Abstract
The gonadotropin-releasing hormone (GnRH) pulse and surge are considered to be generated by arcuate kisspeptin/neurokinin B/dynorphin A (KNDy) neurons and anteroventral periventricular nucleus (AVPV) kisspeptin neurons, respectively, in female rodents. The majority of KNDy and AVPV kisspeptin neurons express κ-opioid receptors (KORs, encoded by Oprk1) in female rodents. Thus, this study aimed to investigate the effect of a conditional Oprk1-dependent Kiss1 deletion in kisspeptin neurons on the luteinizing hormone (LH) pulse/surge and fertility using Kiss1-floxed/Oprk1-Cre rats, in which Kiss1 was deleted in cells expressing or once expressed the Oprk1/Cre. The Kiss1-floxed/Oprk1-Cre female rats, with Kiss1 deleted in a majority of KNDy neurons, showed normal puberty while having a one-day longer estrous cycle and fewer pups than Kiss1-floxed controls. Notably, ovariectomized (OVX) Kiss1-floxed/Oprk1-Cre rats showed profound disruption of LH pulses in the presence of a diestrous level of estrogen but showed apparent LH pulses without estrogen treatment. Furthermore, Kiss1-floxed/Oprk1-Cre rats, with Kiss1 deleted in approximately half of AVPV kisspeptin neurons, showed a lower peak of the estrogen-induced LH surge than controls. These results suggest that arcuate and AVPV kisspeptin neurons expressing or having expressed Oprk1 have a role in maintaining normal GnRH pulse and surge generation, the normal length of the estrous cycle, and the normal offspring number in female rats.
Subject terms: Neuroscience, Reproductive biology
Introduction
Reproductive function is precisely orchestrated by hypothalamic neuropeptides, namely kisspeptin (encoded by the Kiss1 gene) and gonadotropin-releasing hormone (GnRH), pituitary gonadotropins, and gonadal steroids in mammals1–4. There are tonic (pulsatile) or cyclic (surge) modes of GnRH release, which are responsible for folliculogenesis/steroidogenesis or ovulation, respectively, in female mammals1–4. The GnRH pulse is fundamentally important for tonic gonadotropin release because pulsatile GnRH treatment at the physiological frequency restores gonadotropin release in female rhesus monkeys with hypothalamic lesions, whereas continuous administration of GnRH paradoxically suppresses gonadotropin release5. Recently, we have provided direct evidence that KNDy (an acronym for kisspeptin/neurokinin B [NKB]/dynorphin A [Dyn]) neurons in the arcuate nucleus (ARC) serve as the GnRH pulse generator by showing that the rescue of KNDy neurons by transfecting the Kiss1 gene into ARC NKB neurons recovered luteinizing hormone (LH) pulses and folliculogenesis in infertile global Kiss1 knockout (KO) female rats6. Recent in vivo Ca2+ imaging also showed synchronized rhythmic increases in intracellular Ca2+ levels in clusters of KNDy neurons, and rhythmic increases in intracellular Ca2+ levels in KNDy neurons were accompanied by LH pulses in male and female mice7–9. On the other hand, the GnRH surge, which is evoked by the positive feedback action of estrogen derived from mature follicles, is crucially important for the LH surge and ovulation in mammals1. Kisspeptin neurons located in the anteroventral periventricular nucleus (AVPV) serve as a target of estrogen positive feedback action on GnRH/LH surge generation in rodents because estrogen increases AVPV Kiss1 expression or c-Fos (a marker of neuronal activation) in AVPV kisspeptin neurons in female rats10–12 and mice13–15. The notion that AVPV kisspeptin neurons serve as the GnRH surge generator has also been verified by sex differences in the numbers of AVPV kisspeptin neurons (males < females) and the absence of an estrogen-induced LH surge in male rodent models4, 11, 16–19. Importantly, Wiegand et al.20 demonstrated that AVPV lesion disrupted estrogen + progesterone-induced LH surge in female rats. Furthermore, our and other previous studies showed that kisspeptin antagonism by chronic intracerebroventricular infusion of a GPR54 antagonist or by chronic infusion of an anti-kisspeptin antibody into the preoptic area blocked spontaneous or estrogen-induced LH surge in female rats10, 21, 22.
It has been suggested that KNDy neurons synchronize with each other in a paracrine and autocrine manner through stimulatory NKB-neurokinin 3 receptor (NK3R, the primary receptor for NKB) signaling and inhibitory Dyn-κ-opioid receptor (KOR, the primary receptor for Dyn) signaling to form GnRH/gonadotropin pulses because the majority of KNDy neurons express NK3Rs and KORs in rats and sheep23–25. Indeed, central administration of NKB or a KOR antagonist increased the frequency of multiple unit activity (MUA) volleys accompanied by LH pulses, and central Dyn administration decreased the frequency of MUA volleys in female goats26, in which MUA volleys were taken from the cluster of KNDy neurons. In addition, Ruka et al.27 and de Croft et al.28 showed that an NK3R agonist increased and Dyn or a KOR agonist decreased the firing frequency of GFP-labeled KNDy neurons in male mice. Moreover, the LH pulse frequency was increased by peripheral administration of the KOR antagonist and was suppressed by peripheral administration of the NK3R antagonist in female goats29 and ewes30. Importantly, KOR expression was found in some KNDy neurons in rats (62%)25, sheep (98%)24, and mice (6–65%)27, 31–33, but it has not been clarified whether KOR-expressing KNDy neurons play an indispensable role in fertility and GnRH pulse generation. Therefore, it is worth clarifying the effects of a conditional Oprk1-dependent Kiss1 deletion in KNDy cells on fertility and LH pulses in newly generated gene-modified female rats.
The sex steroidal milieu should be taken into account when studying the role of Dyn-KOR signaling in GnRH pulse generation by KNDy neurons because Dyn-KOR signaling mediates the negative feedback action of sex steroids and stress-induced suppression of GnRH/gonadotropin pulses in several mammals, including rodents, ruminants, primates, and humans34. Indeed, Han et al.35 showed that central KOR antagonism promoted synchronization between KNDy neurons in testis-intact Kiss1-Cre male mice, but not in castrated males. Furthermore, our recent study showed that central administration of a KOR antagonist restored glucoprivic suppression of LH pulses in ovariectomized rats treated with a diestrous level of estradiol-17β (OVX + low E2 rats)25. Interestingly, low E2 treatment significantly increased the number of Pdyn (encoding Dyn)-expressing cells in the paraventricular nucleus (PVN) but not in the ARC of OVX rats25, suggesting that PVN Dyn neurons mainly mediate the inhibitory effect of sex steroids and/or malnutrition on KNDy neural activity and GnRH/LH pulses. Therefore, the contribution of Dyn-KOR signaling in controlling pulsatile GnRH/LH release could be changed depending on the steroidal milieu.
It is also known that KORs are expressed in some (50%) AVPV kisspeptin neurons in mice32, while the role of Dyn-KOR signaling in AVPV kisspeptin neurons has not yet been clarified. If KOR-expressing AVPV kisspeptin neurons are needed for GnRH/LH surge generation, a conditional Oprk1-dependent Kiss1 deletion in AVPV kisspeptin cells may affect the LH surge and ovulation.
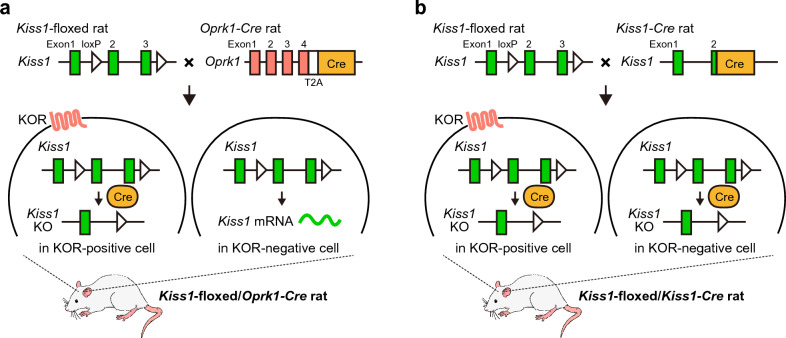
Thus, the present study aimed to investigate the effect of a conditional Oprk1-dependent Kiss1 deletion in kisspeptin cells on LH pulses, the LH surge, and fertility using newly generated Kiss1-floxed/Oprk1-Cre rats (Fig. 1a) to clarify whether KOR-negative kisspeptin neurons could maintain fertility and whether KOR-positive kisspeptin neurons are a prerequisite for the pulsatile and surge modes of GnRH/LH release. Puberty onset, ovarian weight, vaginal cyclicity, and fertility were examined in ovary-intact Kiss1-floxed/Oprk1-Cre rats and Kiss1-floxed control rats. Furthermore, ARC Kiss1 mRNA expression; pulsatile LH release; and pituitary Gnrhr, Lhb, and Fshb mRNA (encoding GnRH receptor, and LH and FSH β-subunit, respectively) expression were examined in Kiss1-floxed/Oprk1-Cre rats and Kiss1-floxed control rats under different steroidal milieus (OVX vs. physiological estrogenic condition, i.e., OVX + low E2 condition). In addition, we examined effects of the conditional Kiss1 deletion on the number of AVPV Kiss1-expressing cells and on the LH surge induced by a proestrous level of E2 (high E2) by comparing Kiss1-floxed/Oprk1-Cre rats and Kiss1-floxed control rats. Moreover, for comparison with Kiss1-floxed/Oprk1-Cre rats, we generated rats with conditionally deleted Kiss1 in all kisspeptin neurons (Kiss1-floxed/Kiss1-Cre rats) by crossing Kiss1-Cre rats36 with Kiss1-floxed rats (Fig. 1b), and reproductive indicators, such as puberty onset, ovarian weight, ARC Kiss1 mRNA expression, pulsatile LH release, and pituitary Gnrhr, Lhb, and Fshb mRNA expression, were also examined in the Kiss1-floxed/Kiss1-Cre rats.
Figure 1.
