Abstract
Intranasal (i.n.) vaccination with adjuvant-free plasmid DNA encoding the leishmanial antigen LACK (LACK DNA) has shown to induce protective immunity against both cutaneous and visceral leishmaniasis in rodents. In the present work, we sought to evaluate the safety and effectiveness of d,l-glyceraldehyde cross-linked chitosan microparticles (CCM) as a LACK DNA non-intumescent mucoadhesive delivery system. CCM with 5 μm of diameter was prepared and adsorbed with a maximum of 2.4 % (w/w) of DNA with no volume alteration. Histological analysis of mouse nostrils instilled with LACK DNA / CCM showed microparticles to be not only mucoadherent but also mucopenetrant, inducing no local inflammation. Systemic safeness was confirmed by the observation that two nasal instillations one week apart did not alter the numbers of bronchoalveolar cells or blood eosinophils; did not alter ALT, AST and creatinine serum levels; and did not induce cutaneous hypersensitivity. When challenged in the footpad with Leishmania amazonensis, mice developed significantly lower parasite loads as compared with animals given naked LACK DNA or CCM alone. That was accompanied by increased stimulation of Th1-biased responses, as seen by the higher T-bet / GATA-3 ratio and IFN-γ levels. Together, these results demonstrate that CCM is a safe and effective mucopenetrating carrier that can increase the efficacy of i.n. LACK DNA vaccination against cutaneous leishmaniasis.
Keywords: Intranasal vaccine, DNA-based vaccine, Vaccine delivery systems, Leishmaniasis, Chitosan microparticles, Glyceraldehyde crosslinking
Introduction
Leishmaniasis is a vector borne neglected disease caused by intracellular protozoan parasites of the genus Leishmania. It may display a wide spectrum of clinical manifestations ranging from benign cutaneous lesions to fatal visceral infection. Around 600,000 to 1 million new cases of cutaneous leishmaniasis (CL) and 50,000–90,000 cases of visceral leishmaniasis (VL) are estimated to occur in 98 countries worldwide [1]. Despite the recent advances in the discovery of new antileishmanial compounds, chemotherapy remains inadequate [2]. Although vaccination is considered the most effective means for disease prevention, there is as yet no vaccine approved for human leishmaniasis [3].
A successful leishmanial infection relies on the parasite ability to rapidly induce Th2-type immune responses that in turn prevent activation of effective Th1 and other pro-inflammatory responses involved in parasite killing [4]. We and others have hypothesized that prevention of the robust Th2 responses can be a more effective way to allow the peripheral expansion of protective Th1 responses than the classical vaccination with weaker Th1-biasing antigens [5]. That assumption is supported by the early study by Julia V et. al (1996) showing that mice rendered tolerant to the strong Th2-biasing antigen LACK (Leishmania homologue of receptors for activated C kinase) are more resistant to L. major infection than wild-type animals [6]. As an alternative to thymic expression from birth as used in that study, we have successfully achieved tolerance to LACK through mucosal vaccination. LACK DNA given by the intranasal (i.n.) route have induced protection in mice and hamsters against both cutaneous and visceral leishmaniasis [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. The superiority of the i.n. route in relation to the classical subcutaneous (s.c.) and intramuscular routes was demonstrated with both LACK-containing whole Leishmania amazonensis lysate (LaAg) and LACK DNA itself [7], [13], [15], [17], [18], [19].
However, nasal mucus may reduce antigen contact and uptake by the nasal epithelium and reduce sensitization of the mucosal immune system. Polymeric nanoparticles and microparticles have been extensively explored as effective drug carriers [20]. Chitosan particles are particularly interesting due to their strong positive charge that enables binding to mucins and mucoadhesiveness, besides electrostatic interaction with negatively charged DNA. Their strong cationic nature can also promote opening of intercellular tight junctions and penetration through the mucosal epithelium [21], [22], [23]. Since chitosan particles are highly hygroscopic, polymer crosslinking is important to avoid hydrogel formation and nasal obstruction. Crosslinking with aldehydes such as glutaraldehyde and formaldehyde can be very effective, but their toxicity limits pharmaceutical utilization. On the other hand, as a metabolic product of fructose, d,l-glyceraldehyde is expectedly non-toxic and more adequate for mucosal administration [24].
