Abstract
Recently, the asymmetric bifunctionalization of alkenes has received much attention. However, the development of enantioselective alkoxyalkenylation has posed a considerable challenge and has lagged largely behind. Herein, we report a new palladium-catalyzed enantioselective alkoxyalkenylation reaction, using a range of primary, secondary, and tertiary γ-hydroxy-alkenes with alkenyl halides. By employing newly identified Xu-Phos (Xu8 and Xu9) with a suitable side-arm adjacent to the PCy2 motif, a series of allyl-substituted tetrahydrofurans were obtained in good yields with up to 95% ee. Besides (E)-alkenyl halides, (Z)-alkenyl halide was also examined and provided the corresponding (Z)-product as a single diastereomer, supporting a stereospecific oxidative addition and reductive elimination step. Moreover, deuterium labeling and VCD experiments were employed to determine a cis-oxypalladation mechanism. DFT calculations helped us gain deeper insight into the side-arm effect on the chiral ligand. Finally, the practicability of this method is further demonstrated through a gram-scale synthesis and versatile transformations of the products.
Subject terms: Synthetic chemistry methodology, Reaction mechanisms
Recently, the asymmetric bifunctionalization of alkenes has received much attention but the development of enantioselective alkoxyalkenylation has posed a considerable challenge and has lagged largely behind. Here, the authors report a palladium-catalyzed enantioselective alkoxyalkenylation reaction, using a range of primary, secondary, and tertiary γ-hydroxyalkenes with alkenyl halides.
Introduction
Tetrahydrofuran derivatives bearing substituents at the C2 position are important subunits in pharmaceuticals, materials, and natural products (Fig. 1)1–8. Thus, many methods for stereoselective synthesis of multisubstituted tetrahydrofurans have been developed9–24, among which metal-catalyzed cyclizations of readily available unsaturated alcohols have shown good performance in the synthesis of diverse tetrahydrofuran products with predictable regio- and diastereo-selectivity25–39. The groups of Zurek, Chemler40,41, and Liu42 independently developed copper-catalyzed asymmetric radical alkene carboalkoxylation of γ-hydroxyalkenes, which provide facile access to chiral tetrahydrofurans with high enantioselectivities. Sigman and co-workers reported an efficient Pd/Cu co-catalyzed enantioselective alkene bifunctionalization reaction of γ-hydroxyalkenes via intramolecular oxypalladation and subsequent addition43,44. Using chiral salen-Co complexes, Kang achieved an elegant instance of enantioselective intramolecular iodoetherification to furnish such chiral five-membered oxygen heterocycles45. Regarding the significance of chiral tetrahydrofuran, the development of highly enantioselective methods for their synthesis from readily available starting materials, especially under mild conditions is still highly desirable.
Fig. 1. Biologically active compounds containing 2-substituted tetrahydrofuran skeletons.
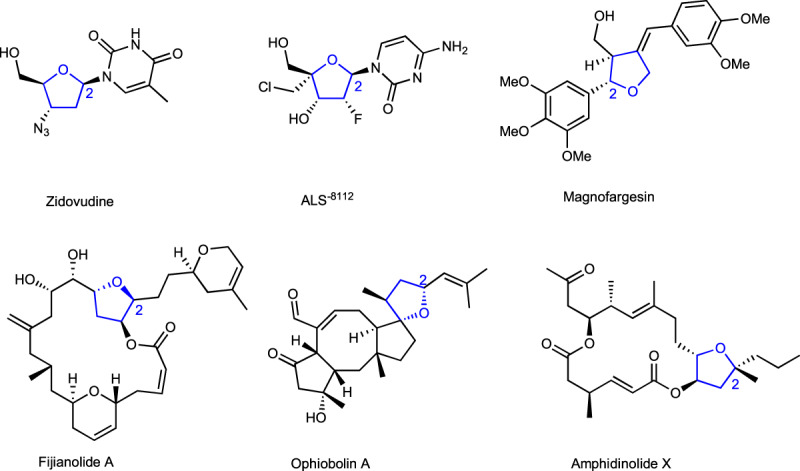
Substructures colored in blue highlight the core 2-substituted tetrahydrofuran skeleton with stereocenter. Zidovudine, an antimetabolite and a HIV-1 reverse transcriptase inhibitor. ALS−8112, respiratory syncytial virus (RSV) polymerase inhibitor. Magnofargesin, a natural product found in Magnolia biondii. Fijianolide A, a natural compound isolated from Spongia mycofijiensis. Ophiobolin A, a fungal secondary metabolite has activity against glioblastoma. Amphidinolide X, a natural product isolated from Amphidinium sp.
Because alkenyl group can be converted into various functional groups, which is particularly important for increasing the diversity of compounds46. Several progresses have been achieved in alkenylation reactions, which provided efficient access to compounds with alkenyl framework. For instance, Sigman realized the aryl-alkenylation of unactivated alkenes with vinyl triflates and boronic acids furnishing skipped dienes, however, the substrates were limited to aryl 1,3-dienes47. Recently, Shu reported a cross-electrophile reaction of unactivated alkenes with vinyl triflates, which led to highly enantioselective aryl-alkenylation products including dihydrobenzofurans, indolines, and indanes in good yields with up to >99% ee48. Unfortunately, the related synthesis of enantiopure tetrahydrofurans with 2-allylic substituent from unactivated alkenols is rarely explored.
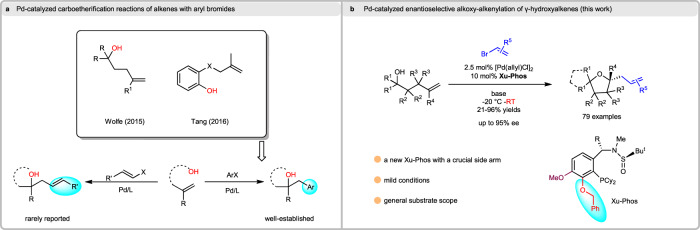
Wolfe and Tang’s works have constructed a series of asymmetric oxygen-containing heterocyclic by Pd-catalyzed carboetherification reactions of alkenes with aryl bromides (Fig. 2a). However, the involvement of alkenyl bromides in the reaction has not been reported successfully, although Wolfe and Tang have made related attempts. The result indicates that the reactivity and enantioselectivity control of alkenyl bromine and aryl bromine in the reaction are very different, even though they are both C(sp2)-Br in form. Inspired by the good performance of sulfinamide-phosphine (Sadphos) in asymmetric catalysis49–58 and the unsuccess of the alkoxyalkenylation in Wolfe and Tang’s previous work59–61, we became very interested in the Pd-catalyzed asymmetric alkoxyalkenylation reaction of γ-hydroxy alkenes with alkenyl halides, which remains as an unsolved problem and pose considerable challenges: 1) the catalyst inhibition caused by competitive side reactions, including Heck reaction, β-hydride elimination and double bond migration of the γ-hydroxyalkene;31,59–61 2) whether metal-catalyzed terminal olefin nucleopalladation is cis or trans-attack has always been an open question49,50; 3) the asymmetric synthesis of tetrahydrofurans with a tertiary carbon center in one-step, especially under mild conditions41,42; 4) primary, secondary and tertiary γ-hydroxyalkenes provide products in good yield and ee59–61.
Fig. 2. Background and reaction design.
a Pd-catalyzed carboetherification reactions of alkenes with aryl bromides. b Pd-catalyzed enantioselective alkoxy-alkenylation of γ-hydroxyalkenes.
