement limit of ICP is ~0.1 ppm boron concentration. About 2–5 ml of solution is required for measurement. The sample is pretreated by being exposed to a strong acid at high temperature, a procedure known as wet ashing (Fig. 2). Removal of insoluble components in the sample is necessary before measurement. Due to the influence of the boron memory effect [17], after measuring a sample containing high concentrations of boron, the sample line should be thoroughly cleaned until the background value sufficiently decreases.
Fig. 2.

Wet-ashing procedure of boron-containing samples using dry block heating system. (a)Wet-ashing sample in centrifuge tube. (b) Dry block heater.
PGA method
PGA is a measurement technique for the analysis of 10B concentration and it involves measuring the prompt gamma rays emitted from the 10B(n, a)7Li* reaction [18]. PGA can be applied to any sample as long as it can be contained in a sample tube. The sample should be stored in a polytetrafluoroethylene (PTFE) container to reduce the generation of unwanted gamma rays. The detection limit of the system is 0.1–0.5 ppm 10B concentration. In practice, the 10B concentration inside a 1-g tissue sample can be measured in <30 s with 10% accuracy. Moreover, the system has the advantage that pretreatment of the sample is not required. To reduce the measurement uncertainty, the measurement time may be extended if the boron concentration in the sample is low.
Other methods
Analytical methods using enzyme-linked immuno sorbent assay (ELISA)[19] and fluorescence with molecular probes[20] are being investigated as methods for measuring the boron concentration.
These methods can detect lower concentrations in smaller amounts of samples compared with ICP-MS and/or PGA. However, the measurement of boron concentration by ELISA or fluorescence method using molecular probes is not yet a standard method. ELISA requires the use of antibodies to boron compounds [21], and these anti-boron compound antibodies are not commercially available. The fluorescence sensor method requires the use of molecular probes for boron compounds [22–25]. The fluorescence sensor for boron cluster compounds such as carborane, decaborane or dodecaborate is not yet reported; the sensor for boron compounds is only developed for compounds containing boric acid. Such methods will eventually be accepted as a standard and common method to determine boron concentration.
RBE or CBE
The biological effects of heavy particle radiation are different from those of X-rays due to the large LET. For the same physical dose of 1 Gy, the biological effect of 1 Gy of heavy particle radiation is larger than that of 1 Gy of X-rays. Therefore, when evaluating the effects of heavy particle radiation on tumors and normal tissues, the coefficient of conversion to X-ray equivalent dose, the relative biological effectiveness (RBE), is used to convert the dose.
The advantage of the RBE is that it can be used as a guide to the tolerable dose for each normal tissue, which has been empirically obtained with X-rays. In other words, the RBE is a tool to optimally and safely use heavy-ion beams, for treatment, by using the rule of thumb that states at what total dose (or higher a tumor effect) is observed or serious adverse events occur when converted to 2 Gy per dose of X-rays.
The concept of RBE applied to BNCT is the compound biological effectiveness (CBE). CBE values differ from one boron agent to another and from one tissue to another. Therefore, after the organ accumulation of a new boron drug is measured using the method described in this paper, it is recommended to evaluate the CBE of tumor tissue and normal tissue (organs at risk in radiotherapy) for that boron drug by performing animal experiments [26,27].
A recommended protocol for preparation of ICP samples
Preparation of cell culture sample for measuring intracellular 10B concentration. Cells were seeded and incubated. The medium was replaced with an equivalent amount of medium containing the boron compounds. After incorporation of the boron compound, the medium was removed by aspiration. The cells were washed thrice with ice-cold PBS(−), harvested by trypsinization and then counted. However, the washing conditions should be considered for each compound because low-molecular-weight compounds tend to be washed out. Trypsinization is performed with 0.05–0.25% trypsin at 37°C for 5–10 min. Trypsin is used at the lowest possible concentration to prevent drug efflux. When normalizing boron concentration per cell using protein amount per cell, collect about 1% of the cell suspension and measure the amount of protein in the cells by BCA (bicinchoninic acid) assay. Each sample was wet-ashed, and the boron concentration was measured by ICP-AES or ICP-MS [28].
Selection of optimum wavelength. When ashing blood samples, care should be made to prevent blowout and explosion, such as covering the sample with a lid before processing. When measuring boron concentration by ICP-AES, there are several candidate wavelengths, and the sensitivity and the effect of foreign substances in the sample differ depending on the wavelength used. For example, some boron wavelengths are interfered by tracer amounts of metals in the blood. Therefore, 208.9 nm is recommended for the measurement of boron concentration in blood to minimize the influence of these tracer amounts of metals. The user should select the optimal wavelength based on the samples and the experimental conditions.
In vitro evaluation methods
The drugs for BNCT must deliver boron selectively to tumors without causing toxicity to normal cells. To evaluate the boron-containing drug for BNCT, cytotoxicity test and cellular uptake test for tumor cells are examined. To confirm the accuracy of these experiments, it is important to verify that the results obtained with conventional boron agents such as BPA and BSH are consistent with the results shown in previous studies. In particular, the usefulness of newly developed drugs can be clarified by comparing them with BPA, which has been proven to have a therapeutic effect. In these evaluations, it is important to clarify the physicochemical characteristics of the drugs in order to properly interpret the experimental results. For example, the high hydrophobicity of drugs is likely to cause aggregation at certain concentrations and compromise the intended function of the molecular design, leading to a risk of overlooking the advantage of the drugs. Thus, at the very least, the solubility of the drugs in water should be evaluated to properly interpret the obtained results. After the demonstration of these functions, BNCT effects with neutron irradiation are investigated if possible. Here, we describe the general methods of each experiment, their limitations and perspectives.
Preparation of compound solutions
It is preferable that sufficient water solubility of a drug is ensured before the in vitro evaluations. Exact solubility is not always necessary, but water solubility should be simply indicated with the following information: how much drug concentration was used to prepare an aqueous solution in in vitro studies or how much concentration was used to prepare a stock solution. It is also preferable to clarify that water solubility is guaranteed in the drug concentration range used in in vitro studies. If a stock solution is prepared with an organic solvent including DMSO, it is important to clearly describe how the stock solution was diluted with the aqueous solution for reproducibility.