Schematic illustration of conditional Kiss1 knockout (KO) in κ-opioid receptor (KOR)-positive or all kisspeptin neurons in female rats. (a) Generation of rats with conditionally deleted Kiss1 in KOR-positive anteroventral periventricular nucleus (AVPV) and arcuate nucleus (ARC) kisspeptin neurons (Kiss1-floxed/Oprk1-Cre rats) by crossing Oprk1-Cre rats with Kiss1-floxed rats. (b) Generation of rats with conditionally deleted Kiss1 in all kisspeptin neurons (Kiss1-floxed/Kiss1-Cre rats) by crossing Kiss1-Cre rats with Kiss1-floxed rats.
Results
Colocalization of Oprk1 and Oprk1-Cre-activated tdTomato mRNA expression
We first generated Oprk1-Cre rats (see Supplementary Fig. S1 online) and confirmed the specificity of Cre expression in Oprk1-expressing cells by crossing Oprk1-Cre rats and Cre-dependent tdTomato reporter rats37 (see Supplementary Fig. S2a online). Double in situ hybridization (ISH) revealed the presence of a number of Oprk1-expressing cells and tdTomato-expressing cells in the hypothalamus, including the ARC (Fig. S2b), PVN (Fig. S2c), and supraoptic nucleus (SON, Fig. S2d), in OVX + low E2 Oprk1-Cre/tdTomato reporter female rats in adulthood. Quantitative analysis revealed that the majority of Oprk1-expressing cells coexpressed tdTomato in the ARC (92.96 ± 1.35%), PVN (98.06 ± 0.35%), and SON (97.95 ± 0.40%) in Oprk1-Cre/tdTomato reporter rats, indicating that most Oprk1-expressing cells in these regions were labeled with the tdTomato reporter (Fig. S2b-d). On the other hand, many tdTomato-expressing cells without Oprk1 expression were observed in these regions because theoretically cells that have expressed Oprk1/Cre at least once should exhibit persistent tdTomato expression after Oprk1/Cre expression in Oprk1-Cre/tdTomato reporter rats. Quantitative analysis revealed that 31.16 ± 3.75% (ARC), 35.63 ± 5.72% (PVN), and 46.07 ± 8,68% (SON) of tdTomato-expressing cells coexpressed Oprk1.
Oprk1-driven Cre-activated tdTomato expression in KNDy neurons in female rats at prepubertal and adult stages
Double ISH for tdTomato and Tac3 (encoding NKB, a marker of KNDy neurons) revealed Oprk1-Cre-activated tdTomato expression in a small population of ARC Tac3-expressing cells (19.48 ± 1.93%) in ovary-intact Oprk1-Cre/tdTomato reporter rats in the prepubertal period (3 weeks of age), whereas tdTomato expression was found in most Tac3-expressing cells (92.14 ± 1.12%) in OVX + low E2 Oprk1-Cre/tdTomato reporter rats in adulthood (9–10 weeks of age, Fig. S2e). The number of ARC Tac3- and tdTomato-coexpressing cells in Oprk1-Cre/tdTomato reporter rats in prepubertal period was significantly lower than that in adulthood (t(4) = 11.34, p < 0.01, Student’s t-test), although the total number of Tac3-expressing cells was comparable between the prepubertal and adult groups (t(4) = 1.13, p = 0.32, Student’s t-test). On the other hand, many tdTomato-expressing cells without Tac3 expression were observed in these regions, consistent with our previous study showing that Oprk1 expression was found in both KNDy and non-KNDy neurons in the ARC of female rats25.
Kiss1-floxed/Oprk1-Cre female rats showed puberty onset, the estrous cycle, and fertility, whereas Kiss1-floxed/Kiss1-Cre female rats lacked puberty onset
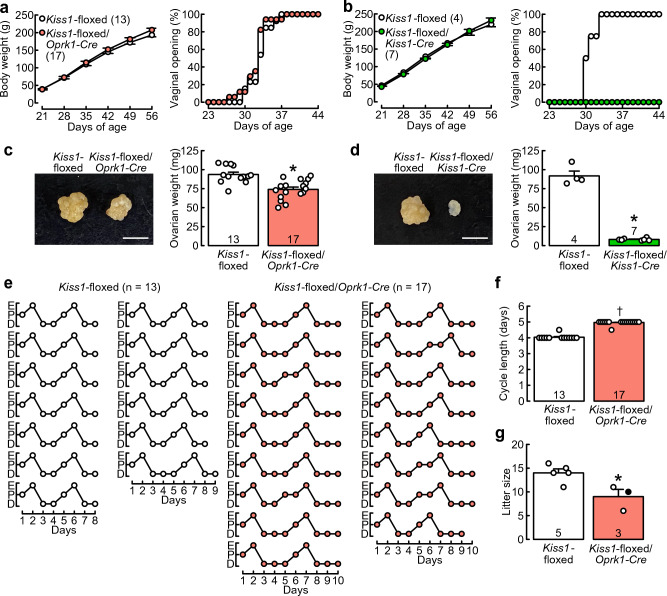
Kiss1-floxed/Oprk1-Cre female rats and Kiss1-floxed/Kiss1-Cre female rats showed normal growth, similar to that of Cre(−)/Kiss1-floxed control rats (Fig. 2a,b). Two-way ANOVA revealed a significant main effect of days of age on the body weight (Kiss1-floxed/Oprk1-Cre, F(5,140) = 3536.12, p < 0.01; Kiss1-floxed/Kiss1-Cre, F(5,45) = 1590.38, p < 0.01), a significant interaction effect between the genotype and days of age on the body weight (Kiss1-floxed/Oprk1-Cre, F(5,140) = 6.78, p < 0.01; Kiss1-floxed/Kiss1-Cre, F(5,45) = 4.41, p < 0.01), and no significant differences in body weights between the genotypes at each time point. Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats showed vaginal opening (VO) between 27 and 38 days of age (Fig. 2a), and no significant difference was found in the occurrence probability of VO between Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats (χ2 = 0.8, p = 0.4, log-rank test). In contrast, Kiss1-floxed/Kiss1-Cre rats showed no VO until 56 days of age, whereas Cre(−)/Kiss1-floxed control rats showed VO between 30 and 33 days of age (Fig. 2b), and there was a significant difference in the occurrence probability of VO between Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed control rats (χ2 = 12.2, p < 0.01, log-rank test).
Figure 2.
Kiss1-floxed/Oprk1-Cre female rats showed puberty onset, estrous cycles, and fertility, whereas Kiss1-floxed/Kiss1-Cre female rats lacked puberty onset. Growth curves and puberty onset in Kiss1-floxed/Oprk1-Cre female rats and their counterpart Cre(−)/Kiss1-floxed female rats (a) or Kiss1-floxed/Kiss1-Cre female rats and their counterpart Cre(−)/Kiss1-floxed female rats (b). Body weights are the means ± SEM. Numbers in parentheses indicate the number of animals used. The timing of vaginal opening as an external sign of puberty onset is expressed as a percentage of the total number of animals in each group. Representative photographs of ovaries and ovarian weights of Kiss1-floxed/Oprk1-Cre rats and their counterpart Cre(−)/Kiss1-floxed rats (c) or Kiss1-floxed/Kiss1-Cre rats and their counterpart Cre(−)/Kiss1-floxed rats (d). Scale bars, 5 mm. Open circles indicate the individual data. (e) Individual data of estrous cycles from the Kiss1-floxed/Oprk1-Cre group and Cre(−)/Kiss1-floxed control group. E, estrus; P, proestrus; and D, diestrus. (f) Cycle length (on average of two consecutive cycles) of Kiss1-floxed/Oprk1-Cre and Cre(−)/Kiss1-floxed groups. A dagger indicates a statistically significant difference (p < 0.05) between the groups based on the Wilcoxon rank sum test. Open circles indicate the individual data. (g) Litter sizes of Kiss1-floxed/Oprk1-Cre and Cre(−)/Kiss1-floxed female rats, which were mated with Iar:Wistar-Imamichi stud male rats. Values are the means ± SEM. Open (pups were delivered on gestational Day 22) and closed (pups were delivered on gestational Day 23) circles indicate the individual data. Numbers in (or on) each column indicate the number of animals used. Asterisks indicate statistically significant differences (p < 0.05) between the groups based on Student’s t-test.
The ovaries of Kiss1-floxed/Oprk1-Cre and Kiss1-floxed/Kiss1-Cre rats were smaller than those of Cre(−)/Kiss1-floxed control rats, as shown in photographs of the ovaries obtained from representative animals of each genotype (Fig. 2c,d). The ovarian weights in Kiss1-floxed/Oprk1-Cre rats (77.88 ± 3.19% of the control) and Kiss1-floxed/Kiss1-Cre rats (8.83 ± 0.61% of the control) were significantly lower than those in Cre(−)/Kiss1-floxed control rats (Kiss1-floxed/Oprk1-Cre, t(28) = 4.46, p < 0.01; Kiss1-floxed/Kiss1-Cre, t(9) = 17.94, p < 0.01, Student’s t-test; Fig. 2c,d). Most (12 out of 13) Cre(−)/Kiss1-floxed control rats exhibited 4-day (on average of two consecutive estrous cycles) estrous cycles, and remaining one showed a 4-day and 5-day estrous cycle, so that the average value was 4.5-day (Fig. 2e). Notably, most (16 out of 17) Kiss1-floxed/Oprk1-Cre rats exhibited 5-day (on average) estrous cycles with a 1-day longer diestrus than controls, and remaining one showed a 4-day and 5-day estrous cycle, so that the average value was 4.5-day (Fig. 2e). As a result, the length of the estrous cycle was significantly longer in Kiss1-floxed/Oprk1-Cre rats than in Cre(−)/Kiss1-floxed control rats (p < 0.01, Wilcoxon rank sum test, Fig. 2f).
Two out of three Kiss1-floxed/Oprk1-Cre female rats and all five Cre(−)/Kiss1-floxed female rats that mated with wild-type stud male rats at the proestrous stage exhibited spontaneous delivery at gestational Day 22. One remaining Kiss1-floxed/Oprk1-Cre female rat exhibited spontaneous delivery one day later. The litter size in Kiss1-floxed/Oprk1-Cre rats was significantly smaller than that in Cre(−)/Kiss1-floxed control rats (t(6) = 3.17, p = 0.02, Student’s t-test, Fig. 2g).