Herein, we report new sulfinamide phosphine ligands (Xu8 and Xu9) with a side-arm adjacent to the PCy2 motif, which are crucial to address the challenging enantioselective alkoxyalkenylation reaction of γ-hydroxy-alkenes with alkenyl halides under mild conditions (Fig. 2b). Moreover, VCD method and DFT calculation are utilized to verify the cis-oxypalladation mechanism, which is usually ambiguous in similar reactions. Transition states for the cis-oxypalladation step are located and analyzed, revealing the origin of the side-arm effect in our ligands.
Results
Reaction Development
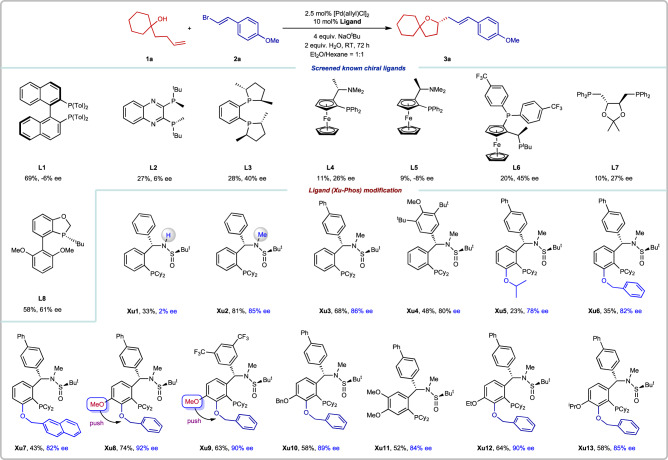
In our initial study, tertiary γ-hydroxyalkene 1a and alkenyl bromide 2a were selected as the model substrates. A series of privileged chiral ligands L1-L8 were investigated, such as (R)-Tol-BINAP (L1), (R)-QuinoxP (L2), (S,S)-Me-duphos (L3), ferrocene ligands (L4-L6), (S,S)-DIOP (L7), (R)-BIDIME (L8), in which the (R)-BIDIME L8 showed the promising result to deliver 3a in 58% yield with 61% ee (Fig. 3). Next, we turned our attention to our developed sulfinamide-phosphine (Sadphos) ligand such as Xu-Phos (Xu1) with free N-H bond, unfortunately, it gives 33% yield and 2% ee (Fig. 3). To our delight, the N-Me-Xu-Phos (Xu2) could deliver the desired product in 81% yield with 85% ee. Then, we focused on adjusting the R group in the Xu-Phos (Xu3, Xu4), but no better results were obtained.
Fig. 3. Screened chiral ligands.
Reaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), NaOtBu (4 equiv.), H2O (2 equiv.), [Pd(allyl)Cl]2 (2.5 mol%), Xu8 (10 mol%) in 1 mL Et2O/Hexane under N2 at RT for 72 h. Isolated yields. ee was determined by chiral HPLC analysis.
Inspired by Tang’s findings that introducing a side-arm in the ligand is an efficient strategy to improve enantioselectivity by adjusting the electronic properties and space environment of the catalyst, like SaBOX ligands62,63, we next designed and prepared new ligands Xu5-Xu8. These ligands bear a sterically congested substituent at the ortho position of the phosphine group (Fig. 3). The absolute configuration of Xu6 was well established by X-ray crystallography analysis. Gratifyingly, ligand Xu8, easily prepared from 2-bromo-3-hydroxy-4-methoxybenz-aldehyde, delivers much better ee (92% ee) compared to Xu6 (82% ee) under the conditions with the 2.5 mol% of [Pd(allyl)Cl]2 and 10 mol% ligand, NaOtBu as the base, H2O as the additive in Et2O/hexane at RT. The introduction of a methoxy group into the meta-position of the phosphine group in ligand Xu8 was critical to the stereoselectivity and reactivity. By changing p-Ph-C6H4 to 3,5-CF3-C6H3, Xu9 afforded the product in 63% yield with 90% ee. While changing OMe to OBn or OEt, Xu10, and Xu12 gave comparative ee (89% ee, 90% ee) compared to Xu8. Furthermore, changing OMe to OiPr (Xu13), led to a lower ee (85% ee) compared to Xu8. Compared to Xu3, Xu11, which contained a methoxy group in the para- and meta-position of the ligand Xu3, yielded slightly lower results (52% yield, 84% ee). We proposed that the methoxy group substituent pushes the side-arm OBn much closer to the catalytic center, and steric effects between methoxy or other alkyloxy (OR) and OBn groups may be crucial for the high stereoselectivity of Xu-Phos.
To obtain better enantioselectivity (vide infra), further screening of the reaction conditions was examined. As shown in Table 1, the absence of H2O resulted in 3a with slightly inferior enantioselectivity (Table 1, entries 2–6). Investigation of palladium catalysts revealed that [Pd(allyl)Cl]2 was most effective in this transformation (Table 1, entries 7–9). Changes of bases to KOtBu, LiOtBu, and Cs2CO3 led to a significant decrease in enantioselectivity (Table 1, entries 10–13). Using the NaOH instead of NaOtBu, can improve the enantioselectivity to 97% ee, but decrease the yield to 29% (Table 1, entry 13). And further solvent screening failed to improve the enantioselectivity (Table 1, entries 14–17).
Table 1.
Optimization of the reaction conditionsa
| Entry | Additive | [Pd] | Base | Solvent | Yield% | Ee% |
|---|---|---|---|---|---|---|
| 1 | H2O | [Pd(allyl)Cl]2 | NaOtBu | Et2O/Hexane = 1:1 | 74 | 92 |
| 2 | -- | [Pd(allyl)Cl]2 | NaOtBu | Et2O/Hexane = 1:1 | 84 | 84 |
| 3 | 4 Å MS | [Pd(allyl)Cl]2 | NaOtBu | Et2O/Hexane = 1:1 | 79 | 91 |
| 4 | MeOH | [Pd(allyl)Cl]2 | NaOtBu | Et2O/Hexane = 1:1 | 75 | 79 |
| 5 | EtOH | [Pd(allyl)Cl]2 | NaOtBu | Et2O/Hexane = 1:1 | 54 | 70 |
| 6 | iPrOH | [Pd(allyl)Cl]2 | NaOtBu | Et2O/Hexane = 1:1 | 52 | 60 |
| 7 | H2O | Pd(dba)2 | NaOtBu | Et2O/Hexane = 1:1 | 43 | 93 |
| 8 | H2O | Pd(OAc)2 | NaOtBu | Et2O/Hexane = 1:1 | 72 | 92 |
| 9 | H2O | PdCl2 | NaOtBu | Et2O/Hexane = 1:1 | 12 | 78 |
| 10 | H2O | [Pd(allyl)Cl]2 | KOtBu | Et2O/Hexane = 1:1 | – | – |
| 11 | H2O | [Pd(allyl)Cl]2 | LiOtBu | Et2O/Hexane = 1:1 | 41 | 7 |
| 12 | H2O | [Pd(allyl)Cl]2 | Cs2CO3 | Et2O/Hexane = 1:1 | 15 | 85 |
| 13 | H2O | [Pd(allyl)Cl]2 | NaOH | Et2O/Hexane = 1:1 | 29 | 97 |
| 14 | H2O | [Pd(allyl)Cl]2 | NaOtBu | Toluene | 90 | 91 |
| 15 | H2O | [Pd(allyl)Cl]2 | NaOtBu | THF | 51 | 90 |
| 16 | H2O | [Pd(allyl)Cl]2 | NaOtBu | Et2O | 36 | 94 |
| 17 | H2O | [Pd(allyl)Cl]2 | NaOtBu | Hexane | 55 | 88 |
aReaction conditions: 1a (0.1 mmol), 2a (0.2 mmol), NaOtBu (4 equiv.), H2O (2 equiv.), [Pd(allyl)Cl]2 (2.5 mol%), Xu8 (10 mol%) in 1 mL Et2O/Hexane under N2 at RT for 72 h. Isolated yields. ee was determined by chiral HPLC analysis.
