Key Clinical Message
Treatment of recurrent myelodysplastic syndrome (MDS) after hematopoietic cell transplantation (HCT) remains challenging. We present a 4‐year‐old girl experiencing early MDS relapse post‐HCT treated with a multimodal strategy encompassing a second HCT and innovative targeted therapies. We underscore the potential of a comprehensive treatment approach in managing recurrent pediatric MDS.
Keywords: disease control, hematopoietic stem cell transplantation, myelodysplastic syndromes, relapse
1. INTRODUCTION
Survival is dismal for the 40%–60% of children with myelodysplastic syndrome (MDS) who relapse post allogeneic hematopoietic cell transplantation (HCT). 1 , 2 , 3 Strategies to decrease relapse risk includes use of cytoreduction prior to HCT or maintenance treatment after HCT, data on the utility of these approaches remains limited. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 Rapid withdrawal of immune suppression or use of donor lymphocyte infusion (DLI) can enhance the graft versus leukemia effect and achieve disease control in some cases. 13 , 14 Addition of hypomethylating agents to DLI may provide additional benefit 15 and second HCT should be considered. 16 , 17 , 18 , 19 , 20 , 21 While several novel therapies may alter the future landscape of MDS therapy 22 , 23 , 24 , 25 , 26 , 27 , 28 (Table 1), the optimal approach to relapsed pediatric MDS remains unclear. We report the management of a child who relapsed less than 70 days after initial HCT. Our approach demonstrates that multimodal therapy may permit prolonged survival with excellent quality of life (QOL) despite lack of long‐term cure.
TABLE 1.
Novel therapeutic approaches for pediatric AML/MDS.
| Mechanistic categories | Example | Selected references/clinical trial numbers (as of 05/02/2023) |
|---|---|---|
| Hematopoietic stem cell transplant approaches | ‐KIR‐favorable donor selection | Mehta, Rezvani 2016; Davies, Iannone et al. 2020 |
| ‐Selective graft depletion strategies |
Mamcarz, Madden et al. 2020 NCT00566696 ‐ completed |
|
| ‐Expanded cord trial | NCT04990323 | |
| ‐Uproleselan with pre‐transplant conditioning a | NCT05569512 | |
| ‐Maintenance therapy after HCT with decitabine and G‐CSF | NCT05796570 | |
| ‐Preemptive donor‐derived ex‐vivo expanded NK‐cells | NCT04836390 | |
| Cellular therapies | ‐CD33 CAR T cell therapy | NCT03971799, NCT05105152 |
| ‐CD123 CAR T cell therapy | NCT04318678, NCT04678336 | |
| ‐Cytokine‐induced memory‐like NK cell therapy | NCT04024761 | |
| ‐Cytokine‐induced memory‐like NK‐cell + DLI | NCT03068819 | |
| ‐CLL‐1 CAR‐T cell therapy | NCT04219163 | |
| Antibody drug conjugate | ‐CD33 directed treatment with fractionated gemtuzumab | Debureaux, Labopin et al. 2020 |
| ‐CD123 directed treatment with tagraxofusp / IMGN632 | Lane 2020 | |
| Immunotherapies | ‐Decitabine plus ipilimumab | Garcia, Flamand et al. 2020 |
| ‐Azacytidine plus nivolumab | NCT03825367 | |
| ‐Magrolimab | NCT03248479 | |
| ‐CD38 directed treatment with daratumomab | NCT03067571 ‐ completed | |
| Novel small molecule therapies | ‐Azacytidine, venetoclax, and trametinib (RAS mutation) | NCT04487106 ‐ completed |
| ‐Venetoclax plus selinexor | NCT04898894 | |
| ‐Lenalidomide (Monosomy7 or 5q‐) | Adema, Kerr et al. 2019 |
Abbreviations: CAR, chimeric antigen receptor; KIR, killer immunoglobulin‐like receptor; G‐CSF, granulocyte‐colony stimulating factor; HCT, Hematopoietic stem cell transplant; NK, Natural Killer.
MDS not included.