In the present study, we aimed to investigate the d,l-glyceraldehyde-crosslinked chitosan microparticle (CCM) biocompatibility with the nasal mucosa, and its capacity to maximize the protective effect of i.n. LACK DNA vaccine against cutaneous leishmaniasis.
Materials and methods
Animals and ethics statement: BALB/c mice (male, eight-week-old) were maintained under controlled conditions of room temperature, autoclaved bedding, pelleted food and filtered water. All animal experiments in this study were conducted in compliance with the principles stated in the Guide for the Care and Use of Laboratory Animals (8th Edition, 2011), pre-approved by the Animal Care and Use Committee of the Federal University of Rio de Janeiro (Brazil) under the code CAUAP118.
d,l-glyceraldehyde cross-linked chitosan microparticles (CCM): Chitosan microparticles were prepared by spray drying technique as adapted from Oliveira et al. [24]. Briefly, a solution of 0.7 % w/v acetic acid (Sigma-Aldrich) containing 2.5 % of chitosan of medium molecular weight (Sigma-Aldrich) and subjected to spray drying (Büchi, Switzerland). For cross-linking, particles were suspended in an acetone:water solution (2:1) containing 1.5 % of d,l-glyceraldehyde under agitation at 500 rpm for 30 min at room temperature. Then, CCM were dried under vacuum at room temperature for 24 h and stocked under desication. Particle size distribution was measured by laser diffraction technique (MasterSizer model E, Malvern, UK), while the zeta potential was measured using a Zetamaster (Malvern, UK). For scanning electron microscopy (SEM), dry microparticles were sputtered on double-face tape and these with gold (JFC-129 1300, JEOL) prior to analysis in electron microscope (JPC 1100, JEOL) at 15 kV [25].
LACK DNA adsorption to CCM (LACK DNA/CCM): The pCI-neo plasmid with p36LACK insert (LACK DNA) was used to transform Escherichia coli DH-5αTM (Invitrogen). LACK DNA was extracted from E. coli cultures by the alkaline lysis method using a DNA lipopolysaccharide (LPS)-free extraction kit (Qiagen Giga-Prep - USA) according to manufacturer's instructions. For adsorption onto CCM, varying amounts of LACK DNA (0.2 mg, 0.4 mg, 0.6 mg, 0.8 mg, 1 mg, 1.2 mg, 1.4 mg, 1.6 mg, 1.8 mg and 2 mg) were mixed with 50 mg of CCM in 25 mL solution of citrate–phosphate buffer and ethanol (2:1) pH 5.5. The suspension was incubated for 2 h at 37 °C under agitation of 100 rpm. After this time, samples were washed two times with 10 mL of citrate–phosphate /ethanol buffer above at 1500 rpm / 10 min, and the rate of adsorbed LACK DNA was determined in triplicate in a spectrophotometer (NanoDrop 2000, Thermo Scientific) at 260 nm. Adsorption was calculated by subtracting the amount of DNA in the supernatant from added DNA at the start of incubation. After adsorption, LACK DNA/CCM (1.2 mg:50 mg) was resuspended in PBS to 1 mg of CCM/mL One hundred microliters of that suspension were labeled with 125 μM of ethidium bromide (EtBr) (Sigma-Aldrich). CCM was treated with EtBr in the same way. Images were obtained under visible and ultraviolet lamp in a fluorescence microscope (AxioVision).