Substrate scope of the reaction
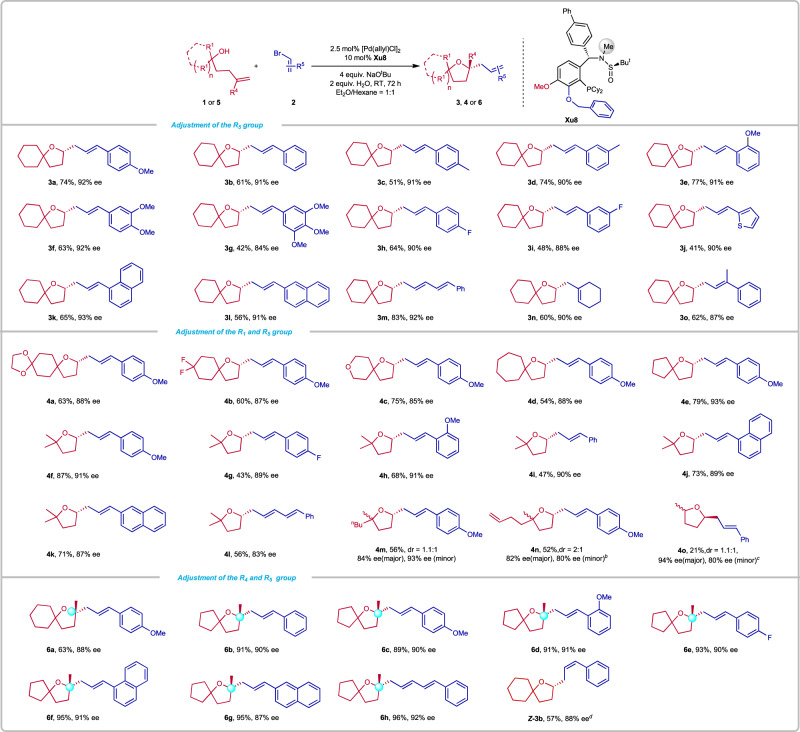
With the optimal reaction conditions in hand (Table 1, entry 1), we evaluated the scope of alkenyl bromides (Fig. 4). The (E)-styrenyl bromide and phenyl substituents with electron-donating groups (Me, MeO) at ortho-, meta- and para-position worked well to form the corresponding compounds 3a-3e in 51–74% yields with 90–92% ees. Disubstituted and trisubstituted phenyl rings with OMe at different positions were also compatible, delivering the corresponding products 3f-3g in 42–63% yields with 84–92% ees. On the other hand, substrates with electron-withdrawing groups at meta- and para-position also proceeded well and generated 3h-3i in 48–64% yields with 88–90% ees. The phenyl group could be replaced by heteroaryl or other aryl groups, such as 2-thienyl, 2-naphthyl, and 1-naphthyl, delivering the desired 3j-3l in 41–65% yields with 90–93% ees. Furthermore, chain alkenyl bromide, cycloalkenyl bromide, and multi-substituted bromide were investigated, furnishing the relevant products 3 m, 3n, and 3o in 60–83% yields with 87–92% ees. Next, we investigated different types of tertiary γ-hydroxyalkenes. We found that functionalized tertiary γ-hydroxyalkenes bearing the electron-donating (MeO) and electron-withdrawing (F) groups on the six-membered rings were well tolerated to afford the corresponding products 4a and 4b in 60–63% yields with 87–88% ees. The pyran-derived tertiary γ-hydroxyalkene was also suitable for this transformation, delivering 4c in 75% yield with 85% ee. Other cyclic γ-hydroxyalkenes containing seven- or five-membered rings were well compatible to furnish the corresponding products 4d and 4e in 54–79% yields with 88–93% ees. The acyclic γ-hydroxyalkene has a good performance in this reaction, offering the alkoxyalkenylation products 4f-4l in 43–87% yields with 83–91% ees.
Fig. 4. Synthesis of 3, 4, or 6.
Reaction conditions: 1 or 5 (0.2 mmol), 2 (0.4 mmol), NaOtBu (4 equiv.), H2O (2 equiv.), [Pd(allyl)Cl]2 (2.5 mol%), Xu8 (10 mol%) in 2 mL Et2O/Hexane under N2 at RT for 72 h. Isolated yields. ee was determined by chiral HPLC analysis. b with Xu6 (10 mol%) in 2 mL toluene under N2 at RT for 72 h. c 1 (0.5 mmol), 2 (1 mmol), EtONa (4 equiv.), [Pd(allyl)Cl]2 (2.5 mol%), (R, RS)-Xu9 (10 mol%) in toluene under N2 at −20 °C for 72 h. d 5 mol% [Pd(allyl)Cl]2, 20 mol% Xu2.
The cyclization of racemic γ-hydroxyalkene as the substrate furnished a 1.1:1 diastereomeric mixture of the product 4 m in 56% yield with 84% and 93% ee. The desymmetrization of mesomeric γ-hydroxyalkene was realized by using Xu6, delivering 4n in 52% yield as a 2:1 mixture of diastereomer with considerable ee. The secondary γ-hydroxyalkene also produced a mixture of diastereomers of 4o in 21% yield with 94% ee and 80% ee. The relatively lower yield may be attributed to the competitive side oxidation reaction of the alcohol59.
Further studies showed that a series of products bearing a tertiary stereocenter could be furnished in moderate to good yields with high enantioselectivities from the corresponding methyl-substituted tertiary γ-hydroxyalkenes (Fig. 4). Alkenes with a five- or six-membered ring were well tolerated to give the desired products 6a-6b with 63–91% yields and 88–90% ees. The reactions with a range of alkenyl bromides bearing a monosubstituted phenyl ring with an electron-donating group (OMe) or an electron-withdrawing group (F) at the ortho- or para-position furnished the desired products 6c-6e in 89–93% yields with 90–91% ees. The use of 1-naphthyl alkenyl bromides and 2-naphthyl alkenyl bromides delivered the enantiopure products 6f-6g bearing a quaternary stereocenter in 95% yields with 87–91% ees. In addition, the 1,3-dienyl bromide was well compatible under the reaction condition, furnishing 6 h in 96% yield with 92% ee.
It is well known that Z-olefins are the basic structural unit of organic molecules and thermodynamic unstable, rendering a jumbo challenge for the synthesis of functionalized Z-olefins with highly selectivity64. When the Z-alkenyl bromide 2b was utilized in the reaction, the target product Z-3b could be obtained in 57% yield with 88% ee by using Xu2 as the ligand (Fig. 4). This result indicated the alkene configuration was retained in this process.