2. RESULTS
A previously healthy 4‐year‐old girl presented with fever. Physical exam at presentation was normal; laboratory studies demonstrated a white blood cell count of 3820 cells/μL with 6% circulating blasts, absolute neutrophil count 640 cells/μL, hemoglobin 11.5 g/dL and platelets 74,000 cells/μL. Bone marrow (BM) testing was diagnostic for MDS with excess blasts‐2 (Figure 1). Next generation sequencing panel showed PTPN11 p.A72V, 32% of 1331 reads and WT1 p.S382‐frameshift, 17% of 848 reads. Fluorescence in situ hybridization (FISH) detected monosomy 7. An underlying germline disorder, which is present in at least 30% of pediatric MDS cases, 3 was not identified. Extensive testing included telomere lengths, chromosome breakage, pancreas iso‐amylase, and whole exome sequencing.
FIGURE 1.
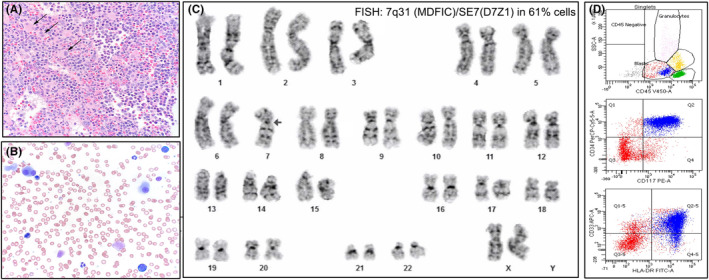
Myelodysplastic syndrome with excessive blasts. (A) Histology of bone marrow biopsy core (H&E) showing dysplastic megakaryocytes and 10%–15% aberrant blasts. (B) Bone marrow aspirate (Wright‐Giemsa) with dysplastic micro‐megakaryocytes, erythroid with nuclear irregularities, and hypo‐granular myeloids. (C) Karyotype from the time of initial diagnosis, with monosomy 7 detected in 61% cells by FISH. (D) Flow cytometry detected 13% myeloid blasts expressing CD13, CD33, CD34, CD117, CD11b, MPO and HLA‐DR.
She received decitabine (20 mg/m2 for 10 days); follow‐up BM evaluation demonstrated a reduction in blasts to 3% with persistent multilineage dysplasia (Figure 2A, B). She proceeded to HCT conditioned with myeloablative busulfan and cyclophosphamide followed by BM graft from her 10/10 HLA matched father (5.84 x 106 CD34+ cell/kg) (Figure 2C). Graft versus host disease (GvHD) prophylaxis included cyclosporine and methotrexate. Engraftment occurred on day 28 and she experienced minimal transplant associated toxicities and no GvHD. BM evaluation on day 30 was without evidence of MDS. However, surveillance BM on day 60 (7 months post diagnosis) demonstrated recurrent disease (Figure 2B). Cyclosporine was rapidly weaned followed by treatment with azacytidine (75 mg/m2 for 7 days) and DLI (1 x 106 CD3+ T cells/kg). Salvage treatment with azacytidine in combination with fludarabine/cytarabine/granulocyte—growth‐factor led to a measurable residual disease (MRD) negative remission. Maintenance therapy was initiated with azacytidine (75 mg/m2 for 7 days, 28‐day cycles) and DLI every other cycle (3 x 106 CD3+ T cells/kg for cycle 1, 2 x 107 CD3+ T cells/kg for cycle 3). Remission was maintained for 4 cycles, until she developed bone pain and recurrent cytopenia. A BM evaluation demonstrated second recurrence of MDS (17 months post diagnosis). She received venetoclax (14 mg/kg, 800 mg adult equivalent) combined with cytarabine (1000 mg/m2 IV every 12 h for 5 days). BM performed on day 22 of treatment was acellular and venetoclax was held. Repeat BM assessment on day 42 showed MRD negative remission by flow cytometry and the patient proceeded to second HCT using a 10/10 HLA matched unrelated donor (2.75 x 106 CD34+ cells/kg) after fludarabine, clofarabine, and busulfan conditioning. Engraftment occurred on day 16. The second HCT was uncomplicated; CD34 chimerism was 100% donor 2 on day 30 and cyclosporine was weaned by 186 days post HCT. She remained disease‐free until 1 year post second transplant when routine surveillance demonstrated 70% peripheral blasts consistent with transformation to AML/MDS (~32 months after diagnosis). Re‐induction with cytarabine and fludarabine resulted in MRD negative remission. An experimental cellular therapy did not mediate a durable remission. She relapsed for a fourth time with a significant blast burden (MDS/AML) and received CPX‐351 with the goal to achieve disease control prior to a planned investigational 3rd HCT. Her disease was refractory to this re‐induction attempt and treatment goals were transitioned to palliative approaches.
