Abstract
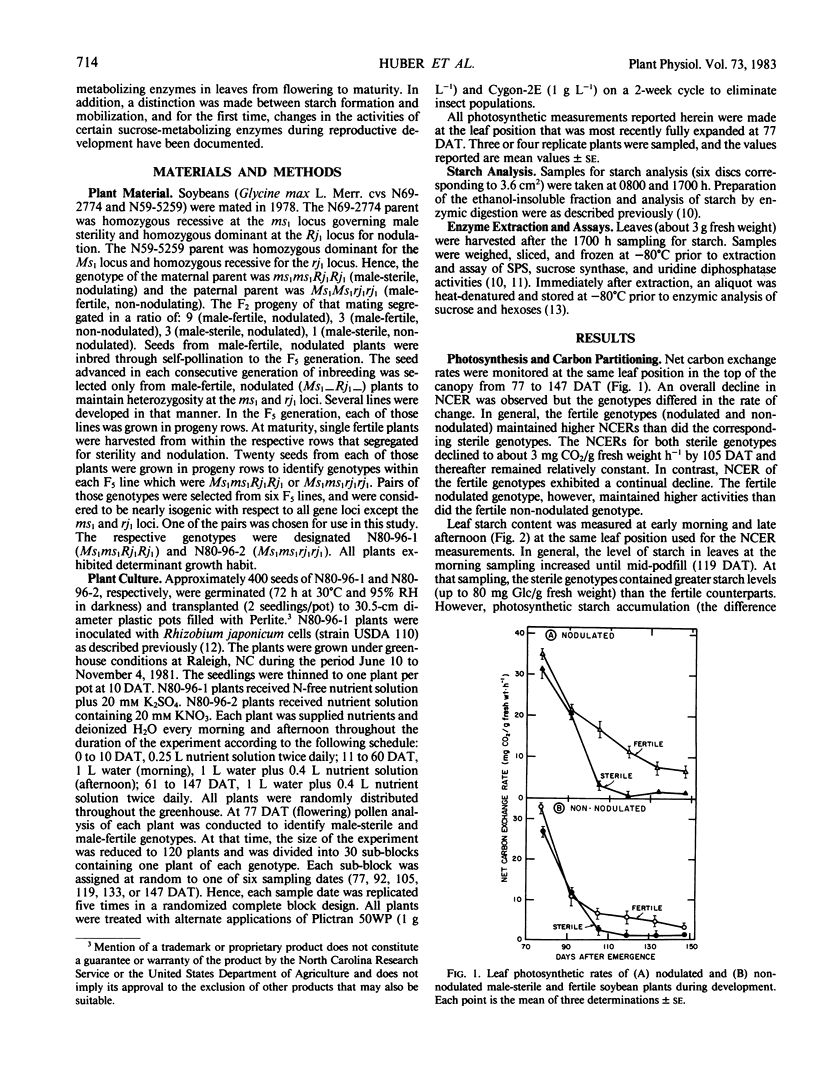
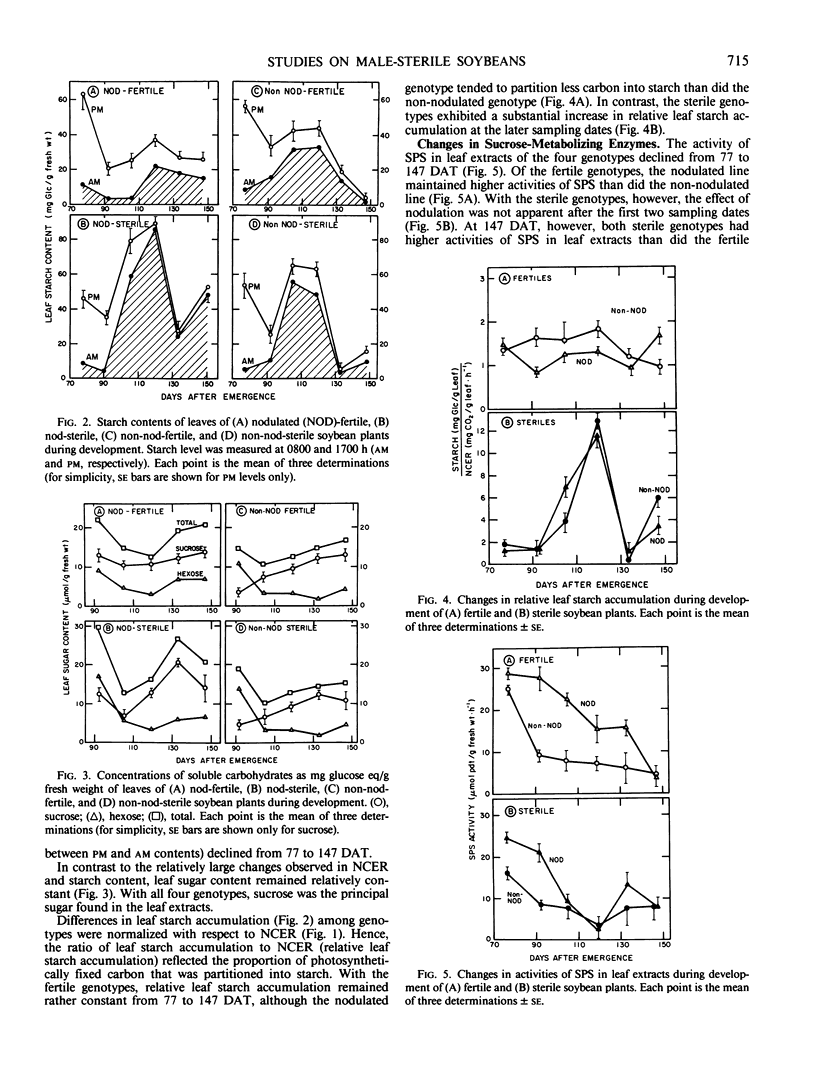
Soybean (Glycine max L. Merr.) germplasm, essentially isogenic except for loci controlling male sterility (ms1) and nodulation (rj1), were developed to study the effects of reproductive development and nitrogen source on certain aspects of photosynthesis. Plants were sampled from flowering (77 days after transplanting) until maturity (150 days after transplanting). With all four genotypes, net carbon exchange rates were highest at flowering and declined thereafter. Photosynthetic rates of the sterile genotypes (nodulated and non-nodulated) declined more rapidly than the fertile genotypes, and after 105 days, both sterile genotypes maintained low but relatively constant carbon exchange rates (<3 milligrams CO2/gram fresh weight per hour). Photosynthetic rates and starch accumulation (difference between afternoon and morning levels) declined with time. The sterile genotypes attained the highest morning starch levels, which reflected reduced starch mobilization. After 92 days, the proportion of photosynthetically fixed carbon that was partitioning into starch (relative leaf starch accumulation) in the sterile genotypes increased dramatically. In contrast, relative leaf starch accumulation in the fertile genotypes remained relatively constant with time. Throughout the test period, all four genotypes maintained leaf sucrose levels between 5 and 15 micromoles glucose equivalents per gram fresh weight.
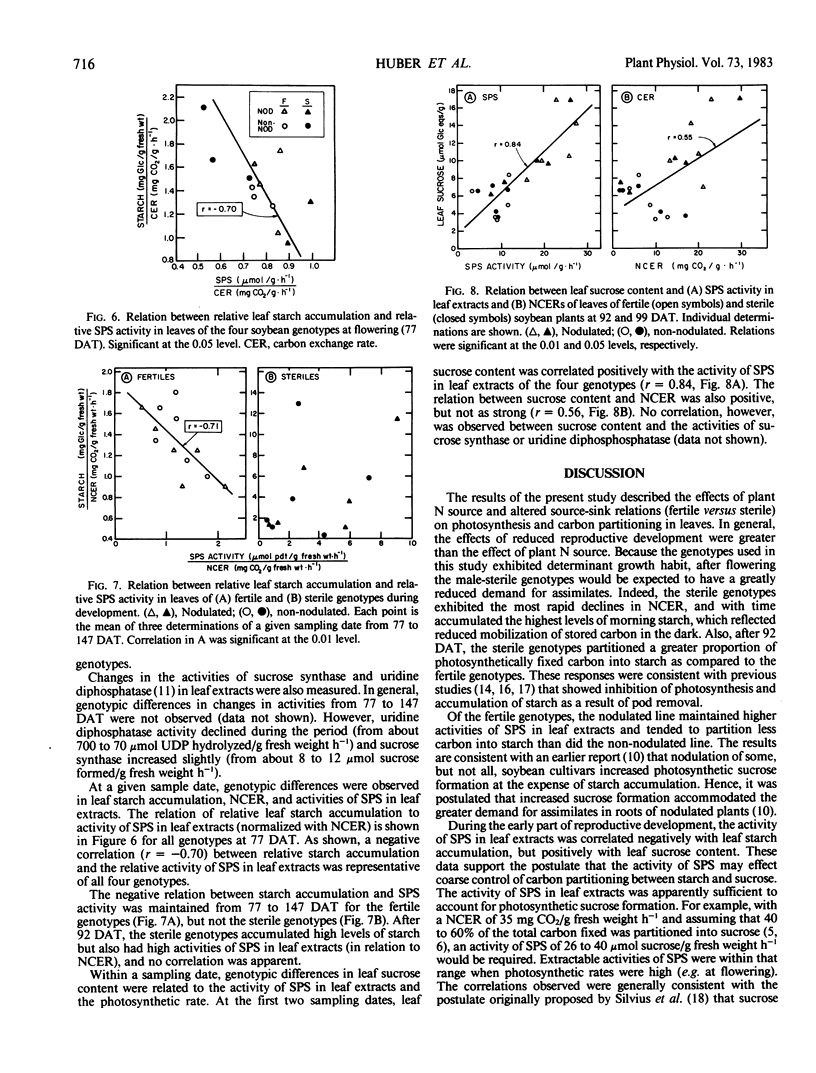
The activities of sucrose phosphate synthase (SPS) in leaf extracts of the four genotypes declined from 77 to 147 days. Nodulated genotypes tended to maintain higher activities (leaf fresh weight basis) than did the non-nodulated genotypes. In general, relative leaf starch accumulation