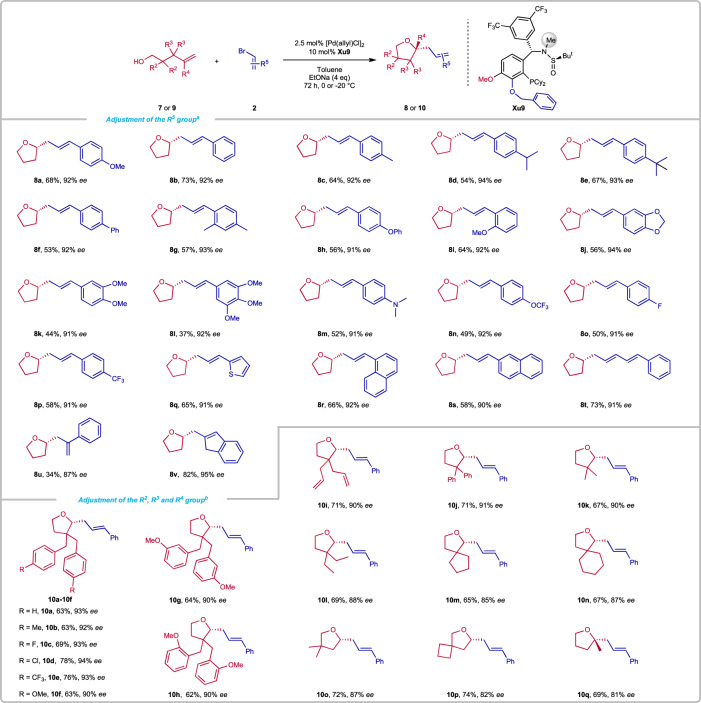
After finished enantioselective alkoxyalkenylation reaction of tertiary γ-hydroxyalkenes, we turned to investigate the more challenging primary γ-hydroxy alkenes due to the competitive side reaction, i.e. the oxidation59 (Fig. 5). Moreover, the enantiocontrol was believed to be more difficult than tertiary alcohols. Indeed, we didn’t get good result (63% yield, 67% ee) at the beginning under standard conditions. Fortunately, another ligand Xu9 was identified as a good ligand, delivering a very exciting result (68% yield, 92% ee) under the conditions with the 2.5 mol% of [Pd(allyl)Cl]2 and 10 mol% ligand (Xu9), NaOEt as the base, in toluene at 0 oC. The scope of alkenyl bromides with primary γ-hydroxyalkenes was then investigated. A range of alkenyl bromides were compatible in the reaction. The alkenyl bromides bearing electron-donating groups on the R2, such as alkyl group (8c, 8d, 8e, 8 g), alkyloxy (8a, 8h-8k), phenyl (8 f), and amino (8 m) coupled with 7 to afford products 44%-73% yields with high enantioselectivities (91–94% ee). However, the R2 changing to 3,4,5-OMe-Ph (8 l), delivered a relatively lower yield, which might be due to the slow oxidation, but the enantioselectivity remains high (94% ee). Electron-withdrawing groups (8n-8p), thiophenyl (8q), and naphthyl (8r, 8 s) on the R2 also could give satisfactory aim products. In these transformations, α-bromostyrene(8 u) and multi-substituted vinyl bromides(8 v) could also give good results.
Fig. 5. Synthesis of 8 or 10.
Reaction conditions: 7 or 9 (0.5 mmol), 2 (1 mmol), EtONa (4 equiv.), [Pd(allyl)Cl]2 (2.5 mol%), Xu9 (10 mol%) in 3.5 mL toluene under N2 for 72 h. Isolated yields. ee was determined by chiral HPLC analysis. aat 0 °C. bat −20 °C.
Then, we turned our attention to investigating other primary γ-hydroxyalkenes bearing various substituents at different positions. The reaction with primary γ-hydroxyalkenes with substituted Bn groups at α-position proceeded smoothly to afford products 10a-10h in moderate to good yields and high ees. Besides Bn group, phenyl (10j), allyl (10i), and alkyl group (10k-10n) were also well tolerated, furnishing the products in 62–78% yields with 87–94% ees. Next, we turned to examine the primary γ-hydroxyalkenes with two substituents at the β-position. The reaction temperature needed to be increased to 0 oC and the desired products 10o and 10p were obtained in 72% and 74% yield with 87% and 82% ee, respectively. Compound 10q containing a tertiary stereocenter could also be obtained in 69% yield with 81% ee from the corresponding primary γ-hydroxyalkene with R4 (methyl) group.
Mechanistic Investigation
To gain deep insight into the reaction mechanism and the origin of the stereoselectivity, further experimental and computational investigations were carried out. The cis- or trans-nucleopalladation attack of terminal alkenes has always been ambiguous since both patterns provide the same products. To determine the product’s relative stereochemistry, we used the reaction of compound 7 labeled with deuterium (Z-7-D) and 2a with Xu9 as the ligand (Fig. 6a). The reaction achieved a high d.r. value (>20:1), indicating a highly specific mechanism of the oxypalladation step. Since the absolute configuration of the stereocenter in the THF ring was confirmed as S by XRD result (vide infra). We narrowed the possible stereochemistry of product 8 down to SS or SR. By comparing the experimental and computational (details provided in SI) VCD spectra (Fig. 6b), we found that 8-D takes an SR configuration, which confirms a cis-oxypalladation operative mechanism. To the best of our knowledge, this is the first VCD application in the mechanism determination of organometallic reactions.
Fig. 6. Investigation of the reaction mechanism.
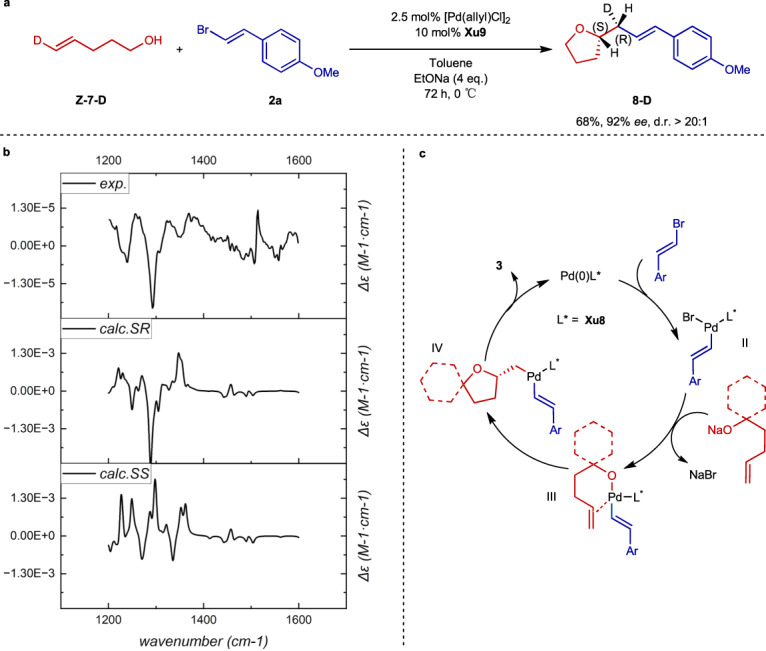
a Deuterium labeling experiment. b Comparison of experimental and computational VCD spectra. c Proposed catalytic cycle.