Allergenicity: Mice were given two i.n. doses with 2 mg of CCM in 20 μL of PBS (10 uL in each nostril) 7 days apart. Controls received 50 μg of s.c. ovalbumin (OVA) (Sigma-Aldrich) in the same days. For early and late cutaneous hypersensitivity, one week after the last dose animals were challenged s.c. in the footpad with 50 μg of CCM or 50 μg of OVA in 20 μL of PBS, and footpad swelling was measured with a dial caliper 2 h and 24 h later. For eosinophilia, 24 h after challenge mouse blood was collected and eosinophils were counted in Fast Panoptic (Instant-Prov, Newprov) - stained blood smears and expressed in relation to total leukocytes (>400 cells/ sample). For IgE, 24 h after challenge this was assessed in the mouse serum by ELISA using a mouse IgE ELISA kit (BD Bioscience) according to the manufacturer’s instructions.
Acute systemic toxicity:, we developed protocols aimed to evaluate the systemic biocompatibility of CCM, based on “Non-Clinical Safety Testing” (TDR/WHO, 2004). Mice were given two i.n. doses with 2 mg of CCM in 20 µL of PBS seven days apart. For liver and kidney toxicity, positive controls received a single intraperitoneal (i.p.) injection with 1 % carbon tetrachloride (CCl4, Sigma-Aldrich) in 100 μL of peanut oil. Mice were euthanized 24 h after the last sensitizing dose, and aspartate aminotransferase (AST), alanine aminotransferase (ALT) and creatinine were determined at the serum by colorimetric laboratory diagnostic kits according to the manufacturer's instructions (Doles, Brazil). Clinical signs of acute toxicity were evaluated twice a day for 7 days. For lung inflammation animals received two i.n. doses of CCM or PBS as above. Positive controls received two i.n. doses with 10 µg of LPS in 20 µL of PBS. Mice were euthanized 2 h after the second dose, the bronchoalveolar lavage (BAL) fluid collected in 1 mL of PBS, and the total number of cells were counted using a Neubauer chamber.
Histology of nasal mucosa: Mice received one dose with 30 μg of LACK DNA/1.2 mg CCM in 20 µL of PBS by i.n. route. Fifteen minutes after the administration, mice were euthanized, their whole heads were fixed for one week in 10 % formaldehyde solution and decalcified during further 2 weeks using a 14 % ethylenediaminetetraacetic acid (EDTA) (Sigma, USA) solution. Then, they were dehydrated, embedded in paraffin and sliced at a thickness of 4 µm. The slices were deparaffinized and stained with Masson's trichrome.
Vaccination and infection: Mice were given two i.n. doses seven days apart with 30 μg of naked LACK DNA; 30 μg LACK DNA/1.2 mg CCM (LACK DNA / CCM); or 1.2 mg of naked CCM in 20 µL of PBS (10 µL in each nostril). Controls received phosphate buffer solution (PBS) alone or naked CCM in equivalent amounts to LACK DNA. One week after the second dose, animals were s.c. injected in the hind footpad with 105 promastigotes of L. amazonensis (MHOM/BR/75/Josefa). The lesion growth was monitored by periodical measuring the thickness of the footpads with a dial caliper. On day 107 of infection (day 121 post vaccination), mice were euthanized and the parasite loads in the infected footpads were determined by limiting-dilution assay (LDA), as previously described [26]. Briefly, the feet were excised at the knee junction, aseptically skinned, and individually homogenized in M199 medium supplemented with antibiotics (50 µg/mL of streptomycin plus 50 U/mL of penicillin) and 20 % heat inactivated fetal calf serum (Cultilab, Brazil). Triplicate serial dilutions were made in 100 µL of the same medium in flat bottom microplates that were incubated at 26 °C in humid chambers for 10 to 14 days. The original numbers of parasites in each footpad was calculated from the reciprocal of the highest dilution containing promastigotes.