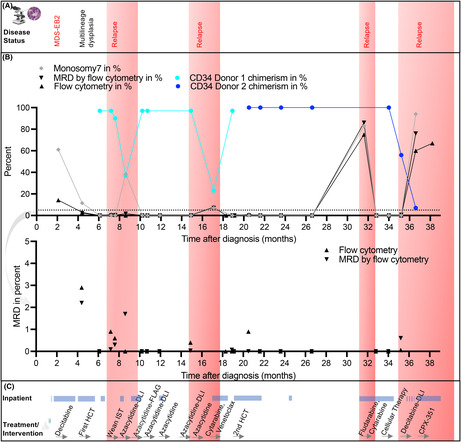
FIGURE 2.
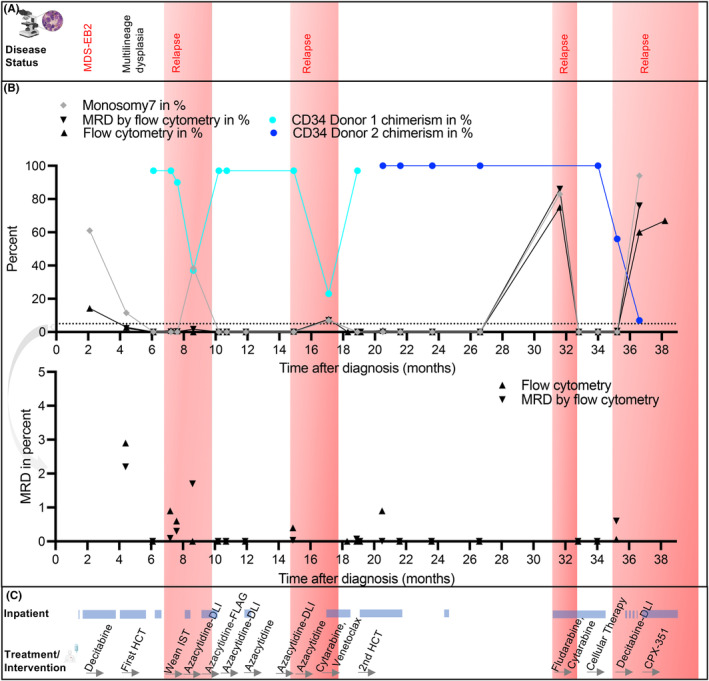
Timeline of disease management and response to treatment. (A) Overview of the disease status over time. (B) Graph showing disease characteristics over time. Monosomy 7 was measured by FISH. CD34 donor chimerism for donor 1 and donor 2 were measured by next generation sequencing at the American Red Cross. Multi‐parameter flow cytometry performed at Boston Children's Hospital was used to measure aberrant blast percentage. AML MRD flow cytometry represents testing done at Hematologics, Inc., Seattle, WA. Lower panel zoomed to improve MRD visualization. (C) Overview of treatment over time, inpatient time is highlighted. Immunosuppressive therapy (IST), Donor Lymphocyte infusions (DLI), Fludarabine, Cytarabine and Granulocyte colony‐stimulating factor (FLAG).
