Abstract
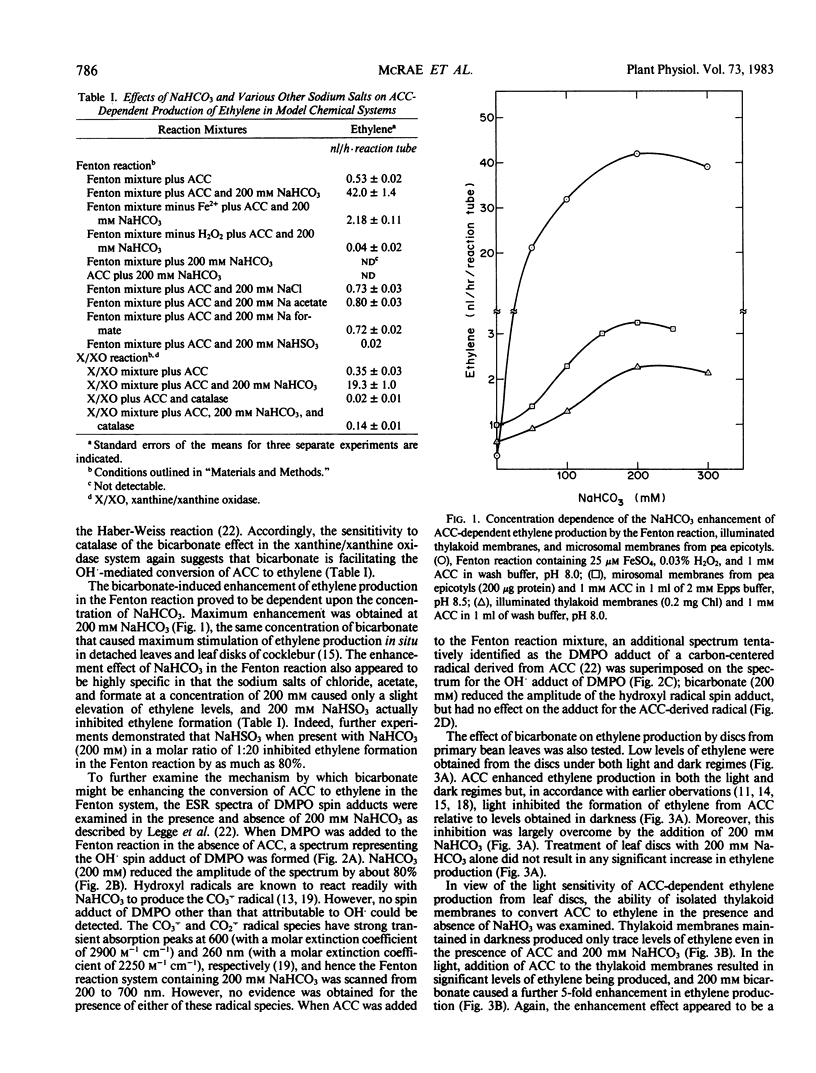
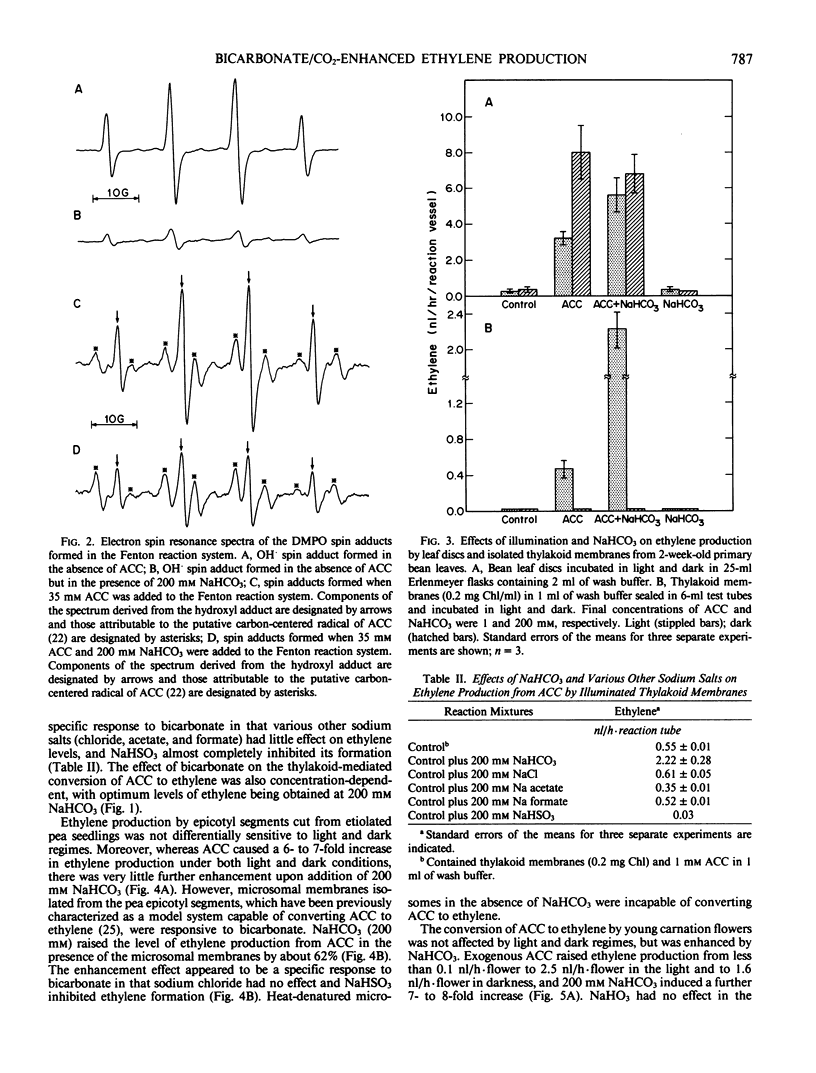
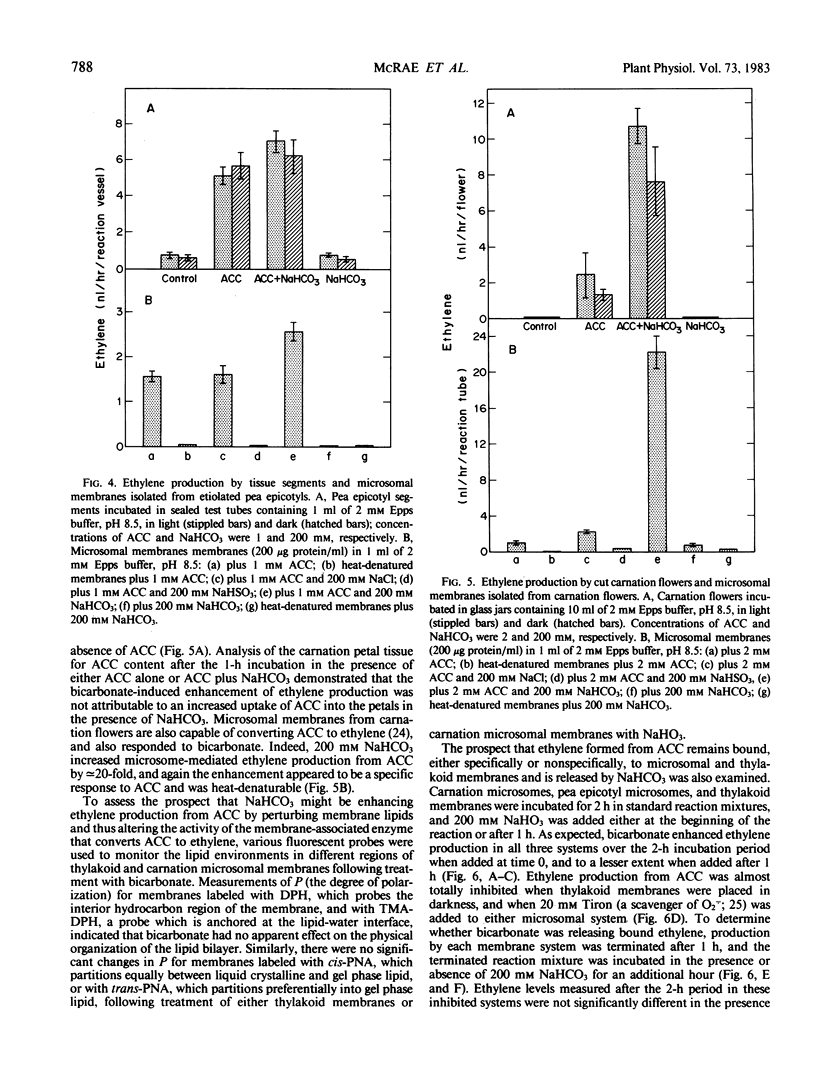
Bicarbonate markedly enhances ethylene production from 1-aminocyclopropane-1-carboxylic acid (ACC) in model chemical systems where the conversion is free radical-mediated, in thylakoid membrane suspensions of Phaseolus vulgaris L. cv Kinghorn where the reaction is light-dependent, and in microsomal membrane suspensions and intact tissues where the reaction is enzymically mediated. In two model systems generating free radicals—the Fenton reaction and a reaction mixture containing xanthine/xanthine oxidase, NaHCO3 (200 millimolar) increased the formation of ethylene from ACC by 84-fold and 54-fold, respectively. Isolated thylakoid membranes also proved capable of ACC-dependent ethylene production, but only upon illumination, and this too was enhanced by added NaHCO3. As well, light-induced inhibition of ACC-dependent ethylene production by leaf discs was relieved by adding 200 millimolar NaHCO3. Finally, NaHCO3 (200 millimolar) augmented ACC-dependent ethylene production from young carnation flowers by about 4-fold, and the conversions of ACC to ethylene by microsomes isolated from carnation flowers and etiolated pea epicotyls were higher by 1900 and 62%, respectively, in the presence of 200 millimolar NaHCO3.