Cytotoxicity
It is necessary to clarify the concentrations at which the drugs do not induce cytotoxicity in the absence of thermal neutrons. WST-1, WST-8 and MTT assays are often used to evaluate acute cytotoxicity. More specifically, many studies have reported the quantification of IC50 values or the comparison of cell viability at specific concentrations. Some drugs may show extremely low toxicity and it may be difficult to calculate the IC50 values. In such cases, a comparison of cell viability at specific concentrations should be useful to demonstrate that drugs do not cause unfavorable cytotoxicity in the following cellular uptake study.
Cellular uptake
This evaluation should be performed at the concentrations at which the drugs do not result in detectable cytotoxicity according to the study mentioned above. Many researchers use cells related to target disease or cells whose BPA uptake behavior has already been studied. It is recommended to use the same cell lines in in vitro and in vivo studies, which permits discussion about the correlation between in vitro and in vivo results.
Cellular uptake is usually indicated by the amount of boron, and it should be normalized by the number of cells, the amount of protein or the weight of the dried cells, because the amount of boron depends on the number of cells at the incineration process. Hence, the cellular uptake is often expressed as [μg boron/1 × 106 cells], [μg boron/mg protein], [μg boron/mg cell], etc. In comparison with cellular uptake among multiple cell lines, it should be kept in mind that cellular uptake efficiency depends on many parameters including expression of transporters, the activity of endocytosis and so on.
Note that it has been reported that replacing the culture medium after administration of BPA with the medium containing low BPA resulted in the export of BPA from the cells, causing a decrease in intracellular boron concentration[29–31]. Thus, it is important to carefully examine the aforementioned procedures.
A recommended protocol of the boron uptake test is shown in “A recommended protocol for preparation of ICP samples” section.
Subcellular distribution
Since the range of α particles and Li recoil nuclei is within 10 μm, the intracellular localization of drugs is expected to induce higher BNCT effects than the extracellular localization. In addition, subcellular distribution of drugs is related to the cellular uptake mechanism. Thus, it is important to investigate the subcellular distribution. To visualize the distribution, previous studies utilized an antibody recognizing a boron compound, a molecular probe, a fluorescently labeled compound[32], NanoSIMS [33], etc. Specifically, subcellular BSH could be visualized by immunocytochemistry (ICC) with anti-BSH antibody [21,34], while BPA could be detected by the fluorescent probe that can react with the boronic acid moiety of BPA[35]. ICC staining using anti-BSH antibodies has been reported to evaluate the intracellular localization of drugs over time after intracellular transport of BSH by DDS or administration of BSH-binding compounds. Anti-BSH antibodies recognize both BSH alone and BSH-bonded peptides and can be used in the same manner as in conventional biological experiments [35].
Cellular uptake mechanism
Since BPA is mainly taken up via LAT1 amino acid transporter, which is overexpressed on many cancer cells, it can selectively be taken inside the tumor [36]. Meanwhile, some boron drugs have been reported to passively accumulate within tumors through diffusion[37]. Other drugs showed selective interaction with receptors or were taken up through endocytosis[38–40]. Clarifying such cellular uptake pathways will lead to the estimation of the selectivity of the therapeutic effect of BNCT. Thus, understanding the cellular uptake mechanism is essential to the development of drugs for BNCT.
To elucidate the mechanism, many studies have utilized inhibition assays with inhibitors or examined the effect of cell culture conditions such as oxygen and glucose concentrations on cellular uptake [41]. For example, in the case of BPA, it has been reported that the uptake amount is reduced by BCH, the inhibitor for system L transporter inhibitor [30,31]. If the uptake mechanism of a newly developed drug can be estimated, it is convenient to use a related inhibitor for a similar inhibition assay.
BNCT effects with neutron irradiation
To examine the BNCT effects of drugs in in vitro, thermal neutrons should be irradiated to the cells; however, in principle, cellular uptake is correlated with BNCT effects. Considering the limited availability of facilities for neutron irradiation (such as reactors and accelerators), it is not always necessary to examine in vitro BNCT effects if cellular uptake is quantitatively investigated.
It should be noted that biological experiments, such as cellular experiments, do not take rich 10B or natural boron compounds into account. This is because isotopes are biologically recognized as identical in cellular uptake and metabolism, and many experiments using isotope-labeled compounds have already been reported [42]. Needless to say, when considering BNCR by neutron irradiation, 10B-rich boron drugs are desirable.
Example of an experimental protocol to examine BNCT effects
The medium is replaced with a fresh medium containing boron compounds, followed by incubation. After removing the medium by aspiration, the cells are washed, trypsinized, collected and counted. Trypsin is then removed by aspiration after centrifugation, and the cells are suspended with the medium. As shown in Fig. 3, the cell suspension in the medium in a plastic vial is irradiated with thermal neutrons. The fluence can be determined by averaging the activation level of the two gold foils that are symmetrically attached to the surface of the plastic vial along the direction of incidence of the thermal neutrons. It should be noted that the vial should be made from a material that is insensitive to thermal neutrons. Although PTFE is the most suitable material for this purpose, polypropylene tubes that are widely used in biological experiments can also be used. Gamma rays that are not intended for BNCT can be determined by thermoluminescent dosimeters (TLDs) attached on the cap of the vial.
Fig. 3.
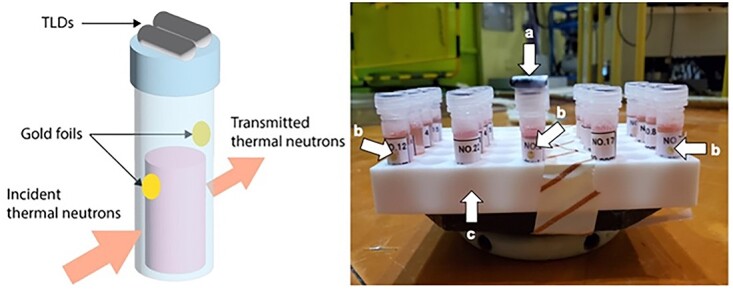
An example of sample preparation. (a) TLDs for measurement of gamma ray. (b) Gold foil for measurement of neutron fluence. (c) PTFE table.
To evaluate the BNCT effect, the cell viability tests are examined such as colony forming test, WST assay or MTT assay using irradiated cells [35,43–45].