3. DISCUSSION
Disease relapse remains the leading cause of mortality for children undergoing HCT for MDS. Treatment options for those who recur early post HCT are limited, and cure is unlikely. Despite the high risk of mortality, a second HCT can achieve long‐term survival in well‐selected patients. 16 , 17 , 19 , 20 , 21 In a retrospective analysis of pediatric patients with acute leukemia and MDS who received a second HCT the single predictor for long term survival was disease control at time of HCT. 16
We report a pediatric patient who received multimodal therapy for recurrent MDS. Given the proximity of her first recurrence to initial HCT, a second HCT was initially not felt to be a therapeutic option given concern for disease refractoriness, and risk of treatment related mortality (TRM). Treatment with azacytidine and DLI followed by a myelosuppressive reinduction achieved a second remission until about 12 months from first HCT, at which point she was felt to be a suitable second transplant candidate. Though there is limited evidence for using an alternative donor for a second HCT 16 we chose an unrelated fully matched donor to facilitate graft versus leukemia effect. 18 While ultimately her disease was incurable, the therapies utilized from time of initial recurrence onward afforded her excellent QOL for 2.5 years—most of her time was spent outpatient with a high‐performance score (Figure 2C).
Low disease burden at the time of HCT for MDS has been associated with improved outcome, 6 , 21 however cytoreductive treatment prior to HCT is associated with inferior outcome 5 , 29 making the role of chemotherapy prior to HCT in pediatric MDS highly controversial. With the increasing utilization of novel targeted therapeutics in pediatric MDS, we may discover that the advantages of lower disease burden due to cytoreduction, outweigh the possible toxicities. The combination of a hypomethylating agent or cytarabine with the Bcl‐2 inhibitor venetoclax has been well tolerated in pediatric myeloid disease and is equally efficacious to conventional chemotherapy in adult MDS. 30 , 31 , 32 , 33 Novel therapeutic approaches include enhancement of GVL effect by checkpoint inhibition but risk of GvHD remains a major concern. 27 , 34 While cure of pediatric MDS recurring early post HCT remains unlikely, novel treatment approaches should be considered. We utilized multiple therapeutic approaches, including second HCT, DLI, maintenance chemotherapy and experimental cellular treatments towards the goal of minimizing toxicity and maximizing QOL while still striving for cure. Investigational approaches in pediatric MDS should be considered (Table 1). 7 , 8 , 9 , 11 , 35 , 36 , 37 The role of a third HCT in relapsed MDS is controversial given the risk of toxicity and should be done within the context of a clinical trial.
4. CONCLUSION
For children with relapsed MDS with a good performance status and absence of uncontrolled infections, GvHD, and other treatment related toxicities, a second HCT should be considered, if disease control can be achieved and if aligned with the family's goals. Acknowledging that early second HCT is associated with increased TRM, 16 , 18 , 21 temporizing disease control with less myelosuppressive agents, like hypomethylating agents in tandem with DLI, may be beneficial. Individualized treatment approaches that utilize targeted therapies with less risk for TRM like Bcl‐2 inhibition (e.g., venetoclax) 11 or immunotherapy (e.g., magrolimab) should be further studied in pediatric MDS. Consolidation strategies in the event of relapse after second HCT are not standardized; selected novel treatments might provide therapeutic benefit with minimal toxicity and therefore warrant consideration.
AUTHOR CONTRIBUTIONS
Franziska Wachter: Conceptualization; funding acquisition; writing – original draft; writing – review and editing. Yana Pikman: Conceptualization; supervision; writing – review and editing. Jacob Bledsoe: Data curation; writing – review and editing. Malika Kapadia: Writing – review and editing. Susanne Baumeister: Data curation; supervision; writing – review and editing. Jared Rowe: Writing – review and editing. Akiko Shimamura: Writing – review and editing. Andrew E. Place: Writing – review and editing. Susan Prockop: Supervision; writing – review and editing. Jennifer Whangbo: Writing – review and editing. Leslie Lehmann: Supervision; writing – review and editing. John Horan: Writing – review and editing. Jessica Pollard: Conceptualization; supervision; writing – review and editing.