This increased production of ethylene appears not to be due to bicarbonate or CO2-induced release of the gas from putative receptor sites, since the addition of NaHCO3 to sealed reaction mixtures after the ACC to ethylene conversion had been terminated had no effect. Spin-trapping studies have confirmed that bicarbonate does not facilitate the formation of free radicals thought to be involved in the conversion of ACC to ethylene. Nor did bicarbonate alter the physical properties of the membrane bilayer, which might indirectly modulate the activity of the membrane-associated enzyme capable of converting ACC to ethylene. Rather, bicarbonate appears to directly facilitate the conversion of ACC to ethylene, and the data are consistent with the view that CO2 derived from bicarbonate is the active molecular species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni N., Anderson J. D., Lieberman M. Production and action of ethylene in senescing leaf discs: effect of indoleacetic Acid, kinetin, silver ion, and carbon dioxide. Plant Physiol. 1979 Nov;64(5):805–809. doi: 10.1104/pp.64.5.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni N., Lieberman M. Ethylene as a regulator of senescence in tobacco leaf discs. Plant Physiol. 1979 Nov;64(5):801–804. doi: 10.1104/pp.64.5.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M., Anderson J. D. Inhibition of ethylene production in fruit slices by a rhizobitoxine analog and free radical scavengers. Plant Physiol. 1978 Jun;61(6):886–888. doi: 10.1104/pp.61.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. Effect of carbon dioxide and light on ethylene production in intact sunflower plants. Plant Physiol. 1982 May;69(5):1222–1225. doi: 10.1104/pp.69.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Oberley L. W. Considerations in the spin trapping of superoxide and hydroxyl radical in aqueous systems using 5,5-dimethyl-1-pyrroline-1-oxide. Biochem Biophys Res Commun. 1978 Jul 14;83(1):69–74. doi: 10.1016/0006-291x(78)90398-4. [DOI] [PubMed] [Google Scholar]
- Dhawan K. R., Bassi P. K., Spencer M. S. Effects of carbon dioxide on ethylene production and action in intact sunflower plants. Plant Physiol. 1981 Oct;68(4):831–834. doi: 10.1104/pp.68.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gepstein S., Thimann K. V. The role of ethylene in the senescence of oat leaves. Plant Physiol. 1981 Aug;68(2):349–354. doi: 10.1104/pp.68.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. The chloroplast at work. A review of modern developments in our understanding of chloroplast metabolism. Prog Biophys Mol Biol. 1978;33(1):1–54. doi: 10.1016/0079-6107(79)90024-5. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Yang S. F., Ichihara A., Sakamura S. Stereospecific conversion of 1-aminocyclopropanecarboxylic Acid to ethylene by plant tissues : conversion of stereoisomers of 1-amino-2-ethylcyclopropanecarboxylic Acid to 1-butene. Plant Physiol. 1982 Jul;70(1):195–199. doi: 10.1104/pp.70.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Seki H., Ilan Y. A., Ilan Y., Stein G. Reactions of the ferri-ferrocytochrome-c system with superoxide/oxygen and CO2-/CO2 studied by fast pulse radiolysis. Biochim Biophys Acta. 1976 Sep 13;440(3):573–586. doi: 10.1016/0005-2728(76)90043-8. [DOI] [PubMed] [Google Scholar]
- Sisler E. C. Measurement of ethylene binding in plant tissue. Plant Physiol. 1979 Oct;64(4):538–542. doi: 10.1104/pp.64.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisler E. C. Partial Purification of an Ethylene-binding Component from Plant Tissue. Plant Physiol. 1980 Sep;66(3):404–406. doi: 10.1104/pp.66.3.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. E., Mayak S., Shinitzky M., Halevy A. H. Acceleration of membrane senescence in cut carnation flowers by treatment with ethylene. Plant Physiol. 1982 Apr;69(4):859–863. doi: 10.1104/pp.69.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]


