Abstract
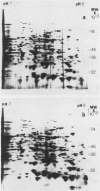
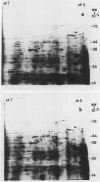
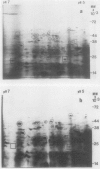
Applications of auxin to the tips of intact aged pea Pisum sativum L. var Alaska epicotyls resulted in an increase in the content of polyribosomes and poly(A) and in the capacity of isolated polysomes to support protein synthesis in vitro. Few changes were seen in the two-dimensional gel patterns of silver-stained proteins accumulated (or degraded) in vivo even after 15 hours of auxin treatment. In contrast, substantial changes were evident in the two-dimensional gel fluorographs of polypeptides generated in vitro by total RNA and by polysomal RNA from tissue treated with auxin for only 6 hours. Of the 200 spots resolved by fluorography, total RNA from auxin-treated tissue generated 33 spots with increased intensity and 10 with decreased intensity; polysomal RNA yielded 33 spots which increased and only three that decreased. In general, the polypeptides that increased in intensity were higher molecular weight and those that decreased were lower molecular weight. These changes occurred prior to growth and might be prerequisite for the auxin-induced slow growth response seen in this aged tissue.
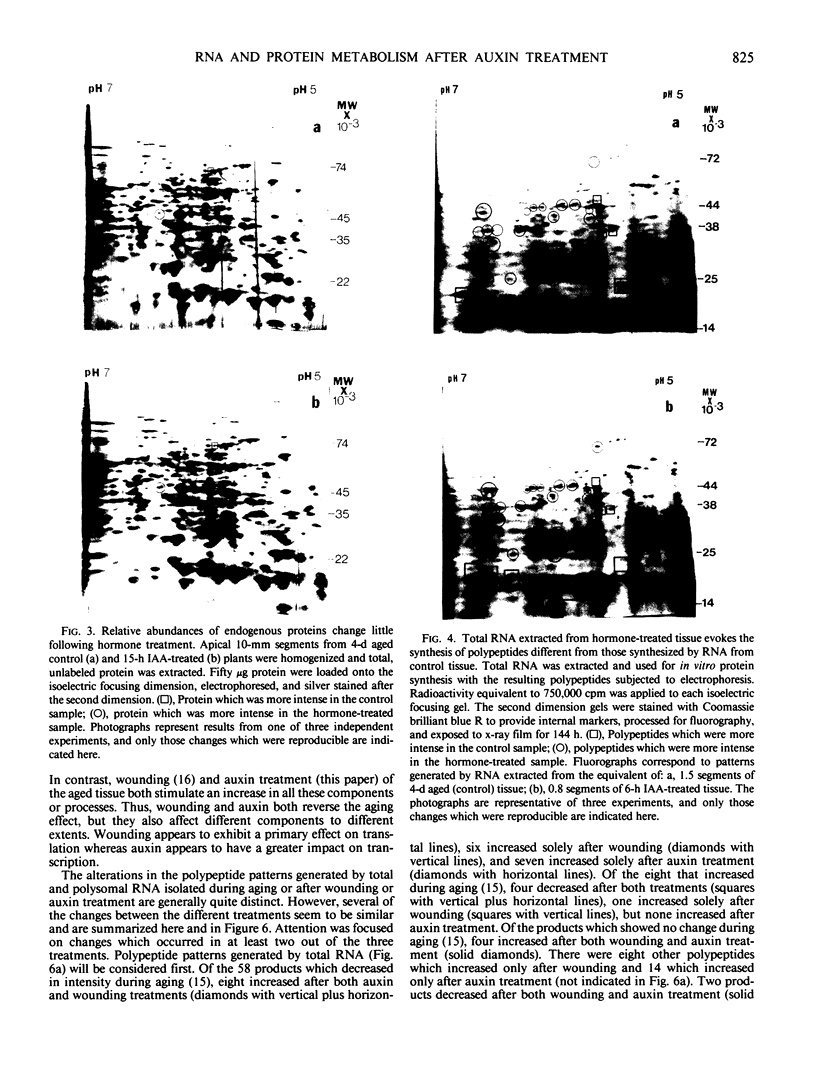
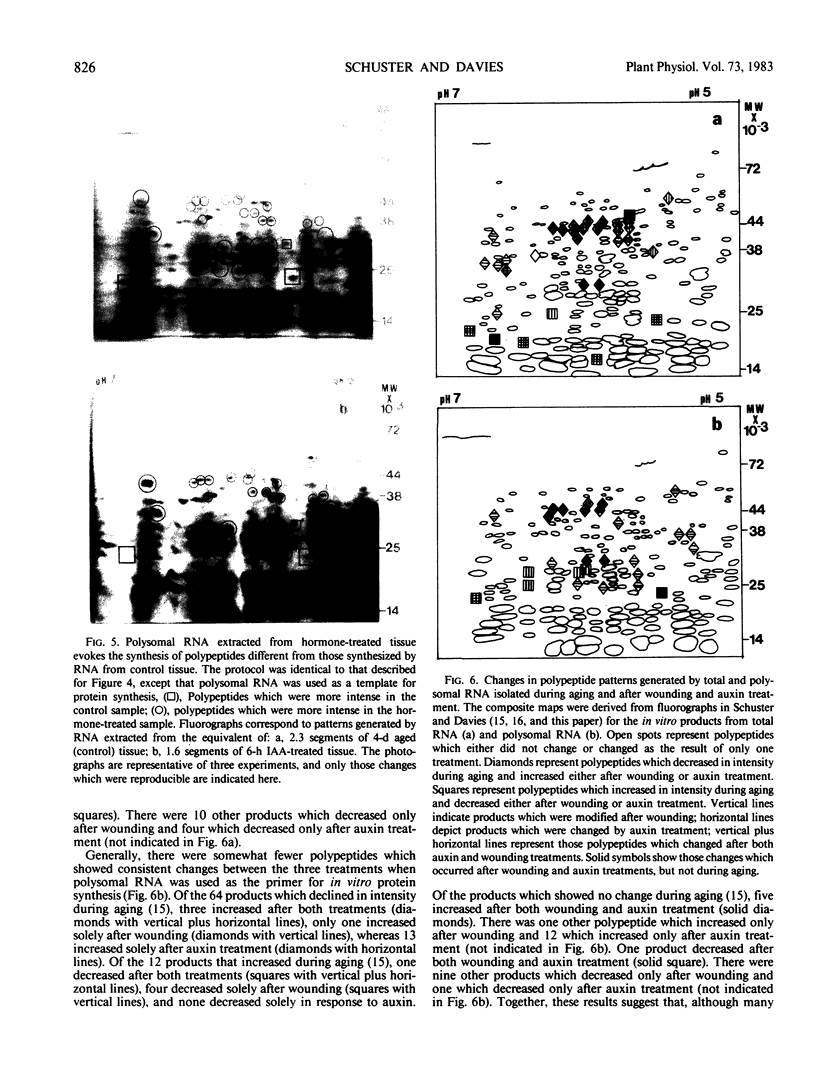
Comparisons were made between the changes in RNA and protein metabolism occuring during aging and after wounding and auxin treatment of aged tissue. Aging causes a decline in poly(A), polysomes, and protein synthesizing capacity, whereas wounding and auxin treatment cause increases. Wounding appears to act primarily at the level of translation, whereas auxin has a greater effect on transcription. It is argued that the use of excised tissue to study auxin effects on RNA and protein metabolism should be avoided.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davies E., Larkins B. A. Polyribosomes from Peas: II. Polyribosome Metabolism during Normal and Hormone-induced Growth. Plant Physiol. 1973 Oct;52(4):339–345. doi: 10.1104/pp.52.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Maclachlan G. A. Effects of indoleacetic acid on intracellular distribution of beta-glucanase activities in the pea epicotyl. Arch Biochem Biophys. 1968 Dec;128(3):595–600. doi: 10.1016/0003-9861(68)90068-4. [DOI] [PubMed] [Google Scholar]