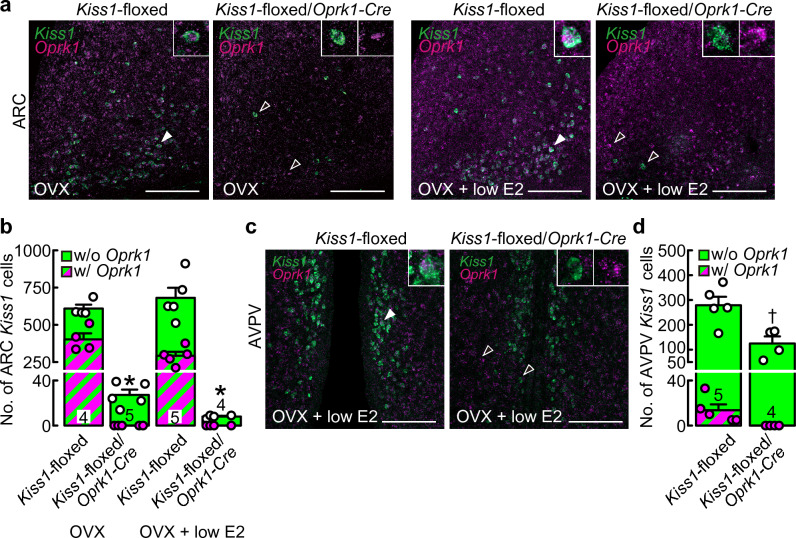
Kiss1 deletion in cells expressing or having expressed Oprk1 reduced the number of ARC and AVPV Kiss1-expressing cells
Double ISH for Kiss1 and Oprk1 revealed that a few Kiss1-expressing cells without Oprk1 signals were located in the ARC of OVX Kiss1-floxed/Oprk1-Cre rats, regardless of E2 treatment, whereas many Kiss1-expressing cells with/without Oprk1 signals were found in the ARC of OVX Cre(−)/Kiss1-floxed control rats, regardless of E2 treatment (Fig. 3a). Two-way ANOVA revealed a significant main effect of the genotype, but not E2 treatment, without the interaction effect between the genotype and E2 treatment on the number of ARC Kiss1-expressing cells. Specifically, the number of ARC Kiss1-expressing cells was significantly lower in Kiss1-floxed/Oprk1-Cre rats (2.87 ± 0.65% of the control on average with/without low E2 treatment; 8.00 ± 0.71 and 27.20 ± 4.73 cells in Kiss1-floxed/Oprk1-Cre rats with or without low E2 treatment, respectively) than in Cre(−)/Kiss1-floxed control rats (F(1,14) = 246.03, p < 0.01, Fig. 3b). Quantitative analysis revealed the absence of Oprk1 expression in the remaining ARC Kiss1-expressing cells in OVX Kiss1-floxed/Oprk1-Cre rats with/without low E2 treatment, whereas Oprk1 coexpression was found in Cre(−)/Kiss1-floxed control rats (52.58 ± 4.40% of the control on average with/without low E2 treatment).
Figure 3.
Kiss1 deletion in cells expressing or having expressed Oprk1 reduced the number of ARC and AVPV Kiss1-expressing cells. (a) Kiss1-expressing (green) and Oprk1-expressing (magenta) cells in the ARC of representative ovariectomized (OVX) or OVX and treated with a negative feedback level of estradiol-17β (OVX + low E2) Kiss1-floxed/Oprk1-Cre rats and their counterpart Cre(−)/Kiss1-floxed rats. (b) The numbers of Kiss1-expressing (green) or Kiss1- and Oprk1-coexpressing (striped) cells in the ARC of OVX or OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats. Asterisks indicate a statistically significant main effect of the genotype (p < 0.05) based on two-way ANOVA. (c) Kiss1-expressing (green) and Oprk1-expressing (magenta) cells in the AVPV of representative OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and their counterpart Cre(−)/Kiss1-floxed rats. The insets indicate representative Kiss1- and Oprk1-coexpressing cells, indicated by the solid white arrowheads, or Kiss1- or Oprk1-expressing cells, indicated by the open white arrowheads. Scale bars, 100 μm. (d) The numbers of Kiss1-expressing (green) or Kiss1- and Oprk1-coexpressing (striped) cells in the AVPV of OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats. A dagger indicates a statistically significant difference (p < 0.05) between the groups based on Student’s t-test. Values are the means ± SEM. Circles indicate the individual data of the number of Kiss1-expressing (white) or Kiss1- and Oprk1-coexpressing (magenta) cells. Numbers in (or on) each column indicate the number of animals used.
Double ISH revealed a number of Kiss1-expressing cells with/without Oprk1 expression in the AVPV of OVX + low E2 Cre(−)/Kiss1-floxed control rats, while in Kiss1-floxed/Oprk1-Cre rats, fewer Kiss1-expressing cells without Oprk1 signals were found in the AVPV (Fig. 3c). The number of AVPV Kiss1-expressing cells was significantly lower in Kiss1-floxed/Oprk1-Cre rats (44.75 ± 10.24% of the control) than in Cre(−)/Kiss1-floxed control rats (t(7) = 3.33, p = 0.013, Student’s t-test, Fig. 3d). Oprk1 coexpression was found in 5.26 ± 1.99% of AVPV Kiss1-expressing cells in OVX + low E2 Cre(−)/Kiss1-floxed control rats.
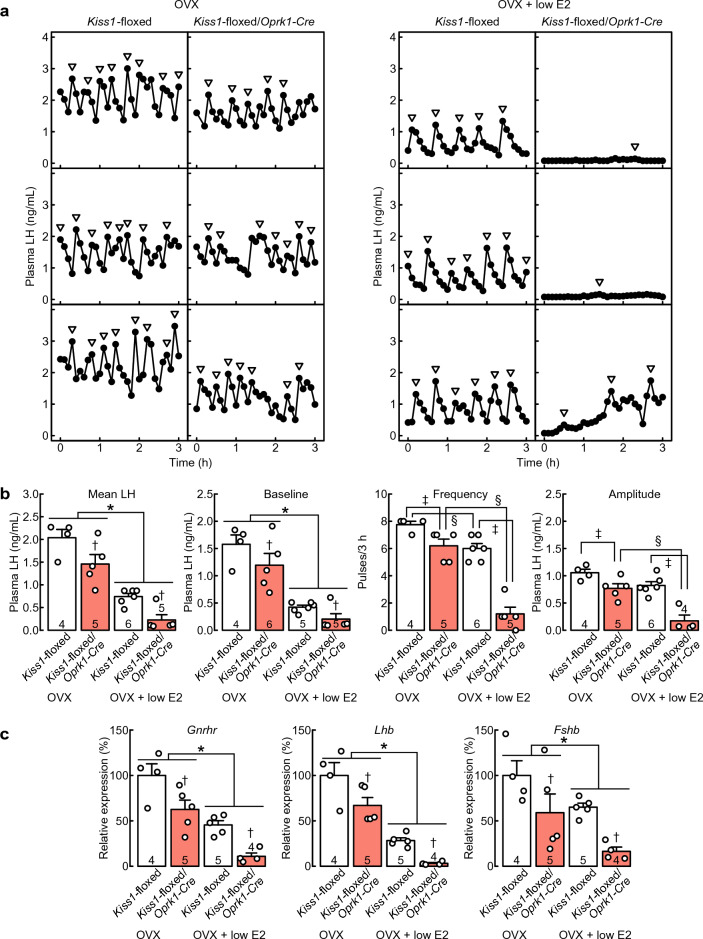
OVX Kiss1-floxed/Oprk1-Cre rats, whose Kiss1 was deleted in kisspeptin neurons expressing or having expressed Oprk1, without E2 treatment showed frequent LH pulses, whereas OVX + low E2 Kiss1-floxed/Oprk1-Cre rats exhibited profound disruption of LH pulses
Frequent LH pulses were found in all OVX Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats (Fig. 4a). Importantly, pulsatile profiles of LH release were profoundly disrupted in all individual OVX + low E2 Kiss1-floxed/Oprk1-Cre rats (Fig. 4a), whereas frequent LH pulses were found in all OVX + low E2 Cre(−)/Kiss1-floxed control rats (Fig. 4a). Two-way ANOVA revealed significant main effects of E2 treatment and the genotype, without the interaction effects between E2 treatment and the genotype on the mean LH concentration and the baseline of LH pulses (Fig. 4b). Specifically, the mean LH concentration and the baseline of LH pulses were significantly lower in OVX + low E2 rats than in OVX rats (E2 treatment as the main effect; mean LH, F(1,16) = 74.36, *, p < 0.01; baseline, F(1,16) = 59.58, *, p < 0.01) and were significantly lower in Kiss1-floxed/Oprk1-Cre rats than in Cre(−)/Kiss1-floxed control rats (genotype as the main effect; mean LH, F(1,16) = 13.96, †, p < 0.01; baseline, F(1,16) = 4.71, †, p = 0.045). Two-way ANOVA revealed significant main effects of E2 treatment and the genotype and significant interaction effects between E2 treatment and the genotype on the frequency (E2 treatment, F(1,16) = 62.21, p < 0.01; genotype, F(1,16) = 55.05, p < 0.01; E2 treatment × genotype, F(1,16) = 14.42, p < 0.01) and amplitude of LH pulses (E2 treatment, F(1,15) = 24.93, p < 0.01; genotype, F(1,15) = 31.80, p < 0.01; E2 treatment × genotype, F(1,15) = 4.76, p = 0.046). Specifically, as shown in Fig. 4b, E2 significantly suppressed the frequency of LH pulses in both Kiss1-floxed/Oprk1-Cre rats (§, p < 0.01) and Cre(−)/Kiss1-floxed control rats (§, p = 0.01) and the amplitude of LH pulses in Kiss1-floxed/Oprk1-Cre rats (§, p < 0.01). In addition, the frequency and amplitude of LH pulses were significantly lower in Kiss1-floxed/Oprk1-Cre rats than in Cre(−)/Kiss1-floxed control rats under OVX (frequency, ‡, p = 0.03; amplitude, ‡, p = 0.03) and OVX + low E2 (frequency, ‡, p < 0.01; amplitude, ‡, p < 0.01) conditions.
Figure 4.