Reverse transcription quantitative PCR (RT-qPCR): Three days after infection challenge, lesion-draining popliteal lymph nodes were homogenized in guanidine isothiocyanate solution 4 M. Complementary DNA (cDNA) was synthetized through ImProm-IITM Reverse Transcription System (Promega, USA) according to manufacturer instructions. RT-qPCR reactions were performed in triplicates for each target in StepOne system (Applied Biosystems, USA) using StepOne v2.1 software. Each reaction consisted of yielded cDNA, 2x SYBER Green Master Mix (Applied Biosystems, USA), forward primer (F) and reverse primer (R) (5 mM each, Promega Corporation, USA). Primers were designed using Primer Express software version 3.0 (Applied Biosystems, USA): Foxp3F: 5́ -GGC CCT TCT CCA GGA CAG A-3′, Foxp3R: 5′ -GGC ATG GGC ATC CAC AGT-3′, GATA3F: 5′ -GAC CCG AAA CCG GAA GAT GT-3′, GATA3R: 5′ -GCG CGT CAT GCA CCT TTT3′, TbetF:5‘ - GCC AGG GAA CCG CTT ATA TG-3′, TbetR: 5′ -AAC TTC CTG GCG CAT CCA-3′. RT-qPCR reaction conditions were: 40 cycles of 95 °C for 30 sec and 60 °C for 1 min. The gene expression of GAPDH was used as endogenous control: GAPDHF 5′ -GGG AAG CCC ATC ACC ATC T-3′, GAPDHR 5′ -GCC TCA CCC CAT TTG ATG TT-3′.
Cytokine production: Infected feet were individually homogenized, and centrifuged at 2000g/10 min for clarification. IFN-γwas quantitated by ELISA using paired rat IgG1 anti-mouse IFN-γ (clone XMG1.2) and biotinylated rat IgG1 anti-mouse IFN-γ (clone R4-6A2) antibodies. Standard curves were prepared with recombinant cytokines according to manufacturer instructions (R&D Systems, USA).
Statistical Analysis: Statistical analysis was employed using One-Way ANOVA and Dunnett post-test for comparison between different groups. These were considered significantly different when p < 0.05.
Results
Microparticle physical properties
Size distribution and zeta potential were determined in CCM showing a mean diameter of 4.77 µm at D (4.3) with an acceptable 2.21 span value, and surface charge (zeta potential) of + 29.8 ± 1.0 mV, compatible with cationic property of chitosan. Adsorption of LACK DNA to CCM reduced the zeta potential to + 20.8 ± 0.6 likely due to the anionic nature of DNA, while the mean particle size remained unchanged (data not shown).
Absence of i.n. CCM allergenicity
The safety of CCM was first evaluated according to its capacity to induce topical allergy. For that, animals were pre-sensitized with two i.n. doses of 2 mg of CCM or two s.c. doses with 50 µg of the known allergen OVA, followed by a s.c. challenge in the footpad with the homologous stimuli one week later. Unlike pre-sensitization with OVA that led to significant footpad swelling 2 and 24 h after homologous challenge, i.n. CCM did not lead to increased swelling in comparison to PBS controls (Fig. 1A). The basal CCM response was more prominent after 24 h than after 2 h probably due to injection itself. Of note, the intended use of CCM is topical in the mucosa not injected s.c., and that animals did not show discomfort after nasal instillation, such as sniffing or scratching as monitored up to 30 h of application. Absence of allergenicity with i.n. CCM, and even s.c. CCM, is confirmed by absence of increased eosinophilia as compared with s.c. OVA (Fig. 1B) or total IgE production (Fig. 1C). Together, these data indicate that in the tested conditions CCM neither induces local nor systemic allergic reactions.
Fig. 1.
Allergenicity of CCM. Mice were immunized with two i.n. doses of CCM (2 mg) with a 7-day interval. Controls were given s.c. OVA or s.c. PBS. One week later, animals were challenged with CCM or OVA. A) Footpad swelling was measured with a caliper 2 and 24 h after challenge. B) Twenty four hours after challenge, the number of blood eosinophils was counted in the blood and C) IgE production was assessed in the mouse serum by ELISA. Arithmetic means ± SD (n = 3) *** p < 0.01 as compared to PBS.