- Davies E. Polyribosomes from Peas: VI. Auxin-stimulated Recruitment of Free Monosomes into Membrane-bound Polysomes. Plant Physiol. 1976 Apr;57(4):516–518. doi: 10.1104/pp.57.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins B. A., Davies E. Polyribosomes from Peas: V. An Attempt to Characterize the Total Free and Membrane-bound Polysomal Population. Plant Physiol. 1975 Apr;55(4):749–756. doi: 10.1104/pp.55.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. EVIDENCE FOR A REQUIREMENT FOR PROTEIN SYNTHESIS FOR AUXIN-INDUCED CELL ENLARGEMENT. Proc Natl Acad Sci U S A. 1963 Aug;50(2):194–200. doi: 10.1073/pnas.50.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noodén L. D., Thimann K. V. Inhibition of protein synthesis and of auxin-induced growth by chloramphenicol. Plant Physiol. 1965 Jan;40(1):193–201. doi: 10.1104/pp.40.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson B. D., Trewavas A. J. Changes in the pattern of protein synthesis induced by 3-indolylacetic Acid. Plant Physiol. 1967 Aug;42(8):1081–1086. doi: 10.1104/pp.42.8.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayle D. L., Cleland R. Control of plant cell enlargement by hydrogen ions. Curr Top Dev Biol. 1977;11:187–214. doi: 10.1016/s0070-2153(08)60746-2. [DOI] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and Protein Metabolism in Pea Epicotyls : II. Response to Wounding in Aged Tissue. Plant Physiol. 1983 Nov;73(3):817–821. doi: 10.1104/pp.73.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster A. M., Davies E. Ribonucleic Acid and protein metabolism in pea epicotyls : I. The aging process. Plant Physiol. 1983 Nov;73(3):809–816. doi: 10.1104/pp.73.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Ray P. M. Early auxin-regulated polyadenylylated mRNA sequences in pea stem tissue. Proc Natl Acad Sci U S A. 1982 Jan;79(2):418–421. doi: 10.1073/pnas.79.2.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin- and ethylene-induced changes in the population of translatable messenger RNA in Basal sections and intact soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):338–340. doi: 10.1104/pp.69.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the patterns of protein synthesis in soybean hypocotyl. Proc Natl Acad Sci U S A. 1980 Jan;77(1):357–361. doi: 10.1073/pnas.77.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurfluh L. L., Guilfoyle T. J. Auxin-induced changes in the population of translatable messenger RNA in elongating sections of soybean hypocotyl. Plant Physiol. 1982 Feb;69(2):332–337. doi: 10.1104/pp.69.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]