OVX Kiss1-floxed/Oprk1-Cre rats, whose Kiss1 was deleted in kisspeptin neurons expressing or having expressed Oprk1, without E2 treatment showed frequent luteinizing hormone (LH) pulses, whereas OVX + low E2 Kiss1-floxed/Oprk1-Cre rats exhibited profound disruption of LH pulses. (a) Plasma LH profiles in three representative animals from the OVX or OVX + low E2 Kiss1-floxed/Oprk1-Cre group and Cre(−)/Kiss1-floxed control group. Arrowheads indicate LH pulses identified with the PULSAR computer program. (b) Mean LH concentrations and the baseline, frequency, and amplitude of LH pulses in OVX or OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats. (c) Pituitary Gnrhr, Lhb, and Fshb mRNA expression in OVX or OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats were determined by reverse transcription-quantitative PCR (RT-qPCR). Open circles indicate the individual data. Values are the means ± SEM. Numbers in (or on) each column indicate the number of animals used. Asterisks indicate statistically significant main effects of E2 treatment (p < 0.05) based on two-way ANOVA. Daggers indicate statistically significant main effects of the genotype (p < 0.05) based on two-way ANOVA. Double daggers indicate statistically significant differences between Kiss1-floxed/Oprk1-Cre and Cre(−)/Kiss1-floxed rats within the OVX or OVX + low E2 groups (p < 0.05, the simple main effect of two-way ANOVA). Section signs indicate statistically significant differences between OVX and OVX + low E2 rats within the Kiss1-floxed/Oprk1-Cre or Cre(−)/Kiss1-floxed groups (p < 0.05, the simple main effect of two-way ANOVA).
Figure 4c shows the relative Gnrhr, Lhb, and Fshb mRNA expression levels in the anterior pituitary gland in Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats. Two-way ANOVA revealed significant main effects of E2 treatment and the genotype, without the interaction effects between E2 treatment and the genotype on pituitary Gnrhr, Lhb, and Fshb mRNA expression (Fig. 4c). Specifically, Gnrhr, Lhb, and Fshb mRNA expression levels were significantly lower in OVX + low E2 rats than in OVX rats (E2 treatment as the main effect; Gnrhr, F(1,14) = 37.65, *, p < 0.01; Lhb, F(1,14) = 69.54, *, p < 0.01; Fshb, F(1,14) = 7.62, *, p = 0.015) and in Kiss1-floxed/Oprk1-Cre rats than in Cre(−)/Kiss1-floxed control rats (genotype as the main effect; Gnrhr, F(1,14) = 17.31, †, p < 0.01; Lhb, F(1,14) = 12.78, †, p < 0.01; Fshb, F(1,14) = 10.18, †, p < 0.01).
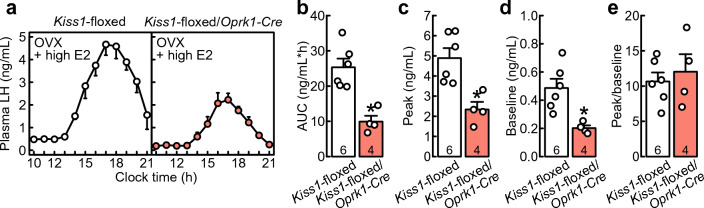
High E2 treatment induced a surge-like increase in LH in OVX Kiss1-floxed/Oprk1-Cre rats, but the LH surge was attenuated by the conditional Kiss1 deletion in cells expressing or having expressed Oprk1
Both OVX + high E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats showed an LH surge in the afternoon (Fig. 5a), while the area under the curve (AUC) of plasma LH levels in OVX + high E2 Kiss1-floxed/Oprk1-Cre rats was significantly lower than that in Cre(−)/Kiss1-floxed control rats (t(8) = 4.60, p < 0.01, Fig. 5b). The peak levels of LH surge (t(8) = 3.70, p < 0.01, Fig. 5c) and the baseline LH levels (t(8) = 3.44, p < 0.01, Fig. 5d) were significantly lower in OVX + high E2 Kiss1-floxed/Oprk1-Cre rats than in Cre(−)/Kiss1-floxed control rats. On the other hand, the ratios of the peak levels of LH surge to the baseline LH levels were comparable between the groups (t(8) = 0.54, p = 0.60, Student’s t-test, Fig. 5e).
Figure 5.
Treatment with a proestrous level of E2 (high E2) induced a surge-like increase in LH in OVX Kiss1-floxed/Oprk1-Cre rats, but the LH surge was attenuated by the conditional Kiss1 deletion in cells expressing or having expressed Oprk1. (a) Mean plasma LH profiles of OVX + high E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats. The area under the curve of the afternoon LH surge (b), the peak levels of the LH surge (c), the baseline LH levels (d), and the ratios of the peak levels of the LH surge to the baseline LH levels (e) in OVX + high E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats. Values are the means ± SEM. Numbers in each column indicate the number of animals used. An asterisk indicates a statistically significant difference (p < 0.05) between the groups based on Student’s t-test.
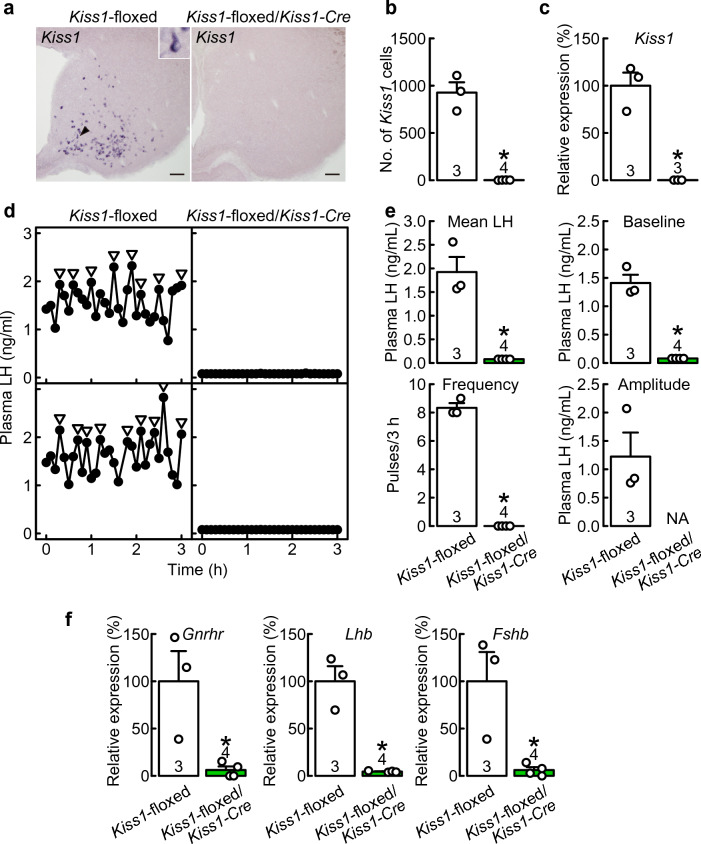
Kiss1-floxed/Kiss1-Cre rats exhibited few ARC Kiss1-expressing cells and complete suppression of LH pulses
OVX Kiss1-floxed/Kiss1-Cre rats exhibited few ARC Kiss1-expressing cells, whereas many Kiss1-expressing cells were detected in the ARC of Cre(−)/Kiss1-floxed control rats (Fig. 6a). The number of Kiss1-expressing cells was significantly lower in the ARC of Kiss1-floxed/Kiss1-Cre rats than in Cre(−)/Kiss1-floxed control rats (t(5) = 10.17, p < 0.01, Student’s t-test, Fig. 6b). The relative Kiss1 mRNA expression level in the ARC was significantly lower in OVX Kiss1-floxed/Kiss1-Cre rats (0.18 ± 0.005% of the control, p < 0.01, Student’s t-test, Fig. 6c) than in OVX Cre(−)/Kiss1-floxed control rats.
Figure 6.
Kiss1-floxed/Kiss1-Cre rats exhibited few ARC Kiss1-expressing cells and complete suppression of LH pulses. (a) Kiss1-expressing cells in the ARC of representative OVX Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed control rats. (b) The numbers of Kiss1-expressing cells in the ARC of OVX Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed control rats. (c) ARC Kiss1 mRNA expression in OVX Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed control rats. The ARC Kiss1 mRNA expression was determined by RT-qPCR. (d) Plasma LH profiles of two representative OVX Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed control rats. Arrowheads indicate LH pulses identified with the PULSAR computer program. (e) Mean LH concentrations and the baseline, frequency, and amplitude of LH pulses in OVX Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed rats. (f) Pituitary Gnrhr, Lhb, and Fshb mRNA expression in OVX Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed rats were determined by RT-qPCR. Values are the means ± SEM. Open circles indicate the individual data. Numbers in (or on) each column indicate the number of animals used. Asterisks indicate statistically significant differences (p < 0.05) between the groups based on Student’s t-test. NA, not applicable, as no LH pulses were detected in OVX Kiss1-floxed/Kiss1-Cre rats.
The plasma LH levels were undetectable, and no LH pulses were found in OVX Kiss1-floxed/Kiss1-Cre rats, whereas frequent LH pulses were found in OVX Cre(−)/Kiss1-floxed control rats (Fig. 6d). The mean LH concentration (t(5) = 6.91, p < 0.01, Student’s t-test) and the baseline (t(5) = 10.94, p < 0.01, Student’s t-test) and frequency of LH pulses (t(5) = 29.88, p < 0.01, Student’s t-test) were significantly lower in Kiss1-floxed/Kiss1-Cre rats than in Cre(−)/Kiss1-floxed control rats (Fig. 6e). Of note, the amplitude of LH pulses in Kiss1-floxed/Kiss1-Cre rats was not available because no LH pulses were detected in OVX Kiss1-floxed/Kiss1-Cre rats.
Figure 6f shows the relative Gnrhr, Lhb, and Fshb mRNA expression levels in the anterior pituitary gland in Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed control rats. Gnrhr (t(5) = 3.46, p = 0.017, Student’s t-test), Lhb (t(5) = 7.14, p < 0.01, Student’s t-test), and Fshb (t(5) = 3.60, p = 0.016, Student’s t-test) mRNA expression levels were significantly lower in Kiss1-floxed/Kiss1-Cre rats than in Cre(−)/Kiss1-floxed control rats (Fig. 6f).
