Abstract
Photoactivation of phosphoenolpyruvate carboxylase was found to occur in several, though not all, C4 species examined; Salsola soda L. was used for a detailed study of this effect of light.
Activity differences between light and darkness are maximized when glycerol (25% v/v) is included in the extraction medium and in the absence of mercaptoethanol. In plants grown in the growth chamber, the night-form of the enzyme, in addition to low activity, shows a positive cooperativity (with phosphoenolpyruvate), which is gradually abolished by light of increasing intensities. This allosteric behavior is absent in plants adapted to a high light environment. Activation and deactivation, under light and darkness respectively, are quite fast, suggesting post-translational regulation. The photoactivation appears to depend on photosynthetic electron flow, since it is saturated at high photon fluxes (around 1000 microeinsteins per square meter per second) and inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea.
Full text
PDF
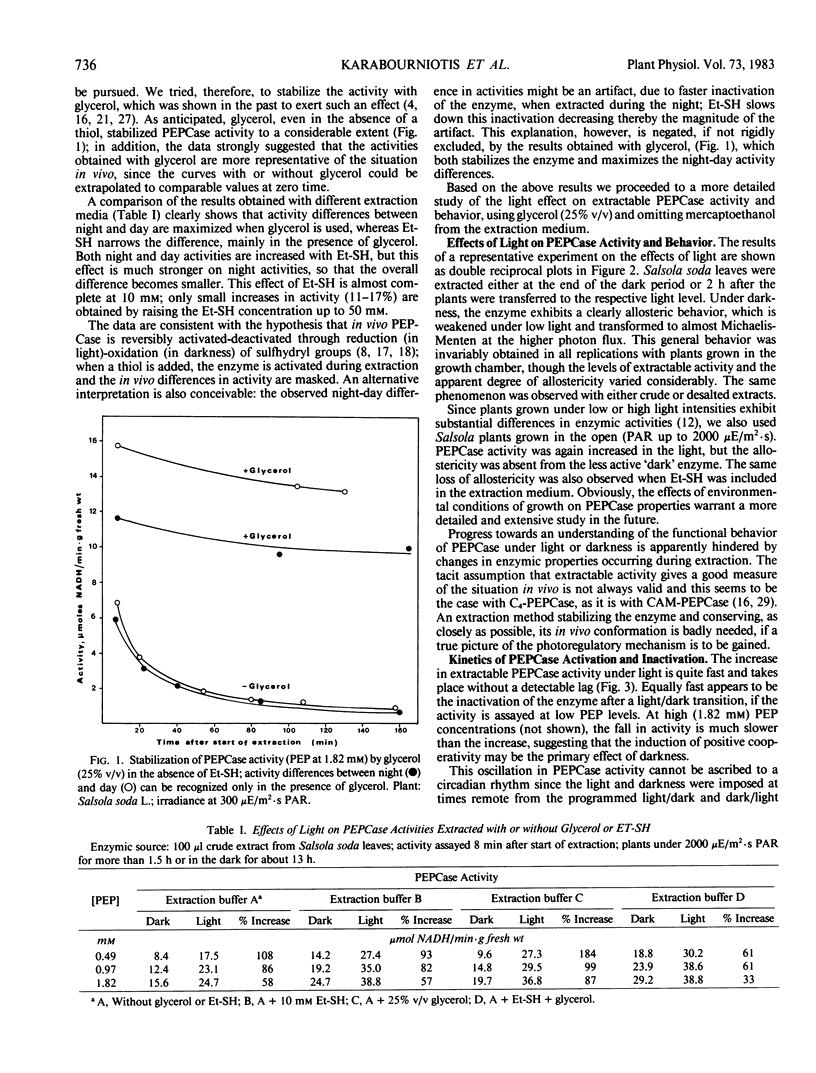


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., Avron M. Light Modulation of Enzyme Activity in Chloroplasts: Generation of Membrane-bound Vicinal-Dithiol Groups by Photosynthetic Electron Transport. Plant Physiol. 1976 Feb;57(2):209–213. doi: 10.1104/pp.57.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Nehrlich S. C. Photosynthetic electron-transport system controls cytoplasmic glucose-6-phosphate dehydrogenase activity in pea leaves. FEBS Lett. 1977 Apr 1;76(1):64–66. doi: 10.1016/0014-5793(77)80121-x. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Studies on the mechanism of activation and inactivation of pyruvate, phosphate dikinase. A possible regulatory role for the enzyme in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 May;112(5):549–558. doi: 10.1042/bj1120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachru R. B., Anderson L. E. Inactivation of pea leaf phosphofructokinase by light and dithiothreitol. Plant Physiol. 1975 Feb;55(2):199–202. doi: 10.1104/pp.55.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerji S. K. Corn leaf phosphoenolpyruvate carboxylases. Purification and properties of two isoenzymes. Arch Biochem Biophys. 1977 Jul;182(1):343–351. doi: 10.1016/0003-9861(77)90315-0. [DOI] [PubMed] [Google Scholar]
- Shomer-Ilan A., Neumann-Ganmore R., Waisel Y. Biochemical Specialization of Photosynthetic Cell Layers and Carbon Flow Paths in Suaeda monoica. Plant Physiol. 1979 Dec;64(6):963–965. doi: 10.1104/pp.64.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R. The photoactivation of a phosphopyruvate synthase in leaves of Amaranthus palmeri. Biochem Biophys Res Commun. 1968 Mar 12;30(5):483–488. doi: 10.1016/0006-291x(68)90077-6. [DOI] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K. Day/Night Changes in the Sensitivity of Phosphoenolpyruvate Carboxylase to Malate during Crassulacean Acid Metabolism. Plant Physiol. 1980 May;65(5):792–796. doi: 10.1104/pp.65.5.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosiuk R. A., Buchanan B. B. Activation of Chloroplast NADP-linked Glyceraldehyde-3-Phosphate Dehydrogenase by the Ferredoxin/Thioredoxin System. Plant Physiol. 1978 Apr;61(4):669–671. doi: 10.1104/pp.61.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]