was correlated negatively with the activity of SPS (normalized with leaf net carbon exchange rate) in leaf extracts for all four genotypes during early reproductive development, and for the fertile genotypes at all sampling dates. In contrast, leaf sucrose content was correlated positively with SPS activity during early reproductive development. These results suggested that a direct relation existed between the activity of SPS and starch/sucrose levels in soybean leaves. However, the interaction between these processes also may be influenced by other factors, particularly when leaf photosynthetic rates and plant demand for assimilates is low, as in the sterile genotypes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Housley T. L., Schrader L. E., Miller M., Setter T. L. Partitioning of C-photosynthate, and long distance translocation of amino acids in preflowering and flowering, nodulated and nonnodulated soybeans. Plant Physiol. 1979 Jul;64(1):94–98. doi: 10.1104/pp.64.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Israel D. W. Biochemical Basis for Partitioning of Photosynthetically Fixed Carbon between Starch and Sucrose in Soybean (Glycine max Merr.) Leaves. Plant Physiol. 1982 Mar;69(3):691–696. doi: 10.1104/pp.69.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Pharr D. M. Occurrence and Regulatory Properties of Uridine Diphosphatase in Fully Expanded Leaves of Soybean (Glycine max Merr.) and Other Species. Plant Physiol. 1981 Dec;68(6):1294–1298. doi: 10.1104/pp.68.6.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C. Role of sucrose-phosphate synthase in partitioning of carbon in leaves. Plant Physiol. 1983 Apr;71(4):818–821. doi: 10.1104/pp.71.4.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. G., Outlaw W. H., Lowry O. H. Enzymic assay of 10 to 10 moles of sucrose in plant tissues. Plant Physiol. 