Abstract
Background/Aims
Covered self-expandable metallic stents (cSEMS) have become popular for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting (EUS-HGS). We compared the time to recurrent biliary obstruction (TRBO), complications, and reintervention rates between EUS-HGS using plastic stent (PS) and cSEMS in patients with unresectable malignancies at multicenter institutions in Japan.
Methods
Patients with unresectable malignant biliary obstruction who underwent EUS-HGS between April 2015 and July 2020 at any of the six participating facilities were enrolled. Primary endpoint: TRBO; secondary endpoints: rate of complications other than recurrent biliary obstruction and technical success rate of reintervention were evaluated.
Results
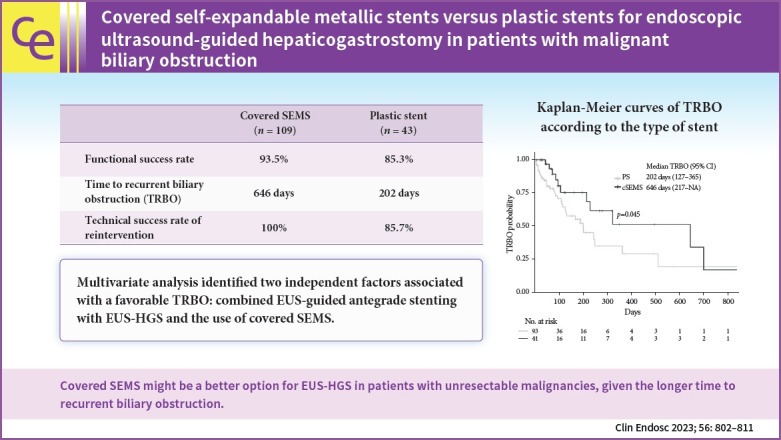
PS and cSEMS were used for EUS-HGS in 109 and 43 patients, respectively. The TRBO was significantly longer in the cSEMS group than in the PS group (646 vs. 202 days). Multivariate analysis identified two independent factors associated with a favorable TRBO: combined EUS-guided antegrade stenting with EUS-HGS and the use of cSEMS. No significant difference was observed in the rate of complications other than recurrent biliary obstruction between the two groups. The technical success rate of reintervention was 85.7% for PS and 100% for cSEMS (p=0.309).
Conclusions
cSEMS might be a better option for EUS-HGS in patients with unresectable malignancies, given the longer TRBO.
Keywords: Bile duct obstruction, Biliary fistula, Drainage, Endoscopic ultrasonography
Graphical abstract
INTRODUCTION
Endoscopic retrograde cholangiopancreatography (ERCP) is the gold standard for palliative treatment to relieve biliary obstructions in patient with unresectable malignancies.1 However, it is sometimes very challenging to access or cannulate the major papilla for ERCP due to surgically altered anatomy or malignant duodenal obstruction. Under these circumstances, percutaneous transhepatic biliary drainage (PTBD) and enteroscopy (including single-balloon enteroscopy and double-balloon enteroscopy)-assisted ERCP (e-ERCP) have been widely performed as alternative procedures to ERCP.2,3 While these techniques have several advantages, they also have disadvantages: PTBD is associated with a higher risk of complications and the need for external drainage, whereas e-ERCP is a time-consuming procedure and is associated with the risk of severe complications, such as perforation and pancreatitis.4,5
In recent years, endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting (EUS-HGS) has been reported as an alternative treatment for ERCP, PTBD, and e-ERCP.6,7 EUS-HGS is feasible even in patients with a surgically altered anatomy or malignant duodenal obstruction. In addition, the risk of post-ERCP pancreatitis caused by traumatic papillary irritation was reduced in EUS-HGS.8 However, EUS-HGS is still challenging because of the complexity of the procedure, risk of severe adverse events, including stent migration, and lack of dedicated devices.9 In addition, there are several problems to be resolved before EUS-HGS can be optimized. One of these is the stent selection. Previous reports indicate that the majority of endoscopists prefer fully/partially covered self-expandable metallic stents (cSEMS) to plastic stents (PS)10 because they are considered to afford longer stent patency, reduced bile leakage, and easier reintervention.9 However, this preference is still based on the opinions of experts and evidence is still lacking, as no studies have been conducted to evaluate the advantages of cSEMS over PS for EUS-HGS in patients with unresectable malignancies.11 Therefore, we performed a retrospective multicenter comparative study of cSEMS and PS to determine which of the two might be optimal for EUS-HGS in patients with unresectable malignancies.
METHODS
Patients
This retrospective multicenter study included patients with unresectable malignancies who underwent EUS-HGS between April 2015 and July 2020 at any of the six participating facilities in Japan. We retrospectively reviewed the hospital medical records and endoscopy reports of the patients and retrieved data on patient characteristics, EUS-HGS procedure used, and complications related to EUS-HGS.