I.n. CCM does not induce detectable systemic toxicity
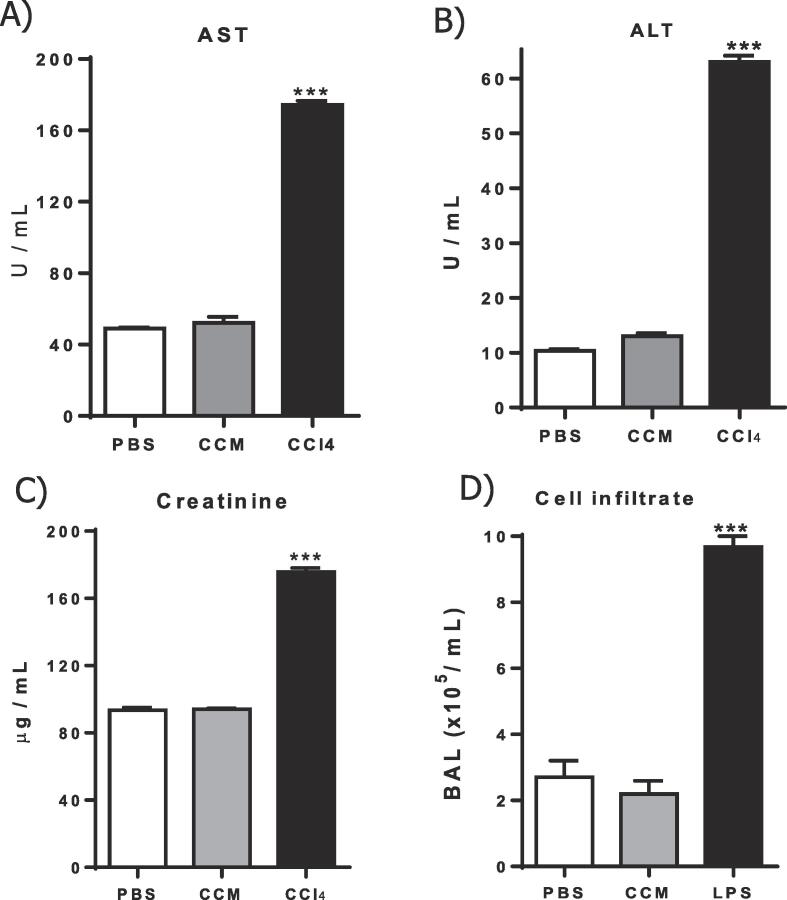
To evaluate the general safety of CCM as a nasal carrier, the serum levels of AST, ALT and creatinine were measured 24 h after the second i.n. dose. Fig. 2A-C show that a total of 4 mg of CCM given intranasally did not induce detectable alterations in any of the biochemical markers, as compared to controls receiving PBS alone.
Fig. 2.
Acute toxicity of CCM. Mice received two i.n. doses of CCM (2 mg), PBS (20 µL), or LPS (10 µL) with a 7-day interval, or a single i.p. injection with 1 % CCl4, as indicated. A-C) Twenty four hours after the second dose, the serum was collected for determination of the indicated injure biomarkers. D) Two hours after de boost dose, the number of cells in the BAL fluid was counted. Arithmetic means ± SD (n = 3). *** p < 0.01 as compared to PBS.
The capacity of i.n CCM to induce lung inflammation was also investigated. For that, the BAL was used to monitor lower airway cell infiltration due both to the difficulty in performing nasal lavage, and the possibility that the nasal particles reach the lungs. Two hours after the second dose of CCM, the BAL was collected, and total leukocytes counted. Unlike instillation with LPS that led to increased BAL cellularity, CCM did not affect cell counts as compared with PBS controls (Fig. 2D). Together, these results show that the glyceraldehyde crosslinked particles are biocompatible and do not induce detectable toxicity to the heart, liver, kidneys and lungs.