In vivo evaluation methods
Acute toxicity test (single dose)
This section describes in vivo toxicity studies conducted as part of academic research for BNCT. In other words, the main objective of the study is to determine whether the planned dose of the new drug that is expected to have a medicinal effect is sufficiently safe. Single-dose toxicity is defined as a toxicological response that is indexed by a change in general condition, including lethality. When conducted as part of preclinical studies for clinical trials, it is necessary to conduct GLP-compliant studies in accordance with predefined guidelines. But when conducted as part of academic research, researchers should follow the GLP-defined guidelines (e.g. ICH-S4 toxicity studies) but can manage depending on the goal of their projects. When evaluating the antitumor effects and adverse events of new boron agents, it is always advisable to have a BPA + neutron irradiation group as a control group. In other words, the evaluation of the antitumor effects and adverse events of BNCT with novel boron agents should always be compared to the antitumor effects and adverse events of BNCT with BPA[46,47]. In in vitro studies, boron drug concentration in the medium is often mentioned in drug discovery research [48], but in in vivo studies, the boron drug dosage per mouse body weight is used for BNCT experiments [46]. It is desirable to convert the drug dose to the boron dose. The toxicity of the drug itself and the toxicity of the drug combined with neutron irradiation should be separately discussed from both perspectives.
Boron uptake study in tumors and various organs
After conducting the above-mentioned acute toxicity studies, in vivo pharmacokinetic studies are conducted. When performing in vivo pharmacokinetic studies, tumor-bearing animal models are often used to simultaneously evaluate the accumulation of the drugs in tumor tissues. PGA and ICP are commonly used to measure boron concentrations, and since PGA requires neutron irradiation [49], ICP has recently been used at the laboratory level [50].
The therapeutic effect ratio of BNCT depends on both the biological effect of the boron drug to each normal and tumor tissues evaluated by CBE and the boron accumulation ratio of tumor tissues compared to organs at risk(s). Therefore, it is common in the field of BNCT to evaluate the distribution of boron agents using the ratio of tumor tissue to normal tissue (T/N ratio) as well as the concentration of boron agents accumulating in each organ. However, in some tumor models such as brain tumors, the commonly used mouse subcutaneous tumor implantation model may have a different boron drug distribution compared to the orthotopic tumor model implanted in the tumor original lesion (e.g. brain in brain tumor model). Therefore, an orthotopic transplant tumor model is used as an ideal animal model to evaluate boron uptake [51]. Since the orthotopic tumor model are not always easy to perform, the allograft model (e.g. subcutaneous injection tumor model) is widely used for boron uptake study. Care must be taken for boron drugs targeting brain tumors, since the blood–brain barrier exists and the distribution of boron drugs may differ between the subcutaneous tumor model and the orthotopic tumor model [52].
The distribution study of boron agents in tissues
The microscopic distribution of the boron drug in the tissue (i.e. the distribution of boron) is a major determinant for the therapeutic effect of BNCT and the occurrence of adverse events. Therefore, it is necessary to evaluate the distribution of the boron drug in the tumor and normal tissues [13,46,53,54].
Various methods have been devised and the appropriate method largely depends on the type of drug. When a drug is labeled in advance with an evaluable atom or radioisotope, it is important to keep in mind that the properties of the drug may become modified. 18F-BPA, for example, is used to evaluate the pharmacokinetics of the BPA and its accumulation in tumor tissues before the application of BPA-based BNCT. However, the chemical properties of the drug change when 18F is bound to BPA; for example, the solubility of 18F in water is slightly different when 18F is bound to BPA [53]. Therefore, it must be rigorously examined whether the pharmacokinetics of 18F-BPA are really the same as those of BPA. To avoid possible changes in the function of the drug itself, radionuclide labeling, such as using 14C as the replacement of the drug constitutional atom, is preferred. However, there are not many laboratories that can use 14C during the drug discovery stage.
Alpha autoradiography (ARG) uses a special plastic detector named CR-39 to visualize the distribution of 10B atoms in a tissue and in a cell [55]. It is also applicable to every 10B-containing drug. However, the disadvantage is that it requires neutron irradiation.
The distribution of the boron agents BPA and BSH in tissue can also be examined by immunohistochemical staining using antibodies against these boron agents [56].
Evaluation of anti-tumor effects by neutron irradiation
Evaluation of antitumor effects with neutron irradiation is an important test for the development of boron drugs for BNCT. Such in vivo experiments are not defined as a GLP standard. To accurately evaluate the effect of BNCT on tumors, it is necessary to perform neutron irradiation only on tumors. A suitable shielding agent, such as a lithium fluoride plate [57], is used for this purpose. In this experiment, 10B-enriched drug compounds should be used to properly evaluate the therapeutic effect.
There are three main methods for evaluating effectiveness. The first is to remove the tumor after BNCT, isolate the cells by mechanical and enzymatic treatment and determine whether the cells are alive or dead by their ability to form colonies in the dish [58]. This is the most sophisticated radiobiological method. With this method, it is possible to determine the relationship between BNCT dose and effect, as in experiments with cultured cells. In other words, the effectiveness of BNCT can be evaluated by comparing the slopes with that of X-rays.
The second method uses the delay time (day) of tumor growth or the prolongation of animal survival time due to an inhibition of tumor growth as an indicator of BNCT effect. The tumor size can be evaluated by measuring the actual size of the subcutaneous tumor. Imaging methods are also used to evaluate the antitumor effects. One of the imaging methods is luminescence imaging [59,60]. To evaluate antitumor effect with this method, fluorescent proteins or enzymes such as luciferase should be induced to the tumor cells beforehand. In addition, diagnostic imaging methods such as CT and MRI can be used for the evaluation of the antitumor effects [61]. In environments where such imaging equipment is not available, direct observation in a time series with an appropriate control group is necessary for the evaluation.
The third method is to use TCD50 (50% radiation dose to control tumor growth) [62]. Using an in vivo carcinoma-bearing mouse model, the radiation dose to the tumor is progressively increased and the tumor control rate is experimentally determined. The TCD50 value is calculated as the value of 50% of the dose at which complete control of the tumor is obtained.
It should be noted that the handling of irradiated mice must conform to the radiation control regulations of each facility, since the standards differ from one country to another. When irradiating mice, the component of the neutron beam used in the neutron irradiation experiment should be recorded (For details regarding the component of neutron beam, please refer to section 6).