Endpoints, outcome measures and definitions
The primary endpoint was time to recurrent biliary obstruction (TRBO). The secondary endpoints were the rate of complications other than recurrent biliary obstruction (RBO) and technical success rate of reintervention. The indications for EUS-HGS were categorized as follows: inaccessible papilla due to malignant duodenal or gastric outlet obstruction; difficulty in cannulation via the papilla; isolated intrahepatic bile duct obstruction for which a transpapillary approach was not possible; surgically altered anatomy; and recurrent ascending cholangitis associated with transpapillary stenting. Technical success was defined as the successful deployment of a cSEMS/PS to the intended location, and functional success was defined as a 50% decrease or normalization of the serum total bilirubin level within 14 days of stent placement, according to the Tokyo criteria 2014.12 In cases of cholangitis without elevation of the serum total bilirubin level, improvement of cholangitis was also defined as functional success. RBO was defined as cholangitis or stent occlusion; both stent occlusion and migration that necessitated reintervention were included under RBO. Stent occlusion was diagnosed if the patients had obstructive jaundice with serum bilirubin elevation and/or biliary duct dilatation and disappearance of air in the biliary tract on computed tomography or abdominal ultrasonography.13 Overall survival (OS) was defined as the period from the day of EUS-HGS to the day of death or final follow-up. TRBO was calculated from the day of EUS-HGS to the day of RBO diagnosis or the day of death of the patient/final follow-up.
Statistical analysis
TRBO and OS were estimated using the Kaplan-Meier method and compared between groups using the log-rank test. Hazard ratios (HR) were calculated using a Cox proportional hazards model. To identify factors independently influencing the TRBO, variables that were identified as statistically significant (p<0.05) by univariate analysis were entered into the multivariate analysis model. Categorical variables were compared using Fisher exact test and continuous variables were compared using the Mann–Whitney U test or Kruskal–Wallis test, as appropriate. All statistical analyses were performed using R ver. 4.0.3 (R Foundation for Statistical Computing).
EUS-HGS technique
Patients received intravenous midazolam and pethidine hydrochloride prior to the procedure for sedation as needed during the procedure. Carbon dioxide insufflation of the abdominal cavity was performed. A curvilinear array echoendoscope (GF-UCT-240 or GF-UCT-260; Olympus Medical Systems; EG-580-UT; Fujifilm Corp.) was used for the procedure, and color Doppler was used to identify the regional vasculature. After visualization of the bile duct from the stomach by endoscopic ultrasound, the bile duct selected for puncture, which was left to the discretion of the endoscopists at each facility, was punctured with a 19-G or 22-G fine-needle aspiration needle. After the puncture, contrast medium was injected to confirm that the tip of the needle was within the bile duct. Then, a sufficient length of 0.025- or 0.018-inch guidewire was inserted into the bile duct or duodenum, followed by deployment of the stent with or without tract dilatation. In patients in whom the tract was dilated, dilatation was performed using a balloon, mechanical, or electrocautery dilator device; selection was also left to the discretion of the endoscopists at each facility. Finally, a PS (7- or 8-Fr×14 or 15 cm, Through & Pass Type-IT; Gadelius Medical; 7- or 8.5-Fr×7, 10, 12, or 15 cm, Flexima; Boston Scientific Japan; 7- or 8.5-Fr×11 or 15 cm; QuickPlace V; Olympus Medical Systems) or a cSEMS (10 mm×6 cm, WallFlex fully covered; Boston Scientific Japan; 6 or 8 mm×10 cm, Hanaro-stent fully covered; MI Tech; 8 or 10 mm×9, 10, or 12 cm; Niti-S S-type partially covered; 6 mm×12 cm, Niti-S S-type fully covered; Taewoong Corporation) was deployed. Antibiotics were administered to all patients before the EUS-HGS procedure.
Ethical statements
This study was reviewed and approved by the Institutional Review Board of the National Cancer Center Hospital East on February 16, 2021 (approval number: 2020-490). The need for informed consent was waived by the institutional review board at each facility because the study was a retrospective chart review and caused no more than minimal risk to the patients. The study was conducted following the criteria set by the Declaration of Helsinki, and patients' information were kept confidential. The authors obtained informed consent from all the patients.
RESULTS
Patient characteristics
A total of 154 patients were enrolled in the study. Of these, a PS was used in 109 patients and a cSEMS in 43 patients, while the stent failed to be deployed in two patients (technical success rate: 98.7%). No significant differences were observed in age, sex ratio, history of prior transpapillary drainage, distribution of the Eastern Cooperative Oncology Group performance status, diagnosis, site of biliary obstruction, and indications for EUS-HGS between the two groups (Table 1).
Table 1.