Discussion
The present study demonstrated that Kiss1-floxed/Oprk1-Cre female rats, in which the Kiss1 gene was deleted in KNDy and AVPV kisspeptin neurons expressing or having expressed Oprk1, showed profound disruption of LH pulses in the presence of a diestrous level of E2 and a reduction in the AUC of the E2-induced LH surge. These results suggest that the Kiss1-deleted kisspeptin neurons in both the ARC and AVPV have a physiological role in maintaining normal reproductive function. Indeed, ovary-intact Kiss1-floxed/Oprk1-Cre female rats showed a 5-day estrous cycle (vs. a 4-day estrous cycle in Cre(−)/Kiss1-floxed control rats), a lower ovarian weight, and a smaller number of pups compared to Cre(−)/Kiss1-floxed controls. These findings suggest that Kiss1 expression in ARC and AVPV kisspeptin neurons, which are expressing or have expressed Oprk1, is likely needed to fully generate the GnRH/LH pulse and surge under physiological estrogen conditions. It is speculated that the estrogen-dependent suppression of LH pulses and LH surge attenuation may have caused a lower ovarian weight and a smaller number of pups in ovary-intact Kiss1-floxed/Oprk1-Cre female rats than in Cre(−)/Kiss1-floxed control rats. This is the first report to show that kisspeptin neurons receiving direct Dyn-KOR signaling are needed to fully generate the GnRH/LH pulse and estrogen-induced GnRH/LH surge in OVX female rats under E2 replacement regimens that mimic diestrous and proestrous levels of estrogen, respectively. Notably, the current ovary-intact Kiss1-floxed/Oprk1-Cre female rats may have maintained effective levels of LH pulses and surges to maintain ovarian function, estrous cycles and fertility even though a longer estrous cycle, a lower ovarian weight, and a smaller number of pups than the wild-type rats.
Importantly, apparent LH pulses were observed in the current OVX Kiss1-floxed/Oprk1-Cre rats without estrogen replacement, suggesting that the remaining small portion of KOR-negative KNDy neurons are sufficient to maintain GnRH/gonadotropin pulse generation, at least in the estrogen-free condition. This notion is consistent with our previous study showing that the rescue of 3–50% of ARC KNDy neurons resulted in the restoration of GnRH/LH pulses in OVX global Kiss1 KO rats6. Indeed, pituitary Gnrhr, Lhb, and Fshb mRNA expression levels, which largely correlate with GnRH/gonadotropin pulses, in OVX Kiss1-floxed/Oprk1-Cre rats seemed to be comparable to OVX Kiss1-floxed control rats treated with low E2. Therefore, we speculate that the remaining KOR-negative KNDy neurons may synchronize with "Kiss1-deficient NDy" neurons in Kiss1-floxed/Oprk1-Cre female rats via mutual contact among KNDy and "Kiss1-deficient NDy" neurons through gap junctions and/or glial connections. Indeed, our previous study showed that gap junction inhibitors blocked synchronized activities among mouse KNDy neurons in vitro38, and Moore et al.9 and Han et al.35 showed using in vivo Ca2+ imaging at a single-cell resolution that all recorded KNDy neurons exhibited synchronous activation prior to pulsatile LH release in female and male mice, respectively. Furthermore, Liu et al.39 showed that KNDy neurons can generate synchronized episodic activity even in Kiss1 KO mice. A recent study by Kauffman and colleagues showed that Kiss KORKO mice, in which KOR signaling in Kiss1 neurons was disrupted, exhibited apparent LH pulses in males, normal attainment of puberty in both sexes, and fertility in females32. In addition, previous studies showed that both sexes of global Oprk1 KO mice40–42 and global Pdyn KO mice43, 44 were fertile. In this context, other opioid receptor signaling pathways, such as enkephalin-δ-opioid receptor (DOR) and β-endorphin-μ-opioid receptor (MOR), may be able to compensate for the lack of KOR signaling in KNDy neurons to maintain GnRH pulse generation. In support of this notion, central DOR and MOR antagonism rescued frequent LH pulses in female rats under glucoprivation45, 46.
The low E2 treatment disrupted the LH pulse in the current OVX Kiss1-floxed/Oprk1-Cre rats. This could be simply due to estrogen-dependent suppression of ARC Kiss1 expression in remaining KOR-negative KNDy neurons, as the E2 treatment tended to reduce the number of ARC Kiss1-expressing cells in OVX Kiss1-floxed/Oprk1-Cre rats. Indeed, the diestrous level of E2 caused a reduction of Gnrhr, Lhb, and Fshb mRNA expression levels in the pituitary of OVX Kiss1-floxed/Oprk1-Cre rats. In addition, it is tempting to speculate that the estrogen-dependent LH pulse disruption in Kiss1-floxed/Oprk1-Cre rats may be due to estrogen-dependent activation of some inhibitory signaling pathway(s). In support of this, our previous studies showed that the suppression of LH pulses by fasting or glucoprivation was dependent on the negative feedback level of E2 in OVX rats47, 48 and that central DOR or MOR antagonism restored LH pulses in OVX + low E2 rats under glucoprivation45, 46. Further studies are required to identify the inhibitory signaling pathway(s) activated by the diestrous level of E2 to suppress GnRH/LH pulse generation in Kiss1-floxed/Oprk1-Cre rats.
The present study showed that Oprk1 expression in KNDy neurons increased after the peripubertal period, as Oprk1-driven Cre-activated tdTomato expression was found in approximately 20% of ARC Tac3 (a marker of KNDy neurons)-expressing cells at 3 weeks of age (prepubertal period) and 92% of ARC Tac3-expressing cells in adulthood. These findings suggest that Kiss1 may have been knocked out in only 20% of KNDy neurons in Kiss1-floxed/Oprk1-Cre female rats at the onset of puberty, which may explain why the timing of puberty onset in the current ovary-intact Kiss1-floxed/Oprk1-Cre female rats was comparable to that in Cre(−)/Kiss1-floxed control rats. It is speculated that after puberty onset, the Kiss1 gene may be gradually knocked out as Oprk1 expression gradually increases in KNDy neurons. Indeed, the findings in the current Oprk1-Cre-activated tdTomato reporter female rats revealed that approximately 90% of KNDy neurons, at least once (even transiently), expressed Oprk1 mRNA by adulthood. Thus, the Kiss1 gene was knocked out in a majority of KNDy neurons in adult Kiss1-floxed/Oprk1-Cre female rats, consequently resulting in a longer estrous cycle, smaller ovarian weight, and smaller litter size than those in Cre(−)/Kiss1-floxed control rats.
The current study suggests that AVPV kisspeptin neurons, which are expressing or have expressed Oprk1, are needed to generate a full amount of LH surge because the AUC of the high E2-induced LH surge in Kiss1-floxed/Oprk1-Cre rats was approximately half of that in Cre(−)/Kiss1-floxed control rats. The reduction in the LH surge could be simply due to the reduction in the number of AVPV Kiss1-expressing cells (approximately half of the control) in the current Kiss1-floxed/Oprk1-Cre rats, in which the ratio of Kiss1 KO in AVPV kisspeptin neurons is largely consistent with a previous study showing Oprk1 mRNA expression in 50% of AVPV kisspeptin neurons in female mice32. On the other hand, it is likely that KOR-negative AVPV kisspeptin neurons still function as the GnRH/LH surge generator because the Kiss1-floxed/Oprk1-Cre rats showed a surge-like increase in LH levels in the afternoon, and the ratios of the peak levels of LH surge to the baseline LH levels were comparable to those in Kiss1-floxed control rats. In support of this notion, previous studies showed that ~ 60% of AVPV kisspeptin neurons were activated at the proestrous and E2-induced LH surge in female rodents10, 12.
Our findings should be interpreted in the context of the following limitation. Kiss1 gene was deleted in > 95% of KNDy neurons and > 50% of AVPV kisspeptin neurons in Kiss1-floxed/Oprk1-Cre female rats, whereas ISH showed Oprk1 mRNA expression only in approximately 50% of KNDy neurons and 5% of AVPV kisspeptin neurons in Kiss1-floxed controls. In addition, Oprk1-Cre/tdTomato reporter rats showed many Cre-activated tdTomato-expressing cells, that did not coexpress Oprk1 mRNA, in the ARC, PVN, and SON. These inconsistencies may be due to the detection threshold of ISH for Oprk1. Namely, Oprk1 mRNA expression might be below the threshold for the current Oprk1 ISH in some Oprk1-expressing cells. Improvement of the methodology to detect Oprk1 mRNA may solve the inconsistency between the Oprk1 expression ratio in ARC and AVPV kisspeptin neurons and the Kiss1 KO rate in Kiss1-floxed/Oprk1-Cre female rats and between the number of Cre-activated tdTomato-expressing cells and Oprk1-expressing cells in Oprk1-Cre/tdTomato reporter rats.
In conclusion, the present study demonstrated that kisspeptin neurons, which are expressing and have expressed Oprk1, in both the ARC and AVPV had a role in maintaining GnRH pulse and surge generation in female rats because the current Kiss1-floxed/Oprk1-Cre female rats showed profound disruption of LH pulses in the presence of a diestrous level of E2 and a reduction in the peak of the E2-induced LH surge. This suggests that KNDy and AVPV kisspeptin neurons, which are expressing and have expressed Oprk1, seem to be needed for normal GnRH/LH pulse generation and for normal peak levels of the estrogen-induced GnRH/LH surge, respectively, to support a normal estrous cycle length and offspring number in ovary-intact female rats. Another important finding of the study is that Kiss1-floxed/Oprk1-Cre rats, in which Kiss1 was expressed only in KOR-negative kisspeptin neurons, were still fertile and maintained the GnRH/gonadotropin pulse-generating mechanism, especially under the steroid-free condition.