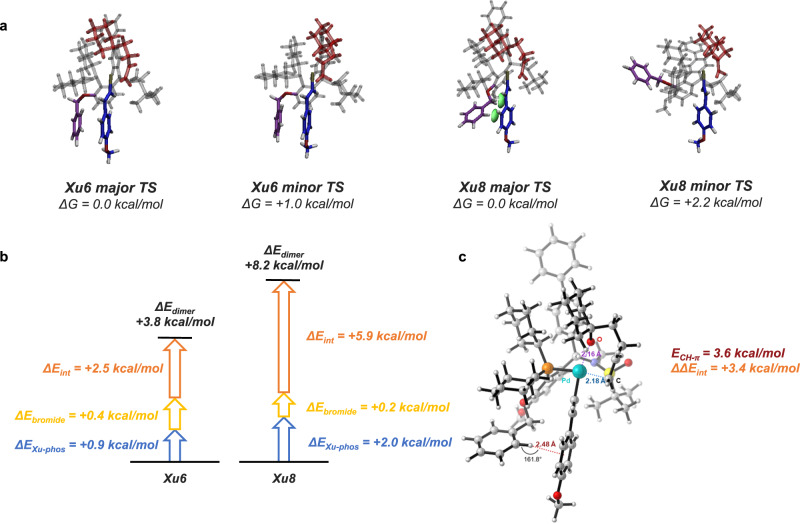
Based on the verified cis-oxypalladation mechanism, the proposed catalytic cycle is shown in Fig. 6c. First, the Pd0 species is transformed into the PdII complex by the oxidative addition of alkenyl bromide (2). In the presence of NaOtBu and alcohol (1), the Pd–O bond is formed to afford complex III via ligand exchange. Then III proceeds a migratory insertion, delivering the Pd complex IV. This is the stereoselectivity-determining step. Through reductive elimination of complex IV, 3 is obtained, and the catalyst can be regenerated. To further interrogate how our ligands may impart stereoselectivity, DFT calculations were performed. Xu1, Xu2, Xu3, Xu6, Xu8, Xu9, and corresponding substrates were taken as subjects. After thorough conformation searchs of the migratory insertion TSs (transition states), the calculated ee values match well with the experimental results (Supplementary Table 2). The TSs structures give us a way to inspect not only the origin of the high stereoselectivity, but also the unique role of the side-arm in enhancing the stereoselectivity.
Taking Xu8 for example, three main factors may benefit the stereoselectivity. First, the conformation of the carbon chain of 1a differs obviously between major and minor TSs. Intuitively, in the minor TS, 1a interacts with the biphenyl substitution group of Xu8 more intensively, causing the scaffold of the ligand to move correspondingly, resulting in some instability. Second, the conformational changes further affect the side-arm (OBn), and a significant conformational change of the side-arm is witnessed (Fig. 7a). In the major TS, a CH-π interaction is formed between the side-arm benzyloxy group and the vinyl substrate (shown as a green isosurface in Fig. 7a), which is absent in the minor TS. This stabilizes the major TS and benefits stereoselectivity (92% ee). Similar CH-π interaction and difference between major and minor TSs can be found in Xu9 as well.
Fig. 7. Visualization study and energy decomposition of the TSs.
a Optimized TS structures for Pd/Xu8 and Pd/Xu6 catalyzed reactions of 1a and 2a. CH-π interaction between side-arms and substrates in Xu8 major is shown as green isosurfaces. b Energy difference decomposition result of Xu-Phos-vinyl substrate dimers. ΔE is calculated as minor structure energy subtracted major structure energy. EXu-Phos represents the energy of Xu-Phos, Evinyl represents the energy of the vinyl substrate, and Eint represents the interaction energy between Xu-Phos and the vinyl substrate. c The core structure and CH- interaction of Xu8 major TS. CH- interaction is denoted with red dashed lines. The value of approximate intensity of the CH- interaction and the difference of interaction energy between dimers in Xu6 and Xu8 are also shown.
To disentangle possible controlling effects and provide quantitative proof for the analysis above, the energy difference ΔΔE between the major and minor TSs of Xu6 and Xu8 was decomposed. We compared the results of Xu6 and Xu8 and found that the energy difference between TSs mainly came from the dimer of Xu-Phos and vinyl substrate (Supplementary Table 4). Further decomposition of the energy indicated that the interaction between Xu-Phos and vinyl substrate is the main contributor (Fig. 7b). We investigated the strength of CH-π interactions between the side-arm benzyloxy group and vinyl substrate. Our computational analysis revealed an interaction energy of 3.6 kcal/mol (Fig. 7c)65, which closely aligns with the Xu-Phos-vinyl substrate interaction energy difference between Xu6 and Xu8 (3.4 kcal/mol).
Furthermore, we observed that the substituent group ortho to the side-arm plays a crucial role in the side-arm effect. Specifically, in Xu6, the OBn group remained in the same position (resting position) in both TSs, and consequently had no impact on stereoselectivity (82% ee). However, when OMe was introduced in Xu8, the restricted space forced OBn to turn away from the resting position, leading to distinct conformations and, as a result, a more significant energy difference between the major and minor TSs. We conducted further theoretical and experimental analysis by substituting OMe with OEt, OiPr, and OtBu groups (Supplementary Table 6), which revealed that bulky groups (i.e. OiPr and OtBu) will interact with vinyl substrate instead of side-arm in minor TSs and reduced the energy difference between major and minor TSs. Consequently, it becomes evident that an appropriate group situated adjacent to the side-arm must serve a dual purpose. Not only should it impart steric hindrance, but it should also strike a balance by avoiding excessive bulkiness that might trigger undesired interactions with the vinyl substrate.
To showcase the synthetic application of this methodology, a gram-scale reaction and several transformations of 6c were carried out (Fig. 8). Under the catalysis of 1.5 mol% [Pd(allyl)Cl]2 at RT, 1.61 g of 6c was obtained in 94% yield with 90% ee. A hydrogenation reaction of 6c delivered the corresponding product 11. Treatment of 6c with 1H-pyrazole under the oxidation of DDQ led to compound 12 with 1.2:1 dr. The enantiopure 6c could undergo a demethylation reaction to produce compound 13, whoseDiscussion.
Fig. 8. Gram-scale synthesis and transformations of products.
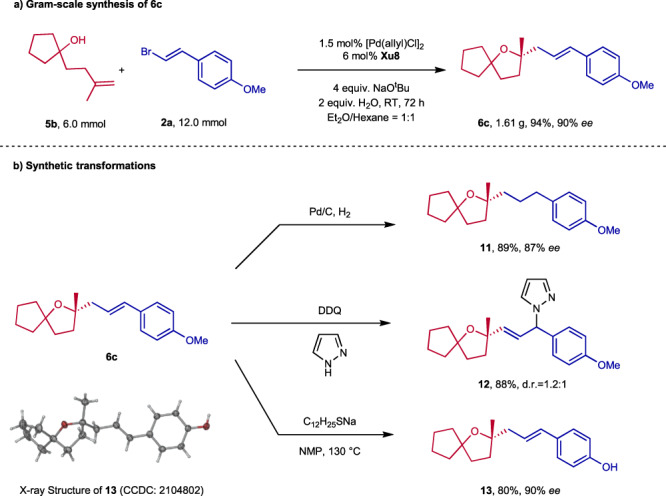
a Gram-scale synthesis of 6c. b Synthetic transformations of 6c.
In summary, we have developed an efficient palladium-catalyzed enantioselective alkoxyalkenylation of a range of primary, secondary, and tertiary γ-hydroxyalkenes with alkenyl halides. This reaction provides facile access to a series of enantiopure tetrahydrofurans containing an olefin framework with a tertiary or quaternary stereocenter. Notably, this process features a broad substrate scope, good functional group tolerance, and mild reaction conditions. Through a combination of experimental and DFT calculation investigation, we verified the cis-oxypalladium mechanism and located the stereo-determining TSs. Additionally, the introduction of a side-arm to the chiral ligand is a crucial strategy for achieving high efficiency and enantioselectivity. These findings offer exciting opportunities in asymmetric reactions, and the investigation of the side-arm effect may help in the rational design of chiral ligands.