Dosimetry for in vitro/in vivo experiments
Biological experiments for boron drug evaluation are usually carried out in a well-thermalized neutron irradiation field mixed with gamma rays and epithermal / fast neutrons. Radiation dose imparted to biological samples by charged particles emitted from 10B(n,α)7Li reactions, caused by thermal neutrons, is of the main interest. Besides that, in such irradiation fields, doses from 14N(n,p)14C reactions caused by thermal neutrons and 1H(n,n)1H reactions by higher energy neutrons should be considered as a background dose. Dose by gamma rays, which are mixed in the irradiation beam and generated by thermal neutron radiative capture reactions in the experimental equipment and the sample itself, is also involved. In this section, the determination methods for thermal neutron fluence, in addition to the background dose components, are described.
Determination of thermal neutron fluence
Biological response to boron drugs depends on the irradiated thermal neutron fluence as well as their intracellular or intratissue accumulation. Therefore, thermal neutron fluence is the most essential quantity to be determined as an index of irradiation dose in drug evaluation. Thermal neutron fluence at the target site can be experimentally determined by using the neutron activation method, which is the most common and reliable technique in dosimetry for BNCT. Small pieces of thin gold foils, which cause little perturbation of the irradiation field, are generally used for this purpose (gold activation). For instance, gold foils are attached to the surface of the cell containers or near the tumor transplantation sites of animals. After the irradiation, the thermal neutron fluence can be determined by measuring the radioactivity of the gold foils. However, gold also reacts to epithermal neutrons. So, to account for this, a correction factor which is experimentally determined during the characteristic evaluation of the irradiation field is necessary.
Thermal neutrons incident on a sample are scattered mainly by hydrogen atoms, resulting in an intensity gradient inside the volume of the target site. For instance, in the case of a parallel beam, attenuation of the neutron fluence occurs in the direction of the neutron beam. This nonuniform dose distribution inside the target site cannot be ignored in typical sized cell containers or transplanted tumors [63]. Consideration should be given to achieving a uniform distribution as much as possible by adopting procedures such as changing the location and/or direction of the samples during the irradiation when the nonuniformity may impair the analysis of the biological response [64]. If this is difficult to achieve, it is desirable to determine the volume-averaged thermal neutron fluence. For such a case, in the experiments using Kyoto University Reactor (KUR), gold foils are placed on both the beam entrance side and the exit side of the sample tube or the transplanted tumor to determine the average thermal neutron fluence. Furthermore, the gradient of the thermal neutron distribution becomes higher when irradiating samples with a large volume, such as stacking of sample tubes or microplates, and so on. In some cases, the gradient may be intentionally formed to have various dose conditions at once [65]. In such experiments, it is desirable to determine the thermal neutron fluence applied to each position of the samples as accurately as possible by increasing the number of measurement points.
Estimation of background doses
In the thermal neutron irradiation, gamma ray and neutron doses are always accompanied as the background dose that is independent of the accumulation of boron drugs. If these accompanying doses are not negligible in the analysis of biological effects, it is desirable to provide their levels as additional information to the thermal neutron fluence.
The gamma-ray dose can be approximated by measurement using a thermoluminescence dosimeter (TLD). Small TLDs, which cause little perturbation of the irradiation field, can be attached to the samples together with the gold foils. Glass dosimeters or optically stimulated luminescence dosimeters are also promising for this purpose. It should be noted that most of the commercially available gamma-ray dosimeters (including the TLD) have relatively high sensitivities to thermal neutrons. In the cases of KUR, a special quartz glass-enclosed BeO (Na) TLD with low sensitivity to thermal neutrons is used. Additionally, the contribution of thermal neutrons to the luminescence is corrected for by using the thermal neutron fluence measured using the gold foil [66]. External shielding cases may be considered to reduce the thermal neutron contribution; however, perturbation of the irradiation field should be carefully considered when using it simultaneously with the sample irradiation.
The background neutron dose is divided into thermal neutron dose and epithermal / fast neutron dose according to their energy ranges. The thermal neutron dose is mostly contributed from 14N(n,p)14C reactions due to nitrogen atoms in the sample. This can be derived by using the thermal neutron fluence, assuming the typical nitrogen content in the samples, as in the expression described below. The epithermal / fast neutron dose is generally difficult to measure, and in situ measurement methods such as the gold activation and TLDs have not been established. Usually, the dose is roughly evaluated based on the nominal value determined in characteristic measurement of the irradiation field. In the cases of KUR, the conversion coefficient from the thermal neutron fluence to the neutron dose is prepared based on the nominal neutron energy spectrum, and the dose in each experiment is quantified from the measured thermal neutron fluence [67].
Derivation of physical or equivalent dose
The use of physical absorbed dose as an index of irradiation dose is often useful for comparing the response to neutron irradiation directly with the control experiments using a reference radiation. The physical dose resulting from the 10B(n,α)7Li reaction due to thermal neutrons is derived by the following expression:
 |
where the numeric constant is the kerma coefficient of 10B for thermal neutron with the temperature of 0.0253 eV, calculated using the evaluated nuclear data file (ENDF) data library [68]. This value is modified according to the temperature 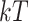 of the thermal neutron field, as in the second term [69]. The macroscopic 10B concentration is expressed as
of the thermal neutron field, as in the second term [69]. The macroscopic 10B concentration is expressed as 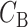 , where the measured value using the determination method such as ICP and PGA described in the previous section can be applied.
, where the measured value using the determination method such as ICP and PGA described in the previous section can be applied. 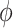 is the thermal neutron fluence, where the measured value using the gold activation method can be used.
is the thermal neutron fluence, where the measured value using the gold activation method can be used.
The thermal neutron dose from 14N(n,p)14C reactions is derived by the following expression:
 |
where the numeric constant is the kerma coefficient of nitrogen for thermal neutron with the temperature of 0.0253 eV, calculated using the ENDF data library [68,70]. This value is modified according to the temperature 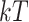 , similarly to the above [70].
, similarly to the above [70]. 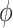 is the thermal neutron fluence. The weight fraction of nitrogen, expressed as
is the thermal neutron fluence. The weight fraction of nitrogen, expressed as 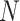 typically in the range of a few percent, is usually assumed from typical nitrogen content of the subjects, such as a culture medium or a targeted tissue for in vitro or in vivo experiments, respectively.
typically in the range of a few percent, is usually assumed from typical nitrogen content of the subjects, such as a culture medium or a targeted tissue for in vitro or in vivo experiments, respectively.