Baseline characteristics
| Characteristic | PS (n=109) | cSEMS (n=43) | p-value |
|---|---|---|---|
| Age (yr) | 70 (32–85) | 69 (32–90) | 0.278 |
| Age (yr) | 0.355 | ||
| <70 | 50 (45.9) | 24 (55.8) | |
| ≥70 | 59 (54.1) | 19 (44.2) | |
| Male sex | 71 (65.1) | 31 (72.1) | 0.528 |
| ECOG PS | 0.310 | ||
| 0, 1 | 84 (77.1) | 37 (86.0) | |
| 2 or more | 25 (22.9) | 6 (14.0) | |
| Diagnosis | 0.170 | ||
| Pancreatic cancer | 44 (40.4) | 18 (41.9) | |
| Bile duct cancer | 25 (22.9) | 16 (37.2) | |
| Gastric cancer | 22 (20.2) | 6 (14.0) | |
| Others | 18 (16.5) | 3 (7.0) | |
| Site of biliary obstruction | 0.396 | ||
| Distal | 61 (56.0) | 28 (65.1) | |
| Perihilar | 48 (44.0) | 15 (34.9) | |
| Prior transpapillary drainage, yes | 51 (46.8) | 18 (41.9) | 0.712 |
| Indication for EUS-HGS | 0.121 | ||
| Inaccessible papilla | 42 (38.5) | 13 (30.2) | |
| Isolated intrahepatic bile duct obstruction | 28 (25.7) | 5 (11.6) | |
| Recurrent ascending cholangitis due to a transpapillary stent | 12 (11.0) | 9 (20.9) | |
| Surgically altered anatomy | 14 (12.8) | 8 (18.6) | |
| Failed biliary cannulation | 13 (11.9) | 8 (18.6) |
Values are presented as median (range) or number (%).
PS, plastic stent; cSEMS, fully or partially covered self-expandable metallic stent; ECOG PS, Eastern Cooperative Oncology Group performance status; EUS-HGS, endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting.
Outcomes of EUS-HGS
The functional success rate was 88.2% (85.3% for PS and 95.3% for cSEMS; p=0.149), with no significant difference between the two groups. A significant difference was observed in the bile duct selected for puncture between the two groups, with a higher frequency of B2 punctures in the PS group and a higher frequency of B3 punctures in the cSEMS group (p=0.007). The device used for tract dilatation also differed significantly between the two groups, with a higher frequency of use of a mechanical dilator in the PS group and a higher frequency of use of a balloon dilator or electrocautery dilator device in the cSEMS group (p<0.001). No significant difference was observed in the frequency of combined biliary drainage of EUS-guided antegrade stenting with EUS-HGS (EUS-HGAS) or procedure time between the two groups (Table 2).
Table 2.
Outcomes of endoscopic ultrasound-guided hepaticogastrostomy and transmural stenting
| PS (n=109) | cSEMS (n=43) | p-value | |
|---|---|---|---|
| Functional success, yes | 93 (85.3) | 41 (95.3) | 0.149 |
| Punctured bile duct | 0.007 | ||
| B2 | 71 (65.1) | 17 (39.5) | |
| B3 | 38 (34.9) | 26 (60.5) | |
| Tract dilator | <0.001 | ||
| Mechanical | 64 (58.7) | 12 (27.9) | |
| Balloon | 19 (17.4) | 17 (39.5) | |
| Electrocautery | 15 (13.8) | 14 (32.6) | |
| None | 11 (10.1) | 0 (0) | |
| EUS-HGAS, yes | 26 (23.9) | 5 (11.6) | 0.144 |
| Procedure time (min) | 30.0 (8.0–187.0) | 41.0 (15.0–150.0) | 0.125 |
Values are presented as number (%) or median (range).
PS, plastic stent; cSEMS, fully/partially covered self-expandable metallic stent; B2, segment 2 bile duct; B3, segment 3 bile duct; EUS-HGAS, combined endoscopic ultrasound-guided antegrade stenting with endoscopic ultrasound-guided hepaticogastrostomy and transmural stenting.
Analysis of OS and TRBO
The median follow-up duration of the surviving patients was 322 days (95% confidence interval [CI], 218-494) (Kaplan-Meier estimate). The OS analysis included 91 deaths (59.9%) among the 152 patients. The median OS was 189 days (95% CI, 99–270) in the PS group and 164 days (95% CI, 95–281) in the cSEMS group (Fig. 1), with no significant difference (p=0.757) in the OS between the two groups. Analysis of TRBO was conducted based on 134 patients who achieved functional success, including 47 cases of RBO (35.1%). The median TRBO was 202 days (95% CI, 127–365) in the PS group and 646 days (95% CI, 217–not available) in the cSEMS group (Fig. 2), which was significantly longer in the cSEMS group than in the PS group (p=0.045). The non-RBO complication rates at 3, 6, and 12 months were 72.9%, 55.4%, and 35.3%, respectively, in the PS group and 85.0 %, 75.5 %, and 51.5 %, respectively, in the cSEMS group.