Methods
General procedure for synthesis of 3, 4, or 6
To a sealed tube was added [Pd(allyl)Cl]2 (2.5 mol%), Xu8 (10 mol%). The flask was evacuated and refilled with argon. Then tertiary γ-hydroxyalkenes (0.2 mmol), alkenyl halides (0.4 mmol), NaOtBu (4.0 equiv.), H2O (2.0 equiv.) and a mixed solution of Et2O/Hexane (1: 1, 2 mL) was added to the tube and stirred at room temperature for 72 h. After the reaction was complete (monitored by TLC), the solvent was removed under reduced pressure. The crude product was then purified by flash column chromatography on silica gel to afford the desired product 3, 4, or 6.
General procedure for synthesis of 8 or 10
To a sealed tube was added [Pd(allyl)Cl]2 (2.5 mol%), Xu9 (10 mol%). The flask was evacuated and refilled with argon. Then γ-hydroxyalkenes (0.5 mmol), alkenyl halides (1 mmol), EtONa (4.0 equiv), and solution of toluene (3.5 mL) was added to the tube and stirred at 0 °C or -20 °C for 72 h. After the reaction was complete (monitored by TLC), the solvent was removed under reduced pressure. The crude product was then purified by flash column chromatography on silica gel to afford the desired product 8 or 10.
Supplementary information
Acknowledgements
We gratefully acknowledge the funding support of NSFC (22031004 and 21921003 for J.Z., and 22071060 for W.L.), National Key R&D Program of China (2021YFF0701600 for J.Z.) Shanghai Municipal Education Commission (20212308 for J.Z.), and Shanghai Science and Technology Commission (23ZR1404800 for Z.L.).
Author contributions
J.Z. conceived the project. J.Z., Z.L., S.Z., M.C., Z.Y. and W.L. wrote the paper. S.Z. and M.C. performed the experiments and analyzed the data. Z.Y. and Z.L. conducted the computational studies. L.W., Z.W., K.Z., and H.D. analyzed the data. All authors discussed the results and commented on the paper.
Peer review
Peer review information
Nature Communications thanks Paul Nicu, and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The data that support the findings of this study are available within the paper and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1977366 (Xu6-borane complex) and 2104802 (13). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The coordinates of DFT calculated structures are provided in Supplementary Information file. All data are also available from authors upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuai Zhu, Zihao Ye, Ming-Jie Chen.
Contributor Information
Zhiming Li, Email: zmli@fudan.edu.cn.
Junliang Zhang, Email: junliangzhang@fudan.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-023-43202-5.
References
- 1.Faul MM, Huff BE. Strategy and Methodology Development for the Total Synthesis of Polyether Ionophore Antibiotics. Chem. Rev. 2000;100:2407–2474. doi: 10.1021/cr940210s. [DOI] [PubMed] [Google Scholar]
- 2.Huo J, Yang S, Ding J, Yue J. Cytotoxic Sesquiterpene Lactones from Eupatorium lindleyanum. J. Nat. Prod. 2004;67:1470–1475. doi: 10.1021/np040023h. [DOI] [PubMed] [Google Scholar]
- 3.Kang EJ, Lee E. Total Synthesis of Oxacyclic Macrodiolide Natural Products. Chem. Rev. 2005;105:4348–4378. doi: 10.1021/cr040629a. [DOI] [PubMed] [Google Scholar]
- 4.Miyazawa M, Kasahara H, Kameoka H. (-)-Magnofargesin and (+)-magnoliadiol, two lignans from Magnolia fargesii. Phytochemistry. 1996;42:531–533. doi: 10.1016/0031-9422(95)00879-9. [DOI] [Google Scholar]
- 5.Noguchi N, Nakada M. Synthetic Studies on (+)-Ophiobolin A: Asymmetric Synthesis of the Spirocyclic CD-Ring Moiety. Org. Lett. 2006;8:2039–2042. doi: 10.1021/ol060437x. [DOI] [PubMed] [Google Scholar]
- 6.Lorente A, Lamariano-Merketegi J, Albericio F, Alvarez M. Tetrahydrofuran-Containing Macrolides: A Fascinating Gift from the Deep Sea. Chem. Rev. 2013;113:4567–4610. doi: 10.1021/cr3004778. [DOI] [PubMed] [Google Scholar]
- 7.Good JD, Mathews N, Cockerill GS. State of the Art in Respiratory Syncytial Virus Drug Discovery and Development. J. Med. Chem. 2019;62:3206–3227. doi: 10.1021/acs.jmedchem.8b01361. [DOI] [PubMed] [Google Scholar]
- 8.Delost MD, Smith DT, Anderson BJ, Njardarson JT. From Oxiranes to Oligomers: Architectures of U.S. FDA Approved Pharmaceuticals Containing Oxygen Heterocycles. J. Med. Chem. 2018;61:10996–11020. doi: 10.1021/acs.jmedchem.8b00876. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe JP. Palladium‐Catalyzed Carboetherification and Carboamination Reactions of γ‐Hydroxy‐ and γ‐Aminoalkenes for the Synthesis of Tetrahydrofurans and Pyrrolidines. Eur. J. Org. Chem. 2007;2007:571–582. doi: 10.1002/ejoc.200600767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe JP, Hay MB. Recent advances in the stereoselective synthesis of tetrahydrofurans. Tetrahedron. 2007;63:261–290. doi: 10.1016/j.tet.2006.08.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalce G, Franck X, Figadère B. Diastereoselective synthesis of 2,5-disubstituted tetrahydrofurans. Tetrahedron: Asymmetry. 2009;20:2537–2581. doi: 10.1016/j.tetasy.2009.10.034. [DOI] [Google Scholar]
- 12.Majumdar KC, Debnath P, Roy B. Metal-Catalyzed Heterocyclization: Formation of Five- and Six-Membered Oxygen Heterocycles through Carbon-Oxygen Bond Forming Reactions. Heterocycles. 2009;78:2661–2728. doi: 10.3987/REV-09-656. [DOI] [Google Scholar]
- 13.Chen G, Ma S. Enantioselective Halocyclization Reactions for the Synthesis of Chiral Cyclic Compounds. Angew. Chem. Int. Ed. 2010;49:8306–8308. doi: 10.1002/anie.201003114. [DOI] [PubMed] [Google Scholar]
- 14.Zafrani Y, et al. CF2H, a Functional Group-Dependent Hydrogen-Bond Donor: Is It a More or Less Lipophilic Bioisostere of OH, SH, and CH3? J. Med. Chem. 2019;62:5628–5637. doi: 10.1021/acs.jmedchem.9b00604. [DOI] [PubMed] [Google Scholar]
- 15.Wolfe JP. Stereoselective Synthesis of Saturated Heterocycles via Palladium-Catalyzed Alkene Carboetherification and Carboamination Reactions. Synlett. 2008;19:2913–2937. doi: 10.1055/s-0028-1087339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolfe JP. Synthesis of Saturated Heterocycles via Metal-Catalyzed Alkene Carboamination or Carboalkoxylation Reactions. Top. Heterocycl. Chem. 2013;32:1–37. doi: 10.1007/7081_2012_98. [DOI] [Google Scholar]