Methods
Animals
Oprk1-Cre/tdTomato reporter rats were generated by crossing newly generated Oprk1-Cre rats (see below for details) and tdTomato reporter rats [LE-Tg(Gt(ROSA)26Sor-CAG-tdTomato)24Jfhy rats37, supplied by the National BioResource Project—Rat, Kyoto University, Kyoto, Japan (NBRP Rat No. 0734) with support in part by the National BioResource Project of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Tokyo, Japan]. Kiss1-floxed/Oprk1-Cre rats were generated by crossing Oprk1-Cre rats and Kiss1-floxed rats (Rat Genome Database identification (RGD ID): 125097497)6. The resulting Kiss1-floxed/Oprk1-Cre (specifically, Kiss1fl/fl/Oprk1Cre+) female rats and Cre(−)/Kiss1-floxed (specifically, Kiss1fl/fl/Oprk1Cre-) female control rats were used. Kiss1-floxed/Kiss1-Cre rats were generated by crossing Kiss1-Cre rats36 and Kiss1-floxed rats. The resulting Kiss1-floxed/Kiss1-Cre (specifically, Kiss1fl/Cre+) female rats and Cre(−)/Kiss1-floxed (specifically, Kiss1fl/Cre-) female control rats were used. Wild-type Iar:Wistar-Imamichi male rats (RGD ID: 125097496, Institute for Animal Reproduction, Kasumigaura, Japan) were used for the mating test. All rats were maintained in a room with a 14:10-h light/dark cycle (lights on at 05:00 h) at 22 ± 3 °C and had free access to food (CE-2; CLEA Japan, Tokyo, Japan) and water. Female rats were housed by groups of 2–4 animals per cage (if not otherwise specified). Female rats with two consecutive 4- or 5-day estrous cycles, as determined by vaginal smears, were mated overnight with male rats on the day of the proestrus. The resultant pregnant females, as determined by the presence of vaginal plugs, were housed individually. The day when the vaginal plug was found in the morning was designated gestational Day 0, and the day when the newborn litter was found at noon was designated postnatal Day 0. Genotypes were analyzed by PCR with DNA obtained from newborn pups. The primers are listed in Supplementary Table S1 online. The litter size was recorded and adjusted to eight by postnatal Day 5 to minimize the growth variation within and between litters. The pups were weaned on postnatal Day 20 or 21. Animals were subjected to brain, pituitary, ovary, or blood collection at 8–13 weeks of age (adulthood) or 21 days of age (prepubertal period). If not otherwise specified, surgeries were conducted under aseptic conditions and anesthesia with an intraperitoneal injection of a ketamine (27 mg/kg)–xylazine (5.3 mg/kg) mixture, followed by inhalation of isoflurane (1–2% in air). After the surgery, female rats were housed individually. Care of the animals and all experimental procedures performed in the present study were reviewed and approved by the Animal Experiment Committees of Nagoya University and the National Institutes of Natural Sciences. This study was conducted in accordance with the Nagoya University Regulations on Animal Care and Use in Research and ARRIVE guidelines.
Gene targeting and generation of Oprk1-Cre rats
To generate Oprk1-Cre rats, a targeting vector was designed to replace the stop codon of the Oprk1 gene with the T2A-Cre sequence (see Supplementary Fig. S1 online). The 5’- and 3’-homology arms and T2A-Cre sequences were inserted into pAAV-MCS3. Production of adeno-associated virus (AAV) type 6 carrying the targeting vector was performed as described previously49. A guide RNA targeted around the stop codon of the rat Oprk1 gene (5′-TAAGCCAGTATGACTAGTCA-3′) was synthesized by and purchased from Integrated DNA Technologies (Coralville, IA). The CRISPR/Cas9 components were introduced by electroporation, and the AAV targeting vector was transfected at the pronuclear stage of rat zygotes collected from Crlj:WI rats (RGD ID: 2312504, Charles River Laboratories Japan, Yokohama, Japan), as described previously50. Two-cell stage embryos were transferred to pseudopregnant female rats under isoflurane anesthesia (2–3% in air). Genotypes of the obtained animals were confirmed by PCR and sequencing. The primers for PCR are listed in Supplementary Table S1 online.
Brain sampling from prepubertal and adult Oprk1-Cre/tdTomato reporter female rats
Adult female Oprk1-Cre/tdTomato reporter rats (n = 3, collected from 3 litters) were OVX and immediately received subcutaneous Silastic tubing (1.57 mm inner diameter; 3.18 mm outer diameter; 25 mm in length; Dow Corning, Midland, MI) filled with E2 (Sigma‒Aldrich, St. Louis, MO) dissolved in peanut oil (Sigma‒Aldrich) at 20 μg/ml for 1 week to produce a negative feedback level of plasma E2 (low E2)47. Ovary-intact prepubertal Oprk1-Cre/tdTomato reporter rats (n = 3, collected from 2 litters) and OVX + low E2 adult Oprk1-Cre/tdTomato reporter rats were deeply anesthetized with sodium pentobarbital (40 mg/kg, Tokyo Chemical Industry, Tokyo, Japan) and then intracardially perfused with phosphate-buffered saline, followed by 4% paraformaldehyde (Sigma‒Aldrich). The brains were immediately removed and postfixed in the same fixative overnight at 4 °C, followed by immersion in 30% sucrose in 0.05 M phosphate buffer until the brains sank at 4 °C. Frozen frontal sections containing the ARC (50-μm thickness) were prepared using a cryostat (CM1800, Leica Biosystems, Wetzlar, Germany). Every fourth ARC section was used for double ISH to visualize tdTomato/Oprk1 or Tac3/tdTomato. Tac3 was used as a marker of KNDy neurons because our previous study showed that Tac3 expression was evident in KNDy neurons before and after puberty onset in female rats51.
Analyses of body weight, puberty onset, and vaginal cyclicity and ovarian sampling from Kiss1-floxed/Oprk1-Cre and Kiss1-floxed/Kiss1-Cre female rats
The body weights of Kiss1-floxed/Oprk1-Cre rats (n = 17) and their counterpart Cre(−)/Kiss1-floxed rats (n = 13), as well as Kiss1-floxed/Kiss1-Cre rats (n = 7) and their counterpart Cre(−)/Kiss1-floxed rats (n = 4), were measured every morning after weaning. Female rats were collected from 2–8 litters. VO as a sign of pubertal onset and then the vaginal smear pattern were examined to monitor stages of the estrous cycle at least until 56 days of age. The estrous cycle length was determined for two consecutive estrous cycles of each individual and the mean length of each individual was calculated. Adult female rats were then OVX, and both ovaries were weighed.
Blood, pituitary, and brain sampling from OVX Kiss1-floxed/Oprk1-Cre and Kiss1-floxed/Kiss1-Cre rats
Two weeks after ovariectomy (as described above), OVX Kiss1-floxed/Oprk1-Cre rats (n = 5) and their counterpart Cre(−)/Kiss1-floxed rats (n = 4), as well as Kiss1-floxed/Kiss1-Cre rats (n = 4) and their counterpart Cre(−)/Kiss1-floxed rats (n = 3), were subjected to frequent blood sampling to determine LH pulses. A silicon cannula for blood sampling (inner diameter 0.5 mm and outer diameter 1.0 mm; Shin-Etsu Polymer, Tokyo, Japan) was inserted into the right atrium through the jugular vein 1 day before the onset of blood sampling. Blood samples (100 μL) were collected from freely moving conscious OVX rats every 6 min for 3 h (13:00 to 16:00 h), and plasma samples were collected after the centrifugation at 4 °C. An equivalent volume of rat red blood cells, which were taken from donor rats and diluted with heparinized saline, was replaced through the atrial catheter after each blood collection to keep the hematocrit constant. One day after blood sampling, the brains and anterior pituitary were collected. The brains were processed as described above. Frozen frontal sections containing the ARC (50-μm thickness) were prepared using a cryostat. Every fourth ARC section was used for ISH to visualize Kiss1 and Oprk1 in Kiss1-floxed/Oprk1-Cre rats or Kiss1 in Kiss1-floxed/Kiss1-Cre rats. The anterior pituitary was stored at − 80 °C for reverse transcription-quantitative PCR (RT-qPCR) analyses for Gnrhr, Lhb, and Fshb mRNA expression.
Blood, pituitary, and brain sampling from E2-treated OVX Kiss1-floxed/Oprk1-Cre rats
Kiss1-floxed/Oprk1-Cre rats (n = 5) and their counterpart Cre(−)/Kiss1-floxed control rats (n = 6) were OVX (as described above) and immediately received Silastic tubing filled with E2 dissolved in peanut oil at 20 μg/mL for 1 week to serve as OVX + low E2 rats. OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats were subjected to frequent blood sampling to determine LH pulses as described above. One day after the frequent blood sampling, the estrogen tubing was replaced with another tube containing E2 dissolved in peanut oil at 1000 μg/mL to produce a positive feedback level of plasma E2 (high E2)6, 52. Blood samples (100 μL) were collected from Kiss1-floxed/Oprk1-Cre rats (n = 4) and their counterpart Cre(−)/Kiss1-floxed control rats (n = 6) every 1 h from 10:00 to 21:00 h 2 days after the high E2 replacement to determine the afternoon LH surge. One day after the second blood sampling to detect the LH surge, the estrogen tubing was replaced with another tube containing E2 dissolved in peanut oil at 20 μg/mL (low E2). Estrogen tubing replacement was performed under isoflurane anesthesia (2–3% in air). One week later, the brains and anterior pituitary of Kiss1-floxed/Oprk1-Cre rats (n = 4) and their counterpart Cre(−)/Kiss1-floxed control rats (n = 5) were collected and processed as described above. Every fourth ARC section and every second AVPV section were used for double ISH to visualize Kiss1 and Oprk1.
Brain sampling from OVX Kiss1-floxed/Kiss1-Cre rats
Two weeks after ovariectomy (as described above), Kiss1-floxed/Kiss1-Cre rats (n = 3) and Cre(−)/Kiss1-floxed rats (n = 3) were decapitated between 13:00 h and 15:00 h. The ARC-median eminence region (approximately 1.8 and 4.0 mm posterior to the bregma) was dissected from the brain with a microblade as previously described53.