CONFLICT OF INTEREST STATEMENT
Franziska Wachter, Yana Pikman, Jacob Bledsoe, Malika Kapadia, Susanne Baumeister, Jared Rowe, Akiko Shimamura, Jennifer Whangbo, Leslie Lehmann, John Horan declare no relevant conflict of interest. Andrew E. Place has received research funding from AbbVie, Inc. Susan Prockop receives support for the conduct of clinical trials through Boston Children's Hospital from AlloVir, Atara, and Jasper, Susan Prockop provides consulting (CellEvolve, Pierre Fabre) and receives honoraria from Regeneron, Susan Prockop is an Inventor related to development of third party viral specific T cells program with all rights assigned to Memorial Sloan Kettering Cancer Center. JAP receives support for the conduct of clinical trials through Boston Children's Hospital / Dana‐Farber Cancer Institute from AbbVie, Ymab Therapeutics and Servier and is on the advisory board for foresee pharmaceuticals.
CONSENT
Written informed consent was obtained from the parent to publish this report in accordance with the journal's patient consent policy. This case report is IRB exempt per institutional guidelines.
ACKNOWLEDGMENTS
FW received salary support provided by the American Society of Hematology Scholar Award, Claudia Adams Barr Award, Wipe Out Kids” Cancer, P4P and Boston Children's Office of Faculty Development Award. This research was supported with a grant from the National Cancer Institute, K08 CA222684 (YP). We thank the Leukemia & Lymphoma Society Leukemia and Lymphoma PedAL OptionSearch team for insightful discussions and support in the clinical trial search.
Wachter F, Pikman Y, Bledsoe J, et al. Treatment of recurrent pediatric myelodysplastic syndrome post hematopoietic stem cell transplantation. Clin Case Rep. 2023;11:e8190. doi: 10.1002/ccr3.8190
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Gerds AT, Gooley TA, Estey EH, Appelbaum FR, Deeg HJ, Scott BL. Pretransplantation therapy with azacitidine vs induction chemotherapy and posttransplantation outcome in patients with MDS. Biol Blood Marrow Transplant. 2012;18(8):1211‐1218. doi: 10.1016/j.bbmt.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maher OM, Silva JG, Wu J, et al. Outcomes of children, adolescents, and young adults following allogeneic stem cell transplantation for secondary acute myeloid leukemia and myelodysplastic syndromes‐the MD Anderson Cancer Center experience. Pediatr Transplant. 2017;21(3). doi: 10.1111/petr.12890 [DOI] [PubMed] [Google Scholar]
- 3. Locatelli F, Strahm B. How I treat myelodysplastic syndromes of childhood. Blood. 2018;131(13):1406‐1414. doi: 10.1182/blood-2017-09-765214 [DOI] [PubMed] [Google Scholar]
- 4. Nakano TA, Lau BW, Dickerson KE, et al. Diagnosis and treatment of pediatric myelodysplastic syndromes: a survey of the North American pediatric aplastic anemia consortium. Pediatr Blood Cancer. 2020;67(10):e28652. doi: 10.1002/pbc.28652 [DOI] [PubMed] [Google Scholar]
- 5. Strahm B, Nollke P, Zecca M, et al. Hematopoietic stem cell transplantation for advanced myelodysplastic syndrome in children: results of the EWOG‐MDS 98 study. Leukemia. 2011;25(3):455‐462. doi: 10.1038/leu.2010.297 [DOI] [PubMed] [Google Scholar]
- 6. Kobos R, Steinherz PG, Kernan NA, et al. Allogeneic hematopoietic stem cell transplantation for pediatric patients with treatment‐related myelodysplastic syndrome or acute myelogenous leukemia. Biol Blood Marrow Transplant. 2012;18(3):473‐480. doi: 10.1016/j.bbmt.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 7. de Lima M, Giralt S, Thall PF, et al. Maintenance therapy with low‐dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116(23):5420‐5431. doi: 10.1002/cncr.25500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pusic I, Choi J, Fiala MA, et al. Maintenance therapy with Decitabine after allogeneic stem cell transplantation for acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2015;21(10):1761‐1769. doi: 10.1016/j.bbmt.2015.