- 17.Garlets ZJ, White DR, Wolfe JP. Recent Developments in Pd0‐Catalyzed Alkene-Carboheterofunctionalization Reactions. Asian J. Org. Chem. 2017;6:636–653. doi: 10.1002/ajoc.201600577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwano R. Catalytic Asymmetric Hydrogenation of 5-Membered Heteroaromatics. Heterocycles. 2008;76:909–922. doi: 10.3987/REV-08-SR(N)5. [DOI] [Google Scholar]
- 19.Tietze LF, Sommer KM, Zinngrebe J, Stecker F. Palladium‐Catalyzed Enantioselective Domino Reaction for the Efficient Synthesis of Vitamin E. Angew. Chem. Int. Ed. 2005;44:257–259. doi: 10.1002/anie.200461629. [DOI] [PubMed] [Google Scholar]
- 20.Jiao Z, et al. Organocatalytic Asymmetric Direct Csp3-H Functionalization of Ethers: A Highly Efficient Approach to Chiral Spiroethers. Angew. Chem. Int. Ed. 2012;51:8811–8815. doi: 10.1002/anie.201204274. [DOI] [PubMed] [Google Scholar]
- 21.Zi W, Toste FD. Gold(I)‐Catalyzed Enantioselective Desymmetrization of 1,3‐Diols through Intramolecular Hydroalkoxylation of Allenes. Angew. Chem. Int. Ed. 2015;54:14447–14451. doi: 10.1002/anie.201508331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rexit AA, Mailikezati M. Asymmetric synthesis of optically active spiroacetals from alkynyl glycols catalyzed by gold complex/Brønsted acid binary system. Tetrahedron Lett. 2015;56:2651–2655. doi: 10.1016/j.tetlet.2015.03.007. [DOI] [Google Scholar]
- 23.Yu S, Wu C, Ge S. Cobalt-Catalyzed Asymmetric Hydroboration/Cyclization of 1,6-Enynes with Pinacolborane. J. Am. Chem. Soc. 2017;139:6526–6529. doi: 10.1021/jacs.7b01708. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, et al. Catalytic enantioselective oxidative coupling of saturated ethers with carboxylic acid derivatives. Nat. Commun. 2019;10:559–567. doi: 10.1038/s41467-019-08473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang G, Cui L, Wang Y, Zhang L. Homogeneous Gold-Catalyzed Oxidative Carboheterofunctionalization of Alkenes. J. Am. Chem. Soc. 2010;132:1474–1475. doi: 10.1021/ja909555d. [DOI] [PubMed] [Google Scholar]
- 26.White DR, Wolfe JP. Stereocontrolled Synthesis of Amino-Substituted Carbocycles by Pd‐Catalyzed Alkene Carboamination Reactions. Chem. Eur. J. 2017;23:5419–5423. doi: 10.1002/chem.201700466. [DOI] [PubMed] [Google Scholar]
- 27.Ward AF, Wolfe JP. Highly Diastereoselective Pd-Catalyzed Carboetherification Reactions of Acyclic Internal Alkenes. Stereoselective Synthesis of Polysubstituted Tetrahydrofurans. Org. Lett. 2010;12:1268–1271. doi: 10.1021/ol1001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Protti S, Dondi D, Fagnoni M, Albini A. Photochemical Arylation of Alkenols: Role of Intermediates and Synthetic Significance. Eur. J. Org. Chem. 2008;2008:2240–2247. doi: 10.1002/ejoc.200701177. [DOI] [Google Scholar]
- 29.Nicolai S, Waser J. Pd(0)-Catalyzed Oxy- and Aminoalkynylation of Olefins for the Synthesis of Tetrahydrofurans and Pyrrolidines. Org. Lett. 2011;13:6324–6327. doi: 10.1021/ol2029383. [DOI] [PubMed] [Google Scholar]
- 30.Nicolai S, Erard S, González DF, Waser J. Pd-Catalyzed Intramolecular Oxyalkynylation of Alkenes with Hypervalent Iodine. Org. Lett. 2010;12:384–387. doi: 10.1021/ol9027286. [DOI] [PubMed] [Google Scholar]
- 31.Hay MB, Wolfe JP. Palladium-Catalyzed Synthesis of 2,1‘-Disubstituted Tetrahydrofurans from γ-Hydroxy Internal Alkenes. Evidence for Alkene Insertion into a Pd−O Bond and Stereochemical Scrambling via β-Hydride Elimination. J. Am. Chem. Soc. 2005;127:16468–16476. doi: 10.1021/ja054754v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hay MB, Hardin AR, Wolfe JP. Palladium-Catalyzed Synthesis of Tetrahydrofurans from γ-Hydroxy Terminal Alkenes: Scope, Limitations, and Stereoselectivity. J. Org. Chem. 2005;70:3099–3107. doi: 10.1021/jo050022+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fries P, Halter D, Kleinschek A, Hartung J. Functionalized Tetrahydrofurans from Alkenols and Olefins/Alkynes via Aerobic Oxidation−Radical Addition Cascades. J. Am. Chem. Soc. 2011;133:3906–3912. doi: 10.1021/ja108403s. [DOI] [PubMed] [Google Scholar]
- 34.Zhu C, Falck JR. Alternative Pathways for Heck Intermediates: Palladium‐Catalyzed Oxyarylation of Homoallylic Alcohols. Angew. Chem. Int. Ed. 2011;50:6626–6629. doi: 10.1002/anie.201101857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuch D, Fries P, Dönges M, Pérez BM, Hartung J. Reductive and Brominative Termination of Alkenol Cyclization in Aerobic Cobalt-Catalyzed Reactions. J. Am. Chem. Soc. 2009;131:12918–12920. doi: 10.1021/ja904577c. [DOI] [PubMed] [Google Scholar]
- 36.Palmer C, et al. Increased Yields and Simplified Purification with a Second-Generation Cobalt Catalyst for the Oxidative Formation of trans-THF Rings. Org. Lett. 2009;11:5614–5617. doi: 10.1021/ol9023375. [DOI] [PubMed] [Google Scholar]
- 37.Ball LT, Green M, Lloyd-Jones GC, Russell CA. Arylsilanes: Application to Gold-Catalyzed Oxyarylation of Alkenes. Org. Lett. 2010;12:4724–4727. doi: 10.1021/ol1019162. [DOI] [PubMed] [Google Scholar]
- 38.McDonald RI, Liu G, Stahl SS. Palladium(II)-Catalyzed Alkene Functionalization via Nucleopalladation: Stereochemical Pathways and Enantioselective Catalytic Applications. Chem. Rev. 2011;111:2981–3019. doi: 10.1021/cr100371y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dohanosova J, Gracza T. Asymmetric Palladium-Catalysed Intramolecular Wacker-Type Cyclisations of Unsaturated Alcohols and Amino Alcohols. Molecules. 2013;18:6173–6192. doi: 10.3390/molecules18066173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bovino MT, et al. Enantioselective Copper‐Catalyzed Carboetherification of Unactivated Alkenes. Angew. Chem. Int. Ed. 2014;53:6383–6387. doi: 10.1002/anie.201402462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karyakarte SD, Um C, Berhane IA, Chemler SR. Synthesis of Spirocyclic Ethers by Enantioselective Copper‐Catalyzed Carboetherification of Alkenols. Angew. Chem. Int. Ed. 2018;57:12921–12924. doi: 10.1002/anie.201808554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng Y, Dong X, Gu Q, Yu Z, Liu X. Achiral Pyridine Ligand‐Enabled Enantioselective Radical Oxytrifluoromethylation of Alkenes with Alcohols. Angew. Chem. Int. Ed. 2017;56:8883–8886. doi: 10.1002/anie.201702925. [DOI] [PubMed] [Google Scholar]