RT-qPCR analyses of ARC Kiss1 mRNA expression and pituitary Gnrhr, Lhb, and Fshb mRNA expression
Total RNA was extracted from the ARC using ISOGEN (Nippon Gene, Tokyo, Japan). Total RNA was extracted from the fixed hemipituitary by using the RNeasy FFPE kit (QIAGEN) as described previously6. cDNA from each sample was synthesized with oligo (deoxythymidine) primers at 37 °C using the high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Gene expression levels were determined using QuantStudio 3 (Applied Biosystems) with the Thunderbird SYBR Green qPCR Mix (TOYOBO, Osaka, Japan) as described previously38, 52. The forward and reverse primers for Kiss1, Gnrhr, Lhb, Fshb, and Actb (encoding β-actin) are listed in Supplementary Table S1 online. The specificity of the amplification products was confirmed by dissociation curve analysis. The relative gene expression levels of Kiss1, Gnrhr, Lhb, and Fshb were normalized to Actb, and the fold changes between the groups were calculated using the 2-ΔΔCT method.
Double ISH for tdTomato/Oprk1 or Tac3/tdTomato in the hypothalamus of Oprk1-Cre/tdTomato reporter rats and for Kiss1/Oprk1 in the hypothalamus of Kiss1-floxed/Oprk1-Cre female rats
Double ISH for tdTomato/Oprk1, Tac3/tdTomato, or Kiss1/Oprk1 was performed as described previously54, 55. Briefly, brain sections were hybridized overnight at 60 °C with a fluorescein isothiocyanate (FITC)-labeled antisense complementary RNA (cRNA) probe and a digoxigenin (DIG)-labeled antisense cRNA probe. The brain sections from Oprk1-Cre/tdTomato reporter rats were hybridized with a tdTomato-specific FITC-labeled cRNA probe (positions 610–1105, AY678269) and an Oprk1-specific DIG-labeled cDNA probe (positions 66–768 and 805–1956; NM_017167) or a Tac3-specific FITC-labeled cRNA probe (positions 180–304, NM_019162) and a tdTomato-specific DIG-labeled cRNA probe. The brain sections from Kiss1-floxed/Oprk1-Cre and their counterpart Cre(−)/Kiss1-floxed control rats were hybridized with a Kiss1-specific FITC-labeled antisense cRNA probe (positions 33–349, AY196983) and the Oprk1-specific DIG-labeled cDNA probe. The hybridized FITC-labeled probe was detected with a peroxidase (POD)-conjugated anti-FITC antibody (Roche Diagnostics, Indianapolis, IN, RRID: AB_840257) and the TSA Plus Fluorescein System (1:100; Akoya Biosciences, Marlborough, MA), and the hybridized DIG-labeled probe was detected with a POD-conjugated anti-DIG antibody (Roche Diagnostics, RRID: AB_514500), the TSA Plus Biotin Kit (1:100; Akoya Biosciences), and DyLight 594-conjugated streptavidin (Thermo Fisher Scientific, Waltham, MA). The fluorescent images were examined under a fluorescence microscope with ApoTome.2 optical sectioning (Carl Zeiss, Oberkochen, Germany).
The numbers of Oprk1- and/or tdTomato-expressing cells in the brain sections that underwent double ISH for tdTomato/Oprk1 were counted unilaterally in the ARC (from 1.72 to 4.36 mm posterior to the bregma), PVN (from 0.96 to 1.92 mm posterior to the bregma), and SON (from 1.20 to 1.80 mm posterior to the bregma) according to the rat brain atlas56. The numbers of Tac3-expressing and Tac3- and tdTomato-coexpressing cells were counted unilaterally in the ARC. The numbers of Kiss1-expressing and Kiss1- and Oprk1-coexpressing cells in the brain sections from OVX + low E2 Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats were counted unilaterally in the ARC and AVPV (from 0.12 mm anterior to 0.60 mm posterior to the bregma). The numbers of Kiss1-expressing and Kiss1- and Oprk1-coexpressing cells in the brain sections from OVX Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats were counted unilaterally in the ARC.
The specificity of antisense cRNA probes was verified by control experiments using sense cRNA probes. No signals were found in the sections incubated with the sense cRNA probes.
ISH for Kiss1 expression in the ARC of OVX Kiss1-floxed/Kiss1-Cre rats
Single ISH for Kiss1 was performed as described previously10. Briefly, brain sections were hybridized overnight at 60 °C with a Kiss1-specific DIG-labeled antisense cRNA probe (positions 33–349, AY196983). The hybridized probe was detected with an alkaline phosphatase-conjugated anti-DIG antibody (1:1,000; Roche Diagnostics, RRID: AB_2734716) and a chromogen solution (337 μg/mL 4-nitro blue tetrazolium chloride and 175 μg/mL 5-bromo-4-chloro-3-indolyl-phosphate, Roche Diagnostics). The Kiss1-expressing cells throughout the ARC were unilaterally counted under a light microscope, BX53 (Olympus, Tokyo, Japan).
Radioimmunoassay (RIA) and analyses of LH pulse and surge parameters
Plasma LH concentrations were determined by a double-antibody RIA with a rat LH-RIA kit provided by the National Hormone and Peptide Program. The concentrations were expressed in terms of rat LH-RP3. The lowest detectable level in the LH assay was 3.9 pg/tube, and the intra- and interassay coefficients of variation were 4.76% and 7.36% at 34 pg/tube, respectively. LH pulses were identified by the PULSAR computer program57, 58. LH pulse parameters, such as the mean LH concentration and the baseline, frequency, and amplitude of LH pulses, were calculated during the 3-h sampling period for each individual and then for the group. For the E2-induced LH surge, LH surge parameters, such as the AUC (from 13:00 to 21:00 h), the peak levels of the LH surge (the highest plasma LH level in the afternoon), the baseline LH levels (mean plasma LH levels at 10:00, 11:00, and 12:00 h), and the ratio of the peak levels of the LH surge to the baseline LH levels were calculated for each individual and then for the group.
Statistical analysis
Statistical differences in the numbers of ARC Tac3-expressing cells and Tac3- and tdTomato-coexpressing cells between prepubertal and adult Oprk1-Cre/tdTomato reporter rats were determined by Student’s t-test. Statistical differences in ovarian weights, litter sizes, the number of Kiss1-expressing cells in the AVPV, and LH surge parameters between Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed rats were determined by Student’s t-test. Statistical differences in ovarian weights, the number of Kiss1-expressing cells in the ARC, ARC Kiss1 expression levels, LH pulse parameters, and pituitary Gnrhr, Lhb, and Fshb expression levels between Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed rats were determined by Student’s t-test. Statistical differences in the timing of VO between Kiss1-floxed/Oprk1-Cre rats or Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed rats were determined by Kaplan‒Meier analysis and the log-rank test. Statistical differences in the lengths of estrous cycles were determined by the Wilcoxon rank sum test. The statistical analyses described above were performed using R version 3.4.2 (https://www.R-project.org/). Statistical differences in body weights between Kiss1-floxed/Oprk1-Cre or Kiss1-floxed/Kiss1-Cre rats and Cre(−)/Kiss1-floxed rats were determined by two-way ANOVA for repeated measures (main effects, groups and days), followed by analyses of simple main effects. Statistical differences in the number of Kiss1-expressing cells in the ARC, LH pulse parameters, and pituitary Gnrhr, Lhb, and Fshb expression levels between OVX Kiss1-floxed/Oprk1-Cre rats and Cre(−)/Kiss1-floxed control rats with/without low E2 treatment were determined by two-way ANOVA (main effects, genotype and E2 treatment), followed by analyses of simple main effects. Two-way ANOVA was performed using SAS OnDemand for Academics (https://welcome.oda.sas.com). Differences were considered statistically significant at p < 0.05.
Supplementary Information
Acknowledgements
We are grateful to the National BioResource Project—Rat (http://www.anim.med.kyoto-u.ac.jp/NBR/) for providing rat strains (LE-Tg(Gt(ROSA)26Sor-CAG-tdTomato)24Jfhy rats NBRP Rat No. 0734). The authors are grateful to the National Hormone and Peptide Program and Dr. AF Parlow for providing the RIA kit. The RIA was performed at the Nagoya University Radioisotope Research Center. We are also grateful to Drs. Toshihiro Kobayashi and Naoaki Mizuno (The University of Tokyo) for their expertise in CRISPR/Cas9-based genome editing. This work was supported in part by the Japan Society for the Promotion of Science KAKENHI grant numbers JP21H05031 and JP21K19186 (to H. Tsukamura), JP19H03103 and JP23H02362 (to N.I.), JP20H03127 and JP22K19245 (to Y.U.), and JP22J10728 and JP22KJ1548 (to M.Nagae), and the Cooperative Study Program (No. 19-256) of the National Institute for Physiological Sciences (to H. Tsukamura). This study was also supported in part by the "Graduate Program of Transformative Chem-Bio Research in Nagoya University"—supported by MEXT (WISE Program) (to M. Nagae and H. Tsuchida), “Nagoya University Interdisciplinary Frontier Fellowship”—supported by Nagoya University and JST, the establishment of university fellowships toward the creation of science technology innovation, Grant Number JPMJFS2120 (to K.Y.), and the Mizutani Scholarship (Graduate School of Bioagricultural Sciences, Nagoya University) (to M. Nagae).
Author contributions
M.Nagae, K.Y., Y.E., N.I., H.Tsukamura, and Y.U. conceived the experiments. M.Nagae, K.Y., Y.E., M.K., H.Tsuchida, A.P., M.Nonogaki, N.M., M.T., S.M., M.H., H.Tsukamura, and Y.U. conducted the experiments. M.Nagae, Y.E., H.Tsukamura, and Y.U. analyzed the results. M.Nagae, H.Tsukamura, and Y.U. wrote the manuscript. All authors reviewed the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hiroko Tsukamura, Email: htsukamura@nagoya-u.jp.
Yoshihisa Uenoyama, Email: uenoyama@nagoya-u.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-47222-5.