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han S, Kim YJ, Lee J, et al. Model‐based adaptive phase I trial design of post‐transplant decitabine maintenance in myelodysplastic syndrome. J Hematol Oncol. 2015;8:118. doi: 10.1186/s13045-015-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oran B, de Lima M, Garcia‐Manero G, et al. A phase 3 randomized study of 5‐azacitidine maintenance vs observation after transplant in high‐risk AML and MDS patients. Blood Adv. 2020;4(21):5580‐5588. doi: 10.1182/bloodadvances.2020002544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei Y, Xiong X, Li X, et al. Low‐dose decitabine plus venetoclax is safe and effective as post‐transplant maintenance therapy for high‐risk acute myeloid leukemia and myelodysplastic syndrome. Cancer Sci. 2021;112(9):3636‐3644. doi: 10.1111/cas.15048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeFilipp Z, Chen YB. How I treat with maintenance therapy after allogeneic HCT. Blood. 2023;141(1):39‐48. doi: 10.1182/blood.2021012412 [DOI] [PubMed] [Google Scholar]
- 13. Gozdzik J, Rewucka K, Krasowska‐Kwiecien A, et al. Adoptive therapy with donor lymphocyte infusion after allogenic hematopoietic SCT in pediatric patients. Bone Marrow Transplant. 2015;50(1):51‐55. doi: 10.1038/bmt.2014.200 [DOI] [PubMed] [Google Scholar]
- 14. Schroeder T, Rautenberg C, Kruger W, et al. Treatment of relapsed AML and MDS after allogeneic stem cell transplantation with decitabine and DLI‐a retrospective multicenter analysis on behalf of the German cooperative transplant study group. Ann Hematol. 2018;97(2):335‐342. doi: 10.1007/s00277-017-3185-5 [DOI] [PubMed] [Google Scholar]
- 15. Huschart E, Miller H, Salzberg D, et al. Azacitidine and prophylactic donor lymphocyte infusions after hematopoietic stem cell transplantation for pediatric high‐risk acute myeloid leukemia. Pediatr Hematol Oncol. 2021;38(2):154‐160. doi: 10.1080/08880018.2020.1829220 [DOI] [PubMed] [Google Scholar]
- 16. Duncan CN, Majhail NS, Brazauskas R, et al. Long‐term survival and late effects among one‐year survivors of second allogeneic hematopoietic cell transplantation for relapsed acute leukemia and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2015;21(1):151‐158. doi: 10.1016/j.bbmt.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoshimi A, Mohamed M, Bierings M, et al. Second allogeneic hematopoietic stem cell transplantation (HSCT) results in outcome similar to that of first HSCT for patients with juvenile myelomonocytic leukemia. Leukemia. 2007;21(3):556‐560. doi: 10.1038/sj.leu.2404537 [DOI] [PubMed] [Google Scholar]
- 18. Christopeit M, Kuss O, Finke J, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem‐cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013;31(26):3259‐3271. doi: 10.1200/JCO.2012.44.7961 [DOI] [PubMed] [Google Scholar]
- 19. Orti G, Sanz J, Bermudez A, et al. Outcome of second allogeneic hematopoietic cell transplantation after relapse of myeloid malignancies following allogeneic hematopoietic cell transplantation: a retrospective cohort on behalf of the Grupo Español de Trasplante Hematopoyetico. Biol Blood Marrow Transplant. 2016;22(3):584‐588. doi: 10.1016/j.bbmt.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 20. Vrhovac R, Labopin M, Ciceri F, et al. Second reduced intensity conditioning allogeneic transplant as a rescue strategy for acute leukaemia patients who relapse after an initial RIC allogeneic transplantation: analysis of risk factors and treatment outcomes. 2019;1‐8. doi: 10.1038/bmt.2015.221 [DOI] [PubMed]
- 21. Ruutu T, de Wreede LC, van Biezen A, et al. Second allogeneic transplantation for relapse of malignant disease: retrospective analysis of outcome and predictive factors by the EBMT. 2019;1‐9. doi: 10.1038/bmt.2015.186 [DOI] [PubMed]