Fig. 1.
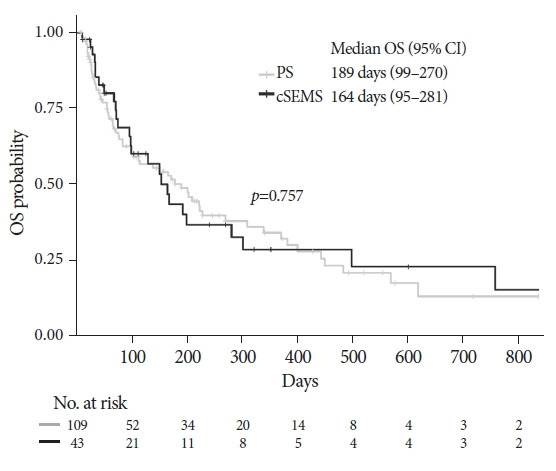
Kaplan-Meier curves of overall survival (OS) according to the type of stent. No significant diferrence was observed between the two groups (p=0.757). The median OS was 189 days (95% confidence interval [CI], 99–270) in the plastic stent (PS) group and 164 days (95% CI, 95–281) in the covered self-expandable metallic stent (cSEMS) group.
Fig. 2.
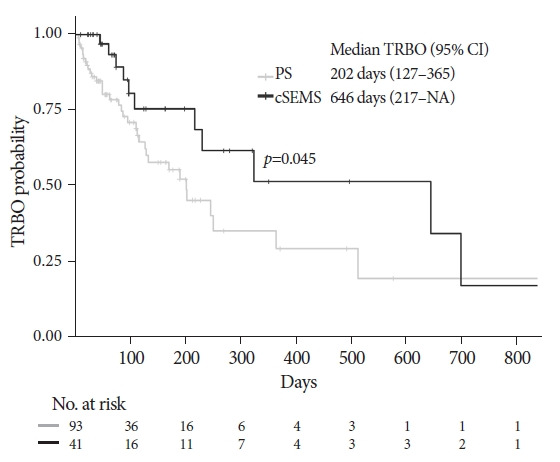
Kaplan-Meier curves of time to recurrent biliary obstruction (TRBO) according to the type of stent. TRBO of the covered self-expandable metallic stent (cSEMS) group was significantly longer than that of the PS group (p=0.045). Median TRBO was 202 days (95% confidence interval [CI], 127–365) in the plastic stent (PS) group and 646 days (95% CI, 217–NA) in the cSEMS group. Non-RBO rates at 3, 6, and 12 months were 72.9%, 55.4% and 35.3% in the PS group and 85.0%, 75.5% and 51.5% in the cSEMS group, respectively. NA, not available.
Factors associated with TRBO
Univariate and multivariate logistic regression analyses were performed to identify the risk factors for TRBO in the study cohort. Univariate analysis identified gastric cancer, EUS-HGAS, and the use of cSEMS as being associated with a significantly lower risk of TRBO. Multivariate analysis performed using these factors identified two factors as being significantly independently associated with a favorable TRBO, including EUS-HGAS (HR, 0.13; 95% CI, 0.03–0.58; p=0.002) and use of cSEMS (HR, 0.28; 95% CI, 0.12–0.66, p=0.003) (Table 3).
Table 3.
Univariate and multivariate analysis to identify the risk factors for recurrent biliary obstruction
| Univariate |
Multivariate |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr) | <70 vs. ≥70 | 1.04 (0.58–1.86) | 0.907 | ||
| Sex | Female vs. male | 0.79 (0.42–1.48) | 0.457 | ||
| ECOG PS | 0, 1 vs. 2 or more | 0.89 (0.35–2.27) | 0.807 | ||
| Diagnosis | Pancreatic cancer | Reference | Reference | ||
| Bile duct cancer | 1.06 (0.55–2.05) | 0.868 | 1.03 (0.47–2.26) | 0.940 | |
| Gastric cancer | 0.20 (0.06–0.66) | 0.008 | 0.27 (0.07–1.00) | 0.050 | |
| Others | 0.54 (0.18–1.57) | 0.257 | 0.27 (0.07–1.10) | 0.067 | |
| Site of biliary obstruction | Distal vs. perihilar | 1.31 (0.73–2.35) | 0.357 | ||
| Prior transpapillary drainage | No vs. yes | 1.55 (0.86–2.79) | 0.142 | ||
| Indication for EUS-HGS | Failed biliary cannulation | Reference | |||
| Isolated intrahepatic bile duct obstruction | 0.96 (0.33–2.80) | 0.947 | |||
| Recurrent ascending cholangitis due to a transpapillary stent | 1.44 (0.47–4.36) | 0.522 | |||
| Surgically altered anatomy | 0.69 (0.21–2.24) | 0.535 | |||
| Inaccessible papilla | 0.97 (0.35–2.71) | 0.953 | |||
| Puncture bile duct | B2 vs. B3 | 1.05 (0.58–1.87) | 0.882 | ||
| EUS-HGAS | No vs. yes | 0.15 (0.04–0.60) | 0.008 | 0.13 (0.03–0.58) | 0.002 |
| Type of stent | PS vs. cSEMS | 0.50 (0.25–1.00) | 0.049 | 0.28 (0.12–0.66) | 0.003 |
HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EUS-HGS, endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting; B2, segment 2 bile duct; B3, segment 3 bile duct; EUS-HGAS, combined endoscopic ultrasound-guided antegrade stenting with endoscopic ultrasound-guided hepaticogastrostomy and transmural stenting; PS, plastic stent; cSEMS, fully/partially covered self-expandable metallic stent.