- 43.Pathak TP, Gligorich KM, Welm BE, Sigman MS. Synthesis and Preliminary Biological Studies of 3-Substituted Indoles Accessed by a Palladium-Catalyzed Enantioselective Alkene Difunctionalization Reaction. J. Am. Chem. Soc. 2010;132:7870–7871. doi: 10.1021/ja103472a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen KH, Webb JD, Sigman MS. Advancing the Mechanistic Understanding of an Enantioselective Palladium-Catalyzed Alkene Difunctionalization Reaction. J. Am. Chem. Soc. 2010;132:17471–17482. doi: 10.1021/ja108106h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SB, Park CM, Kang SH. Catalytic Enantioselective Iodocyclization of γ-Hydroxy-cis-alkenes. J. Am. Chem. Soc. 2003;125:15748–15749. doi: 10.1021/ja0369921. [DOI] [PubMed] [Google Scholar]
- 46.McCammant MS, Liao L, Sigman MS. Palladium-Catalyzed 1,4-Difunctionalization of Butadiene To Form Skipped Polyenes. J. Am. Chem. Soc. 2013;135:4167–4170. doi: 10.1021/ja3110544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao L, Jana R, Urkalan KB, Sigman MS. A Palladium-Catalyzed Three-Component Cross-Coupling of Conjugated Dienes or Terminal Alkenes with Vinyl Triflates and Boronic Acids. J. Am. Chem. Soc. 2011;133:5784–5787. doi: 10.1021/ja201358b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tian Z, et al. Highly Enantioselective Cross-Electrophile Aryl-Alkenylation of Unactivated Alkenes. J. Am. Chem. Soc. 2019;141:7637–7643. doi: 10.1021/jacs.9b03863. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, et al. Palladium/XuPhos-Catalyzed Enantioselective Carboiodination of Olefin-Tethered Aryl Iodides. J. Am. Chem. Soc. 2019;141:8110–8115. doi: 10.1021/jacs.9b04332. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, et al. Palladium‐Catalyzed Enantioselective Reductive Heck Reactions: Convenient Access to 3,3‐Disubstituted 2,3‐Dihydrobenzofuran. Angew. Chem. Int. Ed. 2018;57:10373–10377. doi: 10.1002/anie.201806372. [DOI] [PubMed] [Google Scholar]
- 51.Zhou W, et al. Chiral Sulfinamide Bisphosphine Catalysts: Design, Synthesis, and Application in Highly Enantioselective Intermolecular Cross‐Rauhut–Currier Reactions. Angew. Chem. Int. Ed. 2015;54:14853–14857. doi: 10.1002/anie.201508108. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, Xu B, Xu S, Wu H, Zhang J. Diastereo‐ and Enantioselective Copper(I)‐Catalyzed Intermolecular [3+2] Cycloaddition of Azomethine Ylides with β‐Trifluoromethyl β, β‐Disubstituted Enones. Angew. Chem. Int. Ed. 2016;55:6324–6328. doi: 10.1002/anie.201602542. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Z, et al. A New Type of Chiral Sulfinamide Monophosphine Ligands: Stereodivergent Synthesis and Application in Enantioselective Gold(I)‐Catalyzed Cycloaddition Reactions. Angew. Chem. Int. Ed. 2014;53:4350–4354. doi: 10.1002/anie.201401067. [DOI] [PubMed] [Google Scholar]
- 54.Zhang P, Wang Y, Zhang Z, Zhang J. Gold(I)/Xiang-Phos-Catalyzed Asymmetric Intramolecular Cyclopropanation of Indenes and Trisubstituted Alkenes. Org. Lett. 2018;20:7049–7052. doi: 10.1021/acs.orglett.8b02999. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, et al. Gold‐Catalyzed Asymmetric Intramolecular Cyclization of N‐Allenamides for the Synthesis of Chiral Tetrahydrocarbolines. Angew. Chem. Int. Ed. 2017;56:15905–15909. doi: 10.1002/anie.201709595. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, et al. Enantioselective Synthesis of Isoxazolines Enabled by Palladium-Catalyzed Carboetherification of Alkenyl Oximes. Angew. Chem. Int. Ed. 2020;59:4421–4427. doi: 10.1002/anie.201912408. [DOI] [PubMed] [Google Scholar]
- 57.Wang L, Chen M, Zhang P, Li W, Zhang J. Palladium/PC-Phos-Catalyzed Enantioselective Arylation of General Sulfenate Anions: Scope and Synthetic Applications. J. Am. Chem. Soc. 2018;140:3467–3473. doi: 10.1021/jacs.8b00178. [DOI] [PubMed] [Google Scholar]
- 58.Hu H, et al. Enantioselective gold-catalyzed intermolecular [2 + 2]-cycloadditions of 3-styrylindoles with N-allenyl oxazolidinone. Org. Chem. Front. 2016;3:759–763. doi: 10.1039/C6QO00087H. [DOI] [Google Scholar]
- 59.Wolfe JP, Ross MA. Stereoselective Synthesis of Tetrahydrofurans via the Palladium-Catalyzed Reaction of Aryl Bromides with γ-Hydroxy Alkenes: Evidence for an Unusual Intramolecular Olefin Insertion into a Pd(Ar)(OR) Intermediate. J. Am. Chem. Soc. 2004;126:1620–1621. doi: 10.1021/ja0394838. [DOI] [PubMed] [Google Scholar]
- 60.Hopkins BA, Garlets ZJ, Wolfe JP. Development of Enantioselective Palladium‐Catalyzed Alkene Carboalkoxylation Reactions for the Synthesis of Tetrahydrofurans. Angew. Chem. Int. Ed. 2015;54:13390–13392. doi: 10.1002/anie.201506884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu N, Li K, Wang Z, Tang W. Synthesis of Chiral 1,4‐Benzodioxanes and Chromans by Enantioselective Palladium‐Catalyzed Alkene Aryloxyarylation Reactions. Angew. Chem. Int. Ed. 2016;55:5044–5048. doi: 10.1002/anie.201600379. [DOI] [PubMed] [Google Scholar]
- 62.Liao S, Sun X, Tang Y. Side Arm Strategy for Catalyst Design: Modifying Bisoxazolines for Remote Control of Enantioselection and Related. Acc. Chem. Res. 2014;47:2260–2272. doi: 10.1021/ar800104y. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Zhou J, Tang Y. Sidearm Modified Bisoxazo line Ligands and Their Applications. Chin. J. Chem. 2018;36:1123–1129. doi: 10.1002/cjoc.201800373. [DOI] [Google Scholar]
- 64.Siau, W., Zhang, Y. & Zhao, Y. in Stereoselective Alkene Synthesis, (Springer, Berlin, ed. 327, 2012), pp. 33–58. [DOI] [PubMed]
- 65.Seguin TJ, Lu T, Wheeler SE. Enantioselectivity in Catalytic Asymmetric Fischer Indolizations Hinges on the Competition of π-Stacking and CH/π Interactions. Org. Lett. 2015;17:3066–3069. doi: 10.1021/acs.orglett.5b01349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available within the paper and its Supplementary Information files. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1977366 (Xu6-borane complex) and 2104802 (13). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif. The coordinates of DFT calculated structures are provided in Supplementary Information file. All data are also available from authors upon request.