The total physical dose is the sum of these doses and the epithermal / fast neutron dose 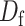 and the gamma-ray dose
and the gamma-ray dose  , estimated by the above methods, as given in the following expression:
, estimated by the above methods, as given in the following expression:
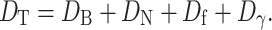 |
Use of the equivalent dose has been often applied, especially to the analysis of in vivo experiments, in the cases that the biological response can be reasonably estimated against the neutron irradiation with/without boron drug administration, relative to a reference radiation [56]. The equivalent dose 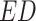 is derived by using the following expression:
is derived by using the following expression:
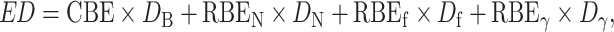 |
where the CBE is the boron CBE, the RBEs, the RBE of each dose component [71,72]. Typically, X-rays or gamma rays are selected as the reference radiation, and CBE and RBEs corresponding to them are used.
Additional consideration for reliable dosimetry
Toward establishing a more reproducible and stable dosimetry, it should be considered for similar experimental conditions to use a standardized irradiation geometry and to quantify the doses based on the well-validated dose characteristics [63,73]. Regarding the reproducibility of a measurement, for example, the results using a gold foil and a TLD may be affected by slight differences in the setup positions. In addition, the thermal neutron fluences, as well as the gamma-ray and neutron doses, in the different types of samples have different distributions depending on the shape and size of the samples. By setting up a standard geometry and defining accurate dose characteristics through measurements and well-validated simulation calculations, a reliable dosimetry protocol can be established for similar experiments. This may also help to improve the effectiveness of the measurement techniques used in each experiment. For instance, in addition to using a standard irradiation device, it should be considered for in vitro experiments to arrange the positioning, size and the number of tubes, and the amount of the medium, etc. to be the same for each irradiation, and for in vivo experiments to arrange the positioning and a number of animals to be the same for each irradiation, and adjust the size and location of the transplanted tumors uniformly as possible.
On the other hand, dosimetry by simulation calculation is a useful tool for dealing with various types of experiments and can lead to a more accurate evaluation in combination with an appropriate experimental validation. For example, it is necessary to consider the large dose gradient in the subject for in vitro experiments using relatively large dishes, flasks, well plates, etc., and for in vivo experiments using the animals with medium to large body size [64,70,71,74]. In such experiments, an accurate measurement is difficult or requires a great deal of effort. Simulation calculation can be quite effective for accurate dose estimation even in such a situation.
SUMMARY AND FUTURE PROSPECTS
BNCT has gained worldwide attention since the turn of the 21st century, and new researchers are participating in the development of boron drugs. As a result, there are some data on the evaluation of the effects that employ unconventional methods. In addition, some of the data of researchers who have been involved in the past have not been correctly evaluated. Therefore, in this paper, we proposed to facilitate new drug development by conducting experiments and evaluations using a unified, standardized method.
First, standard methods were proposed for evaluating the uptake of boron compounds in cultured cells, evaluating the BNCT effect of neutron irradiation and searching for boron distribution. Furthermore, a method for the pharmacokinetics in experimental animals and a method for the evaluation of the effects of boron drugs in combination with neutron irradiation was proposed. In addition, standard methods and notations for the crucial dosimetry were proposed.
This proposal does not always demand a step-by-step evaluation of the effectiveness of the new drug from cultured cells to experimental animals and tumors. Drugs must be effective and safe in clinical BNCT. Although the efficacy of a drug in cultured cells is superior to that of the preceding drug, it is often inferior in the effects on animal tumors. To avoid such wastage, it is necessary to understand the mechanism of the cell-killing effect of the boron neutron capture reaction before proceeding with development. Considering that the cell-killing effect is due to high-LET particles with a range that does not exceed the cell diameter, the microscopic distribution of the drug and the morphological characteristics of the target cells are decisive factors in determining the effectiveness [75,76].
Although the finest distribution must be determined using antidrug antibodies or chemical probes, comparable precision can be achieved with ARG. ARG also has the advantage of being applicable to any drug containing 10B. A neutron source is required but can be provided by domestic research institutes. In the evaluation of effects, it is desirable to precede this microscopic distribution study in cells and tissues.
Since the distribution search can be applied not only to tumors but also to organs, it is very useful for predicting the extent of BNCT effects on normal tissues. After confirming the distribution, it is reasonable to investigate the effect and safety in cultured cells and experimental tumors.
As a rigorous evaluation of effects requires time, labor and money, it is preferable to precede the complete microdistribution study before the validation of effects. The evaluation in tumors should be examined in multiple steps of neutron dose or drug dose. This is because, when compared with previous drugs, a favorable result may be obtained at one level, but in an increased dose of neutron or drug, there may be no difference in the effects.
Since the proposals in this paper are standard at present, we expect a simpler and more useful methods will be developed in the future.
ACKNOWLEDGEMENTS
This work was conducted as part of the activities of JSNCT. We thank Prof Mitsunori Kirihata of Osaka Metropolitan University for his kind advice.
Contributor Information
Yoshihide Hattori, Research Center for BNCT, Osaka Metropolitan University, 1-1 Gakuen-cho, Nakaku, Sakai 599-8531, Japan.
Tooru Andoh, Laboratory of Pharmaceutical Technology, Faculty of Pharmaceutical Sciences, Kobe Gakuin University, Kobe 650-8586, Japan.
Shinji Kawabata, Department of Neurosurgery, Osaka Medical and Pharmaceutical University, 2-7 Daigaku-machi, Takatsuki-shi, Osaka 569-8686, Japan.
Naonori Hu, Kansai BNCT Medical Center, Osaka Medical and Pharmaceutical University, 2-7 Daigaku-machi, Takatsuki-shi, Osaka 569-8686, Japan; Institute for Integrated Radiation and Nuclear Science, Kyoto University, 2, Asashiro-Nishi, Kumatori-cho, Sennan-gun 590-0494 Japan.
Hiroyuki Michiue, Neutron Therapy Research Center, Okayama University, 2-5-1 Shikata-cho, Kita-ku, Okayama 700-8558, Japan.
Hiroyuki Nakamura, Laboratory for Chemistry and Life Science, Institute of Innovative Research, Tokyo Institute of Technology, 4259 Nagatsuta-cho, Midori-ku, Yokohama 226-8503, Japan.