Complications
No significant difference was observed in the rate of complications other than RBO between the two groups (12.8% in the PS group vs. 25.6% in the cSEMS group, p=0.088). Moderate acute pancreatitis occurring in one patient in the PS group who had undergone EUS-HGAS was managed successfully by conservative treatment. Liver abscess in the left lobe was identified in two patients and occurred 11 and 30 days after the EUS-HGS procedure, respectively. A cSEMS was deployed in segment 2 of the bile duct (B2) in both cases. In both patients, the liver abscess was larger than 5 cm in diameter, which necessitated abscess drainage and prolonged hospitalization. However, both patients recovered and were discharged. Mediastinal emphysema occurred in one patient in the cSEMS group, in whom an electrocautery dilator device was used. The only symptom of mediastinal emphysema that the patient presented with was chest pain, which was manageable with analgesic and antibiotic therapy. With this conservative therapy, a follow-up CT performed 10 days after the EUS-HGS procedure showed disappearance of mediastinal emphysema. Asymptomatic stent migration to the gastric side was incidentally observed in two patients in the cSEMS group; however, no treatment was required because neither patient revealed any abnormal laboratory findings (Table 4).
Table 4.
Complications
| PS | cSEMS | p-value | |
|---|---|---|---|
| Complications other than RBO (n=152)a) | |||
| Any | 14 (12.8) | 11 (25.6) | 0.088 |
| Bile leakage | 6 (5.5) | 1 (2.3) | 0.674 |
| Bleeding | 1 (0.9) | 3 (7.0) | 0.069 |
| Peritonitis | 3 (2.8) | 2 (4.7) | 0.622 |
| Pancreatitis | 1 (0.9) | 0 (0) | 1 |
| Liver abscess | 0 (0) | 2 (4.7) | 0.079 |
| Mediastinal emphysema | 0 (0) | 1 (2.3) | 0.283 |
| Ulcer | 0 (0) | 1 (2.3) | 0.283 |
| Septic shock | 4 (3.7) | 0 (0) | 0.578 |
| Stent migration | 0 (0) | 2 (4.7) | 0.079 |
| RBO (n=134)b) | |||
| Any | 35 (37.6) | 12 (29.3) | 0.433 |
| Sludge | 27 (29.0) | 8 (19.5) | 0.471 |
| Food impaction | 1 (1.1) | 1 (2.4) | 0.450 |
| Hyperplasia | 0 (0) | 3 (7.3) | 0.014 |
| Stent migration | 2 (2.2) | 0 (0) | 1 |
| Unknown | 5 (5.4) | 0 (0) | 0.309 |
Values are presented as number (%).
PS, plastic stent; cSEMS, full/partially covered self-expandable metallic stent; RBO, recurrent biliary obstruction.
PS (n=109), cSEMS (n=43);
PS (n=93), cSEMS (n=41).
The percentage of patients who developed RBO was not significantly different between the two groups (37.6% in the PS group and 29.3% in the cSEMS group, p=0.433). The most common cause of RBO was sludge in both the groups. RBO caused by tissue hyperplasia was found in three patients in the cSEMS group but in none of the patients in the PS group. Stent migration related to RBO was observed in two patients in the PS group, and the stent had migrated to the gastric side in both patients.
Reintervention analysis
Reintervention was performed in all patients who developed RBO. No significant difference was observed in the technical success rate of reintervention (85.7% [30/35] in the PS group and 100% [12/12] in the cSEMS group, p=0.309) and median reintervention procedure time (29.5 minutes [range, 11–132] in the PS group and 28.0 minutes [range, 14–75] in the cSEMS group, p=0.754) between the two groups.