- 22. Mehta RS, Rezvani K. Can we make a better match or mismatch with KIR genotyping? Hematology Am Soc Hematol Educ Program. 2016;2016(1):106‐118. doi: 10.1182/asheducation-2016.1.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Davies SM, Iannone R, Alonzo TA, et al. A phase 2 trial of KIR‐mismatched unrelated donor transplantation using in vivo T cell depletion with antithymocyte globulin in acute Myelogenous leukemia: Children's oncology group AAML05P1 study. Biol Blood Marrow Transplant. 2020;26(4):712‐717. doi: 10.1016/j.bbmt.2019.12.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mamcarz E, Madden R, Qudeimat A, et al. Improved survival rate in T‐cell depleted haploidentical hematopoietic cell transplantation over the last 15 years at a single institution. Bone Marrow Transplant. 2020;55(5):929‐938. doi: 10.1038/s41409-019-0750-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Debureaux PE, Labopin M, Mamez AC, et al. Fractionated gemtuzumab ozogamicin in association with high dose chemotherapy: a bridge to allogeneic stem cell transplantation in refractory and relapsed acute myeloid leukemia. Bone Marrow Transplant. 2020;55(2):452‐460. doi: 10.1038/s41409-019-0690-2 [DOI] [PubMed] [Google Scholar]
- 26. Lane AA. Targeting CD123 in AML. Clin Lymphoma Myeloma Leuk. 2020;20(Suppl 1):S67‐S68. doi: 10.1016/S2152-2650(20)30466-3 [DOI] [PubMed] [Google Scholar]
- 27. Garcia JS, Flamand Y, Tomlinson BK, et al. Safety and efficacy of Decitabine plus Ipilimumab in relapsed or refractory MDS/AML in the post‐BMT or transplant Naïve settings. Blood. 2020;136(Supplement 1):15‐17. doi: 10.1182/blood-2020-136235 [DOI] [Google Scholar]
- 28. Adema V, Kerr CM, Walter W, et al. Can monosomy 7 be targeted by next generation Cereblon‐modulating agents? Blood. 2019;134(Supplement_1):1270. doi: 10.1182/blood-2019-128967 31698418 [DOI] [Google Scholar]
- 29. Smith AR, Christiansen EC, Wagner JE, et al. Early hematopoietic stem cell transplant is associated with favorable outcomes in children with MDS. Pediatr Blood Cancer. 2013;60(4):705‐710. doi: 10.1002/pbc.24390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winters AC, Maloney KW, Treece AL, Gore L, Franklin AK. Single‐center pediatric experience with venetoclax and azacitidine as treatment for myelodysplastic syndrome and acute myeloid leukemia. Pediatr Blood Cancer. 2020;67(10):e28398. doi: 10.1002/pbc.28398 [DOI] [PubMed] [Google Scholar]
- 31. Karol SE, Alexander TB, Budhraja A, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose‐escalation study. Lancet Oncol. 2020;21(4):551‐560. doi: 10.1016/S1470-2045(20)30060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Samra B, Konopleva M, Isidori A, Daver N, DiNardo C. Venetoclax‐based combinations in acute myeloid leukemia: current evidence and future directions. Front Oncol. 2020;10:562558. doi: 10.3389/fonc.2020.562558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karol SE, Alexander TB, Budhraja A, et al. Venetoclax in combination with cytarabine with or without idarubicin in children with relapsed or refractory acute myeloid leukaemia: a phase 1, dose‐escalation study. Lancet Oncol. 2020;21(4):551‐560. doi: 10.1016/S1470-2045(20)30060-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghoneim HE, Fan Y, Moustaki A, et al. De novo epigenetic programs inhibit PD‐1 blockade‐mediated T cell rejuvenation. Cell. 2017;170(1):142‐157 e19. doi: 10.1016/j.cell.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gao L, Zhang Y, Wang S, et al. Effect of rhG‐CSF combined with Decitabine prophylaxis on relapse of patients with high‐risk MRD‐negative AML after HSCT: an open‐label, multicenter, randomized controlled trial. J Clin Oncol. 2020;38(36):4249‐4259. doi: 10.1200/JCO.19.03277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ma Y, Qu C, Dai H, et al. Maintenance therapy with decitabine after allogeneic hematopoietic stem cell transplantation to prevent relapse of high‐risk acute myeloid leukemia. Bone Marrow Transplant. 2020;55(6):1206‐1208. doi: 10.1038/s41409-019-0677-z [DOI] [PubMed] [Google Scholar]
- 37. Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021;14(1):4. doi: 10.1186/s13045-020-01017-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