Takahiro Nomoto, Department of Life Sciences, Graduate School of Arts and Sciences, The University of Tokyo, 3-8-1 Komaba, Meguro-ku, Tokyo 153-8902, Japan.
Minoru Suzuki, Institute for Integrated Radiation and Nuclear Science, Kyoto University, 2, Asashiro-Nishi, Kumatori-cho, Sennan-gun 590-0494 Japan.
Takushi Takata, Institute for Integrated Radiation and Nuclear Science, Kyoto University, 2, Asashiro-Nishi, Kumatori-cho, Sennan-gun 590-0494 Japan.
Hiroki Tanaka, Institute for Integrated Radiation and Nuclear Science, Kyoto University, 2, Asashiro-Nishi, Kumatori-cho, Sennan-gun 590-0494 Japan.
Tsubasa Watanabe, Institute for Integrated Radiation and Nuclear Science, Kyoto University, 2, Asashiro-Nishi, Kumatori-cho, Sennan-gun 590-0494 Japan.
Koji Ono, Kansai BNCT Medical Center, Osaka Medical and Pharmaceutical University, 2-7 Daigaku-machi, Takatsuki-shi, Osaka 569-8686, Japan.
CONFLICT OF INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- 1. Barth RF. Boron neutron capture therapy at the crossroads: challenges and opportunities. Appl Radiat Isot 2009;67:S3–6. [DOI] [PubMed] [Google Scholar]
- 2. Moss RL. Critical review, with an optimistic outlook, on boron neutron capture therapy (BNCT). Appl Radiat Isot 2014;88:2–11. [DOI] [PubMed] [Google Scholar]
- 3. Dymova MA, Taskaev SY, Richter VA, Kuligina EV. Boron neutron capture therapy: current status and future perspectives. Cancer Commun 2020;40:406–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suzuki M, Tanaka H, Sakurai Y et al. Impact of accelerator-based boron neutron capture therapy (AB-BNCT) on the treatment of multiple liver tumors and malignant pleural mesothelioma. Radiother Oncol 2009;92:89–95. [DOI] [PubMed] [Google Scholar]
- 5. Tanaka H, Sakurai Y, Suzuki M et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl Radiat Isot 2011;69:1642–5. [DOI] [PubMed] [Google Scholar]
- 6. Kanno H, Nagata H, Ishiguro A et al. Designation products: boron neutron capture therapy for head and neck carcinoma. Oncologist 2021;26:e1250–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirose K, Konno A, Hiratsuka J et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan (10B) for recurrent or locally advanced head and neck cancer (JHN002): an open-label phase II trial. Radiother Oncol 2021;155:182–7. [DOI] [PubMed] [Google Scholar]
- 8. Cartelli D, Capoulat ME, Bergueiro TJ et al. Present status of accelerator-based BNCT: focus on developments in Argentina. Appl Radiat Isot 2015;106:18–21. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura S, Imamichi S, Masumoto K et al. Evaluation of radioactivity in the bodies of mice induced by neutron exposure from an epi-thermal neutron source of an accelerator-based boron neutron capture therapy system. Proc Jpn Acad Ser B Phys Biol Sci 2017;93:821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kiyanagi Y, Sakurai Y, Kumada H, et al. Status of accelerator-based BNCT projects worldwide. In: 25th International Conference on the Application of Accelerators in Research and Industry, 2019. Melville, NY, USA: AIP Publishing. [Google Scholar]
- 11. Savolainen S, Kortesniemi M, Timonen M et al. Boron neutron capture therapy (BNCT) in Finland: technological and physical prospects after 20 years of experiences. Phys Med 2013;29:233–48. [DOI] [PubMed] [Google Scholar]
- 12. Aleynik V, Burdakov A, Davydenko V et al. BINP accelerator based epithermal neutron source. Appl Radiat Isot 2011;69: 1635–8. [DOI] [PubMed] [Google Scholar]
- 13. Ichikawa H, Taniguchi E, Fujimoto T et al. Biodistribution of BPA and BSH after single, repeated and simultaneous administrations for neutron-capture therapy of cancer. Appl Radiat Isot 2009;67:S111–S4. [DOI] [PubMed] [Google Scholar]
- 14. Kiger W III, Palmer M, Riley K. A pharmacokinetic model for the concentration of 10B in blood after boronophenylalanine-fructose administration in humans. Radiat Res 2001;155:611–8. [DOI] [PubMed] [Google Scholar]
- 15. Soloway AH, Tjarks W, Barnum BA et al. The chemistry of neutron capture therapy. Chem Rev 1998;98:1515–62. [DOI] [PubMed] [Google Scholar]
- 16. Javid M, Brownell GL, Sweet WH. The possible use of neutron-capturing isotopes such as boron 10 in the treatment of neoplasms. II. Computation of the radiation energies and estimates of effects in normal and neoplastic brain. J Clin Invest 1952;31:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vanderpool RA, Hoff D, Johnson PE. Use of inductively coupled plasma-mass spectrometry in boron-10 stable isotope experiments with plants, rats, and humans. Environ Health Perspect 1994;102:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kobayashi T, Kanda K. Microanalysis system of ppm-order B-10 concentrations in tissue for neutron-capture therapy by prompt gamma-ray spectrometry. Nucl Instrum Methods Phys Res 1983;204:525–31. [Google Scholar]
- 19. Nakase I, Katayama M, Hattori Y et al. Intracellular target delivery of cell-penetrating peptide-conjugated dodecaborate for boron neutron capture therapy (BNCT). Chem Commun 2019;55:13955–8. [DOI] [PubMed] [Google Scholar]
- 20. Hattori Y, Ishimura M, Ohta Y et al. Detection of boronic acid derivatives in cells using a fluorescent sensor. Org Biomol Chem 2015;13:6927–30. [DOI] [PubMed] [Google Scholar]
- 21. Kirihata M, Uehara K, Asano T. Hapten compound and antibody. WO 2007;2007097065:A1. [Google Scholar]
- 22. Kondo N, Aoki E, Takada S et al. A red-emitting fluorescence sensor for detecting boronic acid-containing agents in cells. Sensors 2022;22:7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takada S, Kondo N, Hagimori M et al. Development of a switching-type fluorescence sensor for the detection of boronic acid-containing agents. Anal Sci 2022;38:1289–96. [DOI] [PubMed] [Google Scholar]