DISCUSSION
EUS-HGS is often performed for palliation in patients with biliary obstruction due to unresectable malignancies, the majority of whom are undergoing chemotherapy. Long-term stent patency and low complication rates are crucial to maintain the performance status of these patients and to allow them to continue to receive chemotherapy and have the best chance of survival. In addition, as the prognosis of patients with unresectable malignancies has improved with advances in chemotherapy, the role of reintervention is also increasing. In view of the aforementioned issues, optimal stent selection is an issue that inevitably needs to be addressed. The use of cSEMS has gained popularity in patients undergoing EUS-HGS because it affords longer stent patency, reduced bile leakage, and easier reintervention.9 However, no comparative studies between PS and cSEMS have been performed; thus, evidence for the advantages of cSEMS is still lacking. This is the first study conducted in a relatively large number of patients from six participating facilities, to compare the TRBO, rate of complications, and rate of reintervention between patients receiving PS and cSEMS for EUS-HGS. In our cohort, multivariate analysis performed using ten variables identified two factors, including the use of cSEMS and EUS-HGAS, as independent predictors of favorable TRBO. We also demonstrated lack of significant differences in the rate of complications, including bile leakage and technical success rate of reintervention, between the PS and cSEMS groups.
Theoretically, EUS-HGS using a cSEMS is considered to afford longer stent patency due to the larger diameter of the stent, as in transpapillary biliary drainage.14 However, the differences between transpapillary biliary drainage and EUS-HGS still need to be considered, such as the location of the stent and whether the stent traverses a malignant stricture, which could affect the risk of RBO. Our results in this study support that even in the case of EUS-HGS, cSEMS affords longer stent patency than PS in patients with unresectable malignancies.11 Recently, EUS-HGAS has been reported as an advanced palliative technique for obtaining long-term stent patency. Ogura et al.13 reported the clinical benefits of long stent patency in 49 patients who underwent EUS-HGAS, and mentioned that double-stent deployment could offer longer stent patency than EUS-HGS alone. Indeed, our study identified EUS-HGAS as an independent predictor of favorable TRBO.
No significant difference was observed in OS between the two groups. Therefore, the impact of patient death on TRBO is not biased. In addition, while the TRBO was significantly shorter in the PS group than in the cSEMS group, the patients’ survival in the latter part of the Kaplan-Meier curve was not significantly different. This suggests that the management of RBO in patients might be appropriate and crucial. The functional success rate in the cSEMS group was higher than that in the PS group (85% and 95%, respectively; p=0.130), although the difference was not statistically significant. This result could also be attributed to the larger diameter of the cSEMS.
In the present study, approximately one-third of the patients in both groups developed RBO, mainly due to sludge. However, the type of stent may also play a role, at least in part; RBO caused by tissue hyperplasia was significantly more frequent in the cSEMS group than in the PS group (7% and 0%, respectively; p=0.014). Nakai et al.15 reported that tissue hyperplasia is the major cause of RBO in patients undergoing EUS-HGS with cSEMS. This is generally believed to be caused by a mismatch between the size of the stent and the luminal diameter of the intrahepatic bile duct, although this hypothesis has not yet been validated and further study is required. Stent migration was observed in four patients, including two from the PS group and two from the cSEMS group. In all cases, the stent migrated to the gastrointestinal side. Interestingly, two patients in the cSEMS group who developed stent migration did not develop RBO, suggesting that the fistula might have been well sustained and served as a drainage route because of the large diameter of the cSEMS. In contrast, two patients in the PS group who developed stent migration developed RBO and required reintervention. In one of these patients, the fistula was identified endoscopically and an uncovered SEMS was deployed; in the other patient, the fistula could not be detected endoscopically, so additional EUS-HGS was performed with successful deployment of the PS. None of the patients developed RBO until the end of the follow-up period.