1977 Sep;60(3):379–383. doi: 10.1104/pp.60.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal M. H., Brun W. A., Brenner M. L. Effects of Sink Removal on Photosynthesis and Senescence in Leaves of Soybean (Glycine max L.) Plants. Plant Physiol. 1978 Mar;61(3):394–397. doi: 10.1104/pp.61.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw W. H., Fisher D. B., Christy A. L. Compartmentation in Vicia faba Leaves: II. Kinetics of C-Sucrose Redistribution among Individual Tissues following Pulse Labeling. Plant Physiol. 1975 Apr;55(4):704–711. doi: 10.1104/pp.55.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A., Brenner M. L. Effect of obstructed translocation on leaf abscisic Acid, and associated stomatal closure and photosynthesis decline. Plant Physiol. 1980 Jun;65(6):1111–1115. doi: 10.1104/pp.65.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setter T. L., Brun W. A. Stomatal closure and photosynthetic inhibition in soybean leaves induced by petiole girdling and pod removal. Plant Physiol. 1980 May;65(5):884–887. doi: 10.1104/pp.65.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvius J. E., Kremer D. F., Lee D. R. Carbon assimilation and translocation in soybean leaves at different stages of development. Plant Physiol. 1978 Jul;62(1):54–58. doi: 10.1104/pp.62.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H., Koller H. R. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974 Aug;54(2):201–207. doi: 10.1104/pp.54.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R. F., Burton J. W., Buck J. A., Brim C. A. Studies on Genetic Male-Sterile Soybeans: I. Distribution of Plant Carbohydrate and Nitrogen during Development. Plant Physiol. 1978 May;61(5):838–841. doi: 10.1104/pp.61.5.838. [DOI] [PMC free article] [PubMed] [Google Scholar]