- 24. Kondo N, Takada S, Hagimori M et al. Development of a 2-(2-Hydroxyphenyl)-1H-benzimidazole-based fluorescence sensor targeting boronic acids for versatile application in boron neutron capture therapy. Cancers 2023;15:1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martínez-Aguirre MA, Flores-Alamo M, Yatsimirsky AK. Thermodynamic and structural study of complexation of phenylboronic acid with salicylhydroxamic acid and related ligands. Appl Organomet Chem 2018;32:e4405. [Google Scholar]
- 26. Morris G, Coderre J, Hopewell J et al. Response of rat skin to boron neutron-capture therapy with p-boronophenylalanine or borocaptate sodium. Radiother Oncol 1994;32:144–53. [DOI] [PubMed] [Google Scholar]
- 27. Morris G, Coderre J, Hopewell J et al. Response of the central-nervous-system to boron neutron-capture irradiation-evaluation using rat spinal-cord model. Radiother Oncol 1994;32:249–55. [DOI] [PubMed] [Google Scholar]
- 28. Pollmann D, Broekaert J, Leis F et al. Determination of boron in biological tissues by inductively-coupled plasma optical-emission spectrometry (ICP-OES). Fresenius J Anal Chem 1993;346:441–5. [Google Scholar]
- 29. Wittig A, Sauerwein WA, Coderre JA. Mechanisms of transport of p-borono-phenylalanine through the cell membrane in vitro. Radiat Res 2000;153:173–80. [DOI] [PubMed] [Google Scholar]
- 30. Nomoto T, Inoue Y, Yao Y et al. Poly(vinyl alcohol) boosting therapeutic potential of p-boronophenylalanine in neutron capture therapy by modulating metabolism. Sci Adv 2020;6:eaaz1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nomoto T, Yao Y, Inoue Y et al. Fructose-functionalized polymers to enhance therapeutic potential of p-boronophenylalanine for neutron capture therapy. J Control Release 2021;332:184–93. [DOI] [PubMed] [Google Scholar]
- 32. Iguchi Y, Michiue H, Kitamatsu M et al. Tumor-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumor model. Biomaterials 2015;56:10–7. [DOI] [PubMed] [Google Scholar]
- 33. Kabata S, Agüi-Gonzalez P, Saal KA et al. Boron-containing probes for non-optical high-resolution imaging of biological samples. Angew Chem Int Ed 2019;58:3438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Michiue H, Kitamatsu M, Fukunaga A et al. Self-assembling A6K peptide nanotubes as a mercaptoundecahydrododecaborate (BSH) delivery system for boron neutron capture therapy (BNCT). J Control Release 2021;330:788–96. [DOI] [PubMed] [Google Scholar]
- 35. Michiue H, Sakurai Y, Kondo N et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014;35:3396–405. [DOI] [PubMed] [Google Scholar]
- 36. Wongthai P, Hagiwara K, Miyoshi Y et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB(0,+), LAT1 and LAT2. Cancer Sci 2015;106:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gregoire V, Begg AC, Huiskamp R et al. Selectivity of boron carriers for boron neutron capture therapy: pharmacological studies with borocaptate sodium, L-boronophenylalanine and boric acid in murine tumors. Radiother Oncol 1993;27:46–54. [DOI] [PubMed] [Google Scholar]
- 38. Nomoto T, Nishiyama N. Design of drug delivery systems for physical energy-induced chemical surgery. Biomaterials 2018;178:583–96. [DOI] [PubMed] [Google Scholar]
- 39. Geninatti-Crich S, Alberti D, Szabo I et al. MRI-guided neutron capture therapy by use of a dual gadolinium/boron agent targeted at tumour cells through upregulated low-density lipoprotein transporters. Chemistry 2011;17:8479–86. [DOI] [PubMed] [Google Scholar]
- 40. Alberti D, Protti N, Toppino A et al. A theranostic approach based on the use of a dual boron/Gd agent to improve the efficacy of boron neutron capture therapy in the lung cancer treatment. Nanomedicine 2015;11:741–50. [DOI] [PubMed] [Google Scholar]
- 41. Hattori Y, Ishimura M, Ohta Y et al. Dodecaborate conjugates targeting tumor cell overexpressing translocator protein for boron neutron capture therapy. ACS Med Chem Lett 2022;13:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Faubert B, Tasdogan A, Morrison S et al. Stable isotope tracing to assess tumor metabolism in vivo. Nat Protoc 2021;16:5123–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suzuki M, Masunaga S, Kinashi Y et al. The effects of boron neutron capture therapy on liver tumors and normal hepatocytes in mice. Jpn J Cancer Res 2000;91:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kawai K, Nishimura K, Okada S et al. Cyclic RGD-functionalized closo-Dodecaborate albumin conjugates as integrin targeting boron carriers for neutron capture therapy. Mol Pharm 2020;17:3740–7. [DOI] [PubMed] [Google Scholar]
- 45. Isono A, Tsuji M, Sanada Y et al. Design, synthesis, and evaluation of Lipopeptide conjugates of Mercaptoundecahydrododecaborate for boron neutron capture therapy. ChemMedChem 2019;14:823–32. [DOI] [PubMed] [Google Scholar]
- 46. Yokoyama K, Miyatake S, Kajimoto Y et al. Pharmacokinetic study of BSH and BPA in simultaneous use for BNCT. J Neurooncol 2006;78:227–32. [DOI] [PubMed] [Google Scholar]
- 47. Masunaga S-I, Sakurai Y, Tanaka H et al. The dependency of compound biological effectiveness factors on the type and the concentration of administered neutron capture agents in boron neutron capture therapy. Springerplus 2014;3:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wongthai P, Hagiwara K, Miyoshi Y et al. Boronophenylalanine, a boron delivery agent for boron neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2. Cancer Sci 2015;106:279–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kobayashi T, Sakurai Y, Ishikawa M. Real-time absorbed dose estimation system for BNCT by PG-SPECT. In: Hawthorne MF, Shelly K, Wiersema RJ (eds). Frontiers in Neutron Capture Therapy, Vol. 1. Boston, MA: Springer US, 2001, 641–5. [Google Scholar]
- 50. Linko S, Revitzer H, Zilliacus R et al. Boron detection from blood samples by ICP-AES and ICP-MS during boron neutron capture therapy. Scand J Clin Lab Invest 2008;68:696–702. [DOI] [PubMed] [Google Scholar]