The reported rate of complications other than RBO after EUS-HGS in previous studies was 7% to 41%.16 From the standpoint of the risk of complications, especially bile leakage, cSEMS are considered preferable. However, endoscopists must be aware of the potential risk of cSEMS, including obstruction of the side branches of the biliary tract, which can lead to cholangitis or liver abscess. In our cohort, the rate of complications other than RBO was 16.4%, with no significant difference between the two groups. Consistent with previous reviews of EUS-HGS, the frequency of bile leakage was higher in the PS group than in the cSEMS group in this study, although the difference did not reach statistical significance.9,10 Therefore, to prevent bile leakage, in addition to the selection of the appropriate stent, attention should also be paid to the distance from the intrahepatic bile duct to the liver surface. Yamamoto et al.17 reported that a short distance of the tract in the liver parenchyma (<2.5 cm) is an independent predictor of the risk of bile leakage. This is because the liver parenchyma may exert tamponade effects. We also encountered two patients who developed a liver abscess in the left hepatic lobe after cSEMS placement in B2. Considering the location of the abscess, we surmised that it might have been caused by blockage of side branches. A partially covered SEMS (pcSEMS) is often used to avoid blockage of the biliary side branches. However, it might still be challenging to prevent side branch blockage because the uncovered portion in a conventional pcSEMS is no greater than 5 mm in length. In EUS-HGS, the stent length in the intrahepatic bile duct should be at least 20 mm to prevent migration. Thus, even if a pcSEMS is used, the covered portion is deployed into the intrahepatic bile duct, which is associated with the risk of side-branch blockage. In fact, the pcSEMS was deployed in both patients. Recently, a dedicated pcSEMS (EGIS biliary stent, double-covered; S&G Biotech Inc.) for EUS-HGS was developed.18 Compared to the conventional pcSEMS, the uncovered portion at the distal end of this new stent is sufficiently long to prevent side branch obstruction. In addition, the stent had a radiopaque marker, indicating the end of the uncovered portion. These advantages may reduce the risk of side-branch blockage. Mediastinal emphysema was observed during the procedure in one patient in the cSEMS group. The transesophageal approach has been reported to carry the potential risk of severe complications, including mediastinal emphysema, mediastinitis, and pneumothorax.19 In this patient, B2 was punctured via the gastric wall just below the esophagus, and the tract was subsequently dilated with an electrocautery dilator. When puncturing B2, we carefully visualized the crus of the diaphragm using EUS to avoid mediastinal punctures. However, both the damaging effect of the self-expandable system of cSEMS and the burning effect of the electrocautery dilator on the diaphragm and the consequent risk of mediastinal emphysema should also be considered.
In the present study, no significant difference was observed in the technical success rate of reintervention or the time taken for the reintervention procedure between the two groups. However, it is worth noting that the technical success rate of the reintervention was 100% in the cSEMS group. In patients in whom a cSEMS was used, reintervention, including stent-in-stent placement and balloon sweeping, was performed using the existing cSEMS. Technically, it was relatively easy to perform because of the large diameter of the cSEMS. In contrast, in patients in whom PS had been used, reintervention usually necessitated stent exchange. From this viewpoint, planned stent exchange of the PS with the cSEMS could contribute to reducing the incidence of cholangitis secondary to stent occlusion. Before the PS is changed, a guidewire needs to be introduced into the intrahepatic bile duct through or along the PS using an ERCP catheter. If this fails, the PS should be retrieved, followed by detection of the fistula using the guidewire. These procedures are technically challenging and could have contributed to the lower success rate of reintervention in the PS group. As the number of patients who underwent reintervention was small in this study, further accumulation of patients would be desirable to obtain more robust results.
This study has several limitations. First, it was a non-randomized retrospective study, which is often associated with a selection bias. Thus, various types of cSEMS and PS were used in this study. A randomized controlled study is required to draw definitive conclusions about the optimal stent selection for EUS-HGS for malignant biliary obstructions. However, we included a relatively large number of patients (n=154) in this study, and EUS-HGS was performed by different endoscopists at the six participating facilities, which could contribute to the validity of our results and show real-world results. Second, the EUS-HGS procedure used, including the bile duct segment puncture and the tract dilator device used, differed significantly between the two groups, which could have influenced the outcomes of EUS-HGS. In terms of TRBO, our multivariate analysis revealed that neither factor had a significant effect on this outcome. However, we could not analyze the influence of these two factors on the rate of complications. A previous study reported that the use of an electrocautery dilator device, including a needle knife, was associated with a higher rate of complications.20-22 Therefore, a high-quality study in a larger cohort is required using a design that excludes the effect of the tract dilator used on the risk of complications. Third, due to the nature of unresectable malignancies, 50 patients (37.3%) died (censored) without RBO. This resulted in an increase in variability in the latter part of the Kaplan-Meier curves of TRBO.
Our study suggests the advantages of cSEMS over PS for EUS-HGS in patients with unresectable malignancies regarding RBO, as shown in similar previous studies. Therefore, it is anticipated that newer cSEMS specifically designed for EUS-HGS can be used to standardize the use of cSEMS.
Footnotes
Conflicts of Interest
The authors have no potential conflicts of interest.
Funding
None.
Author Contributions
Conceptualization: TS, YH; Data curation: KO, MS, IK, AM, YS, YT; Formal analysis: TS; Methodology: TS, YH; Supervision: YH; Visualization: TS; Writing–original draft: TS; Writing–review & editing: all authors.