References
- 1.Uenoyama Y, Inoue N, Nakamura S, Tsukamura H. Kisspeptin neurons and estrogen-estrogen receptor α signaling: Unraveling the mystery of steroid feedback system regulating mammalian reproduction. Int. J. Mol. Sci. 2021;22:9229. doi: 10.3390/ijms22179229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uenoyama Y, Nagae M, Tsuchida H, Inoue N, Tsukamura H. Role of KNDy neurons expressing kisspeptin, neurokinin B, and dynorphin A as a GnRH pulse generator controlling mammalian reproduction. Front Endocrinol. (Lausanne) 2021;12:724632. doi: 10.3389/fendo.2021.724632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman RL, Herbison AE, Lehman MN, Navarro VM. Neuroendocrine control of gonadotropin-releasing hormone: Pulsatile and surge modes of secretion. J. Neuroendocrinol. 2022;34:e13094. doi: 10.1111/jne.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsukamura H. Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen. Comp. Endocrinol. 2022;315:113755. doi: 10.1016/j.ygcen.2021.113755. [DOI] [PubMed] [Google Scholar]
- 5.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science. 1978;202:631–633. doi: 10.1126/science.100883. [DOI] [PubMed] [Google Scholar]
- 6.Nagae M, et al. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc. Natl. Acad. Sci. USA. 2021;118:e2009156118. doi: 10.1073/pnas.2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarkson J, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc. Natl. Acad. Sci. USA. 2017;114:E10216–E10223. doi: 10.1073/pnas.1713897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McQuillan HJ, et al. Definition of the estrogen negative feedback pathway controlling the GnRH pulse generator in female mice. Nat. Commun. 2022;13:7433. doi: 10.1038/s41467-022-35243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore AM, Coolen LM, Lehman MN. In vivo imaging of the GnRH pulse generator reveals a temporal order of neuronal activation and synchronization during each pulse. Proc. Natl. Acad. Sci. USA. 2022;119:e2117767119. doi: 10.1073/pnas.2117767119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi S, et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 2007;53:367–378. doi: 10.1262/jrd.18146. [DOI] [PubMed] [Google Scholar]
- 11.Kauffman AS, et al. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology. 2007;148:1774–1783. doi: 10.1210/en.2006-1540. [DOI] [PubMed] [Google Scholar]
- 12.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- 14.Clarkson J, d'Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: Implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150:3664–3671. doi: 10.1210/en.2009-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. J. Neuroendocrinol. 2009;21:305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- 17.Tsukamura H, Maeda K-I, Uenoyama Y. Fetal/perinatal programming causing sexual dimorphism of the kisspeptin–GnRH neuronal network. In: Herbison AE, Plant TM, editors. The GnRH Neuron and its Control Masterclass in Neuroendocrinology. Wiley; 2018. pp. 43–60. [Google Scholar]
- 18.Tsukamura H, Homma T, Tomikawa J, Uenoyama Y, Maeda K-I. Sexual differentiation of kisspeptin neurons responsible for sex difference in gonadotropin release in rats. Ann. N. Y. Acad. Sci. 2010;1200:95–103. doi: 10.1111/j.1749-6632.2010.05645.x. [DOI] [PubMed] [Google Scholar]
- 19.Homma T, et al. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol. Reprod. 2009;81:1216–1225. doi: 10.1095/biolreprod.109.078311. [DOI] [PubMed] [Google Scholar]
- 20.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31:147–157. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita M, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 22.Pineda R, et al. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151:722–730. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- 23.Amstalden M, et al. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: Colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2010;22:1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weems PW, et al. Kappa opioid receptor is co-localized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157:2367–2379. doi: 10.1210/en.2015-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsuchida H, et al. Paraventricular dynorphin A neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology. 2020;161:bqaa161. doi: 10.1210/endocr/bqaa161. [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi Y, et al. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J. Neurosci. 2010;30:3124–3132. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154:2761–2771. doi: 10.1210/en.2013-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154:2750–2760. doi: 10.1210/en.2013-1231. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki T, et al. Peripheral administration of κ-opioid receptor antagonist stimulates gonadotropin-releasing hormone pulse generator activity in ovariectomized, estrogen-treated female goats. Domest. Anim. Endocrinol. 2019;68:83–91. doi: 10.1016/j.domaniend.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Goodman RL, et al. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154:4259–4269. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navarro VM, et al. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coutinho EA, et al. Conditional deletion of KOR (Oprk1) in kisspeptin cells does not alter LH pulses, puberty, or fertility in mice. Endocrinology. 2022;163:bqac175. doi: 10.1210/endocr/bqac175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro VM, et al. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J. Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uenoyama Y, Tsuchida H, Nagae M, Inoue N, Tsukamura H. Opioidergic pathways and kisspeptin in the regulation of female reproduction in mammals. Front. Neurosci. 2022;16:958377. doi: 10.3389/fnins.2022.958377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SY, et al. Mechanism of kisspeptin neuron synchronization for pulsatile hormone secretion in male mice. Cell Rep. 2023;42:111914. doi: 10.1016/j.celrep.2022.111914. [DOI] [PubMed] [Google Scholar]
- 36.Yamada K, et al. Sex difference in developmental changes in visualized Kiss1 neurons in newly generated Kiss1-Cre rats. J. Reprod. Dev. 2023;69:227–238. doi: 10.1262/jrd.2023-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Igarashi H, et al. A novel reporter rat strain that conditionally expresses the bright red fluorescent protein tdTomato. PLoS One. 2016;11:e0155687. doi: 10.1371/journal.pone.0155687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikegami K, et al. Evidence of involvement of neurone-glia/neurone-neurone communications via gap junctions in synchronised activity of KNDy neurones. J. Neuroendocrinol. 2017;29:1–14. doi: 10.1111/jne.12480. [DOI] [PubMed] [Google Scholar]
- 39.Liu X, et al. Highly redundant neuropeptide volume co-transmission underlying episodic activation of the GnRH neuron dendron. Elife. 2021 doi: 10.7554/eLife.62455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonin F, et al. Disruption of the κ-opioid receptor gene in mice enhances sensitivity to chemical visceral pain, impairs pharmacological actions of the selective κ-agonist U-50,488H and attenuates morphine withdrawal. EMBO J. 1998;17:886–897. doi: 10.1093/emboj/17.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hough LB, et al. Improgan, a cimetidine analog, induces morphine-like antinociception in opioid receptor-knockout mice. Brain Res. 2000;880:102–108. doi: 10.1016/s0006-8993(00)02776-1. [DOI] [PubMed] [Google Scholar]
- 42.Ansonoff MA, et al. Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of κ-opioid receptor-1 knockout mice. J. Pharmacol. Exp. Ther. 2006;318:641–648. doi: 10.1124/jpet.106.101998. [DOI] [PubMed] [Google Scholar]
- 43.Sharifi N, Diehl N, Yaswen L, Brennan MB, Hochgeschwender U. Generation of dynorphin knockout mice. Brain Res. Mol. Brain Res. 2001;86:70–75. doi: 10.1016/s0169-328x(00)00264-3. [DOI] [PubMed] [Google Scholar]
- 44.Loacker S, Sayyah M, Wittmann W, Herzog H, Schwarzer C. Endogenous dynorphin in epileptogenesis and epilepsy: anticonvulsant net effect via kappa opioid receptors. Brain. 2007;130:1017–1028. doi: 10.1093/brain/awl384. [DOI] [PubMed] [Google Scholar]
- 45.Tsuchida H, et al. Central μ-opioid receptor antagonism blocks glucoprivic LH pulse suppression and gluconeogenesis/feeding in female rats. Endocrinology. 2021;162:bqab140. doi: 10.1210/endocr/bqab140. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchida H, et al. Enkephalin-δ opioid receptor signaling mediates glucoprivic suppression of LH pulse and gluconeogenesis in female rats. Endocrinology. 2023;164:bqac216. doi: 10.1210/endocr/bqac216. [DOI] [PubMed] [Google Scholar]
- 47.Cagampang FR, Maeda K-I, Tsukamura H, Ohkura S, Ota K. Involvement of ovarian steroids and endogenous opioids in the fasting-induced suppression of pulsatile LH release in ovariectomized rats. J. Endocrinol. 1991;129:321–328. doi: 10.1677/joe.0.1290321. [DOI] [PubMed] [Google Scholar]
- 48.Nagatani S, et al. Reduction of glucose availability suppresses pulsatile luteinizing hormone release in female and male rats. Endocrinology. 1996;137:1166–1170. doi: 10.1210/endo.137.4.8625885. [DOI] [PubMed] [Google Scholar]
- 49.Mizuno N, et al. Intra-embryo gene cassette knockin by CRISPR/Cas9-mediated genome editing with adeno-associated viral vector. iScience. 2018;9:286–297. doi: 10.1016/j.isci.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oikawa M, et al. Generation of Tfap2c-T2A-tdTomato knock-in reporter rats via adeno-associated virus-mediated efficient gene targeting. Mol. Reprod. Dev. 2022;89:129–132. doi: 10.1002/mrd.23562. [DOI] [PubMed] [Google Scholar]
- 51.Majarune S, et al. Ad libitum feeding triggers puberty onset associated with increases in arcuate Kiss1 and Pdyn expression in growth-retarded rats. J. Reprod. Dev. 2019;65:397–406. doi: 10.1262/jrd.2019-048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horihata K, Inoue N, Uenoyama Y, Maeda K-I, Tsukamura H. Retinoblastoma binding protein 7 is involved in Kiss1 mRNA upregulation in rodents. J. Reprod. Dev. 2020;66:125–133. doi: 10.1262/jrd.2019-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takase K, et al. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. J. Neuroendocrinol. 2009;21:527–537. doi: 10.1111/j.1365-2826.2009.01868.x. [DOI] [PubMed] [Google Scholar]
- 54.Assadullah, et al. Co-expression of the calcitonin receptor gene in the hypothalamic kisspeptin neurons in female rats. Reprod. Med. Biol. 2018;17:164–172. doi: 10.1002/rmb2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ieda N, et al. GnRH(1–5), a metabolite of gonadotropin-releasing hormone, enhances luteinizing hormone release via activation of kisspeptin neurons in female rats. Endocr. J. 2020;67:409–418. doi: 10.1507/endocrj.EJ19-0444. [DOI] [PubMed] [Google Scholar]
- 56.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6. Academic Press; 2008. [DOI] [PubMed] [Google Scholar]
- 57.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am. J. Physiol. 1982;243:E310–318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 58.Uenoyama Y, et al. Lack of pulse and surge modes and glutamatergic stimulation of LH release in Kiss1 knockout rats. J Neuroendocrinol. 2015;27:187–197. doi: 10.1111/jne.12257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding authors on reasonable request.