- 51. Barth RF, Yang W, Coderre JA. Rat brain tumor models to assess the efficacy of boron neutron capture therapy: a critical evaluation. J Neurooncol 2003;62:61–74. [DOI] [PubMed] [Google Scholar]
- 52. Khawli LA, Prabhu S. Drug delivery across the blood–brain barrier. Mol Pharm 2013;10:1471–2. [DOI] [PubMed] [Google Scholar]
- 53. Watanabe T, Hattori Y, Ohta Y et al. Comparison of the pharmacokinetics between L-BPA and L-FBPA using the same administration dose and protocol: a validation study for the theranostic approach using [18F]-L-FBPA positron emission tomography in boron neutron capture therapy. BMC Cancer 2016;16:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yokoyama K, Miyatake S, Kajimoto Y et al. Analysis of boron distribution in vivo for boron neutron capture therapy using two different boron compounds by secondary ion mass spectrometry. Radiat Res 2007;167:102–9. [DOI] [PubMed] [Google Scholar]
- 55. Tanaka H, Sakurai Y, Suzuki M et al. Development of a simple and rapid method of precisely identifying the position of 10B atoms in tissue: an improvement in standard alpha autoradiography. J Radiat Res 2014;55:373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Futamura G, Kawabata S, Nonoguchi N et al. Evaluation of a novel sodium borocaptate-containing unnatural amino acid as a boron delivery agent for neutron capture therapy of the F98 rat glioma. Radiat Oncol 2017;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sakurai Y, Sasaki A, Kobayashi T. Development of neutron shielding material using metathesis-polymer matrix. Nucl Instrum Meth A 2004;522:455–61. [Google Scholar]
- 58. Ono K, Masunaga S, Suzuki M et al. The combined effect of boronophenylalanine and borocaptate in boron neutron capture therapy for SCCVII tumors in mice. Int J Radiat Oncol Biol Phys 1999;43:431–6. [DOI] [PubMed] [Google Scholar]
- 59. Kueffer PJ, Maitz CA, Khan AA et al. Boron neutron capture therapy demonstrated in mice bearing EMT6 tumors following selective delivery of boron by rationally designed liposomes. Proc Natl Acad Sci U S A 2013;110:6512–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wang Z, Chen Z, Zhang Z et al. Multifunctional high boron content MOFs nano-co-crystals for precise boron neutron capture therapy for brain glioma in situ. Nano Today 2022;45:101558. [Google Scholar]
- 61. Protti N, Geninatti-Crich S, Alberti D et al. Evaluation of the dose enhancement of combined (1)(0)B + (1)(5)(7)Gd neutron capture therapy (NCT). Radiat protection dosimetry 2015;166:369–73. [DOI] [PubMed] [Google Scholar]
- 62. Ono K, Kinashi Y, Masunaga S et al. Effect of electroporation on cell killing by boron neutron capture therapy using borocaptate sodium (10B-BSH). Jpn J Cancer Res 1998;89:1352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hu N, Tanaka H, Takata T et al. Evaluation of PHITS for microdosimetry in BNCT to support radiobiological research. Appl Radiat Isot 2020;161:109148. [DOI] [PubMed] [Google Scholar]
- 64. Yamamoto T, Matsumura A, Yamamoto K et al. Characterization of neutron beams for boron neutron capture therapy: in-air radiobiological dosimetry. Radiat Res 2003;160:70–6. [DOI] [PubMed] [Google Scholar]
- 65. Hattori Y, Kusaka S, Mukumoto M et al. Synthesis and in vitro evaluation of thiododecaborated α, α-cycloalkylamino acids for the treatment of malignant brain tumors by boron neutron capture therapy. Amino Acids 2014;46:2715–20. [DOI] [PubMed] [Google Scholar]
- 66. Sakurai Y, Kobayashi T. Characteristics of the KUR heavy water neutron irradiation facility as a neutron irradiation field with variable energy spectra. Nucl Instrum Meth A 2000;453:569–96. [Google Scholar]
- 67. Sakurai Y, Kobayashi T. Spectrum evaluation at the filter-modified neutron irradiation field for neutron capture therapy in Kyoto University research reactor. Nucl Instrum Meth A 2004;531:585–95. [Google Scholar]
- 68. Brown DA, Chadwick MB, Capote R et al. ENDF/B-VIII.0: the 8th major release of the nuclear reaction data library with CIELO-project cross sections, new standards and thermal scattering data. Nuclear Data Sheets 2018;148:1–142. [Google Scholar]
- 69. Yamamoto K, Yamamoto T, Kumada H et al. Evaluation of JRR-4 neutron beam using tumor cells. JAERI-Tech 2001-017 2001;1–38. [Google Scholar]
- 70. International Commission on Radiation Units and Measurements . Nuclear Data for Neutron and Proton Radiotherapy and for Radiation Protection: ICRU Report 63, International Commission on Radiation Units & Measurements, 2000. [Google Scholar]
- 71. Coderre J, Morris G. The radiation biology of boron neutron capture therapy. Radiat Res 1999;151:1–18. [PubMed] [Google Scholar]
- 72. Ono K. An analysis of the structure of the compound biological effectiveness factor. J Radiat Res 2016;57:i83–i9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Tanaka H, Takata T, Watanabe T et al. Characteristic evaluation of the thermal neutron irradiation field using a 30 MeV cyclotron accelerator for basic research on neutron capture therapy. Nucl Instrum Meth A 2020;983:164533. [Google Scholar]
- 74. Liu YH, Lee PY, Lin YC et al. Dose estimation of animal experiments at the THOR BNCT beam by NCTPlan and Xplan. Appl Radiat Isot 2014;88:125–8. [DOI] [PubMed] [Google Scholar]
- 75. Kobayashi T, Kanda K. Analytical calculation of boron-10 dosage in cell nucleus for neutron capture therapy. Radiat Res 1982;91:77–94. [PubMed] [Google Scholar]
- 76. Ono K, Tanaka H, Tamari Y et al. Proposal for determining absolute biological effectiveness of boron neutron capture therapy—the effect of 10B(n,α) 7 li dose can be predicted from the nucleocytoplasmic ratio or the cell size. J Radiat Res 2019;60:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