REFERENCES
- 1.Smith AC, Dowsett JF, Russell RC, et al. Randomised trial of endoscopic stenting versus surgical bypass in malignant low bile duct obstruction. Lancet. 1994;344:1655–1660. doi: 10.1016/s0140-6736(94)90455-3. [DOI] [PubMed] [Google Scholar]
- 2.Aabakken L, Bretthauer M, Line PD. Double-balloon enteroscopy for endoscopic retrograde cholangiography in patients with a Roux-en-Y anastomosis. Endoscopy. 2007;39:1068–1071. doi: 10.1055/s-2007-966841. [DOI] [PubMed] [Google Scholar]
- 3.Itoi T, Ishii K, Sofuni A, et al. Single-balloon enteroscopy-assisted ERCP in patients with Billroth II gastrectomy or Roux-en-Y anastomosis (with video) Am J Gastroenterol. 2010;105:93–99. doi: 10.1038/ajg.2009.559. [DOI] [PubMed] [Google Scholar]
- 4.Nennstiel S, Weber A, Frick G, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: an analysis over 10 years. J Clin Gastroenterol. 2015;49:764–770. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 5.Yamada A, Kogure H, Nakai Y, et al. Performance of a new short-type double-balloon endoscope with advanced force transmission and adaptive bending for pancreaticobiliary intervention in patients with surgically altered anatomy: a propensity-matched analysis. Dig Endosc. 2019;31:86–93. doi: 10.1111/den.13261. [DOI] [PubMed] [Google Scholar]
- 6.Lee TH, Choi JH, Park do H, et al. Similar efficacies of endoscopic ultrasound-guided transmural and percutaneous drainage for malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2016;14:1011–1019. doi: 10.1016/j.cgh.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Khashab MA, El Zein MH, Sharzehi K, et al. EUS-guided biliary drainage or enteroscopy-assisted ERCP in patients with surgical anatomy and biliary obstruction: an international comparative study. Endosc Int Open. 2016;4:E1322–E1327. doi: 10.1055/s-0042-110790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagielski M, Zieliński M, Piątkowski J, et al. Outcomes and limitations of endoscopic ultrasound-guided hepaticogastrostomy in malignant biliary obstruction. BMC Gastroenterol. 2021;21:202. doi: 10.1186/s12876-021-01798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovannini M. EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2019;8(Suppl 1):S35–S39. doi: 10.4103/eus.eus_47_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura T, Higuchi K. Endoscopic ultrasound-guided hepaticogastrostomy: technical review and tips to prevent adverse events. Gut Liver. 2021;15:196–205. doi: 10.5009/gnl20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo J, Giovannini M, Sahai AV, et al. A multi-institution consensus on how to perform EUS-guided biliary drainage for malignant biliary obstruction. Endosc Ultrasound. 2018;7:356–365. doi: 10.4103/eus.eus_53_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isayama H, Hamada T, Yasuda I, et al. TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc. 2015;27:259–264. doi: 10.1111/den.12379. [DOI] [PubMed] [Google Scholar]
- 13.Ogura T, Kitano M, Takenaka M, et al. Multicenter prospective evaluation study of endoscopic ultrasound-guided hepaticogastrostomy combined with antegrade stenting (with video) Dig Endosc. 2018;30:252–259. doi: 10.1111/den.12976. [DOI] [PubMed] [Google Scholar]
- 14.Davids PH, Groen AK, Rauws EA, et al. Randomised trial of self-expanding metal stents versus polyethylene stents for distal malignant biliary obstruction. Lancet. 1992;340:1488–1492. doi: 10.1016/0140-6736(92)92752-2. [DOI] [PubMed] [Google Scholar]
- 15.Nakai Y, Sato T, Hakuta R, et al. Long-term outcomes of a long, partially covered metal stent for EUS-guided hepaticogastrostomy in patients with malignant biliary obstruction (with video) Gastrointest Endosc. 2020;92:623–631. doi: 10.1016/j.gie.2020.03.3856. [DOI] [PubMed] [Google Scholar]
- 16.Ogura T, Higuchi K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J Gastroenterol. 2016;22:3945–3951. doi: 10.3748/wjg.v22.i15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamamoto Y, Ogura T, Nishioka N, et al. Risk factors for adverse events associated with bile leak during EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2020;9:110–115. doi: 10.4103/eus.eus_68_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asai S, Takeshita K, Ichinona T, et al. A novel partially covered metallic stent with a 20-mm long distal bare portion for EUS-guided hepaticogastrostomy. VideoGIE. 2021;6:322–324. doi: 10.1016/j.vgie.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okuno N, Hara K, Mizuno N, et al. Risks of transesophageal endoscopic ultrasonography-guided biliary drainage. Gastrointest Interv. 2017;6:82–84. [Google Scholar]
- 20.Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–1284. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Honjo M, Itoi T, Tsuchiya T, et al. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video) Endosc Ultrasound. 2018;7:376–382. doi: 10.4103/eus.eus_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khashab MA, Messallam AA, Penas I, et al. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175–E181. doi: 10.1055/s-0041-109083. [DOI] [PMC free article] [PubMed] [Google Scholar]