CCM is an efficient LACK DNA carrier system
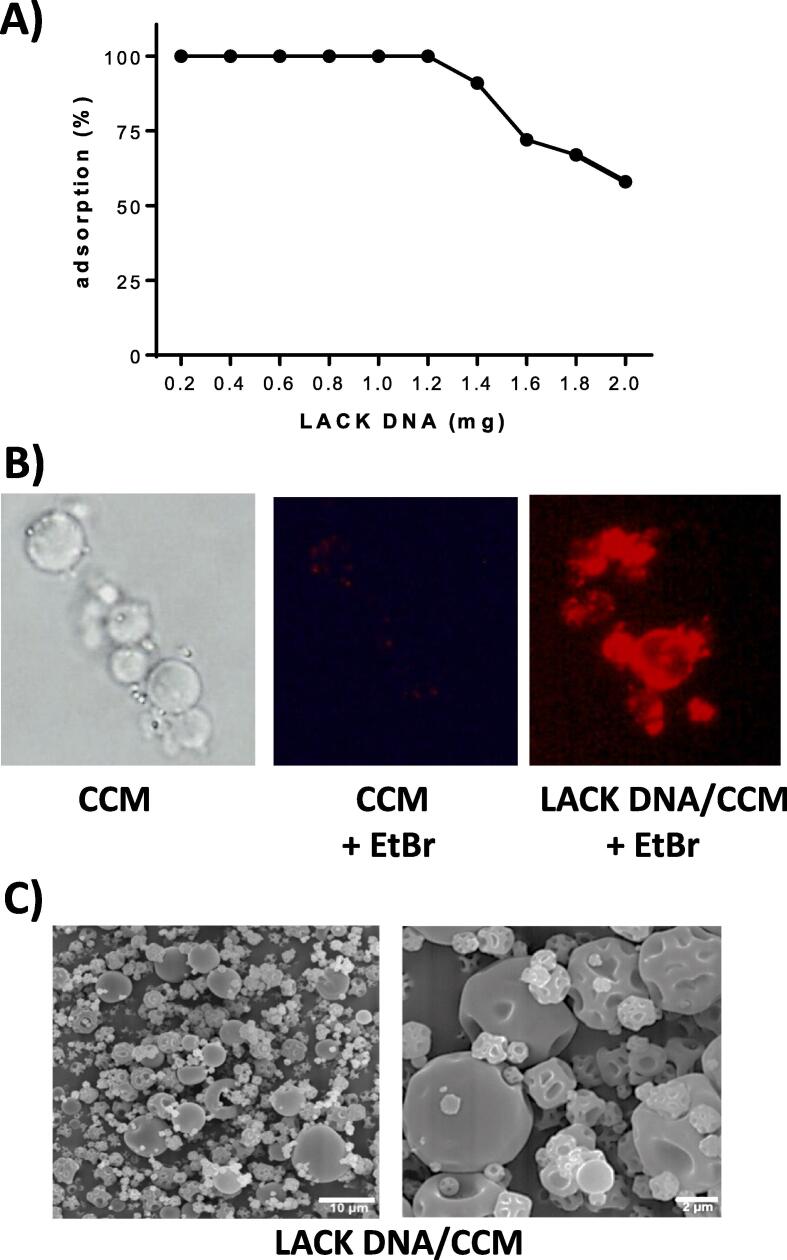
After confirmation of biocompatibility, CCM were tested for the capacity to adsorb and serve as delivery systems for i.n. DNA vaccines. After incubation of CCM with varying amounts of LACK at pH 5.5 for 2 h, DNA was quantified in the supernatant. Fig. 3A shows that as much as 1.2 mg of DNA can be 100 % adsorbed onto 50 mg of CCM, i.e., 0.6 % loading capacity (w/w). Addition of higher LACK DNA amounts results in decreased loading efficiency.
Fig. 3.
Adsorption of LACK-DNA to CCM. A) Varying amounts of LACK DNA were incubated with 50 mg of CCM in citrate–phosphate buffer pH 5.5 and ethanol (2:1) for 2 h at 37 °C under agitation. Then, microparticles were centrifuged and the amount of LACK DNA in the supernatants was determined at 260 nm wavelength. B) Naked CCM and LACK DNA/CCM (1.2 mg) were stained with ethidium bromide and observed under fluorescent microscopy. C) Dried microparticles were imaged by SEM in lower (left) and higher (right) magnification.
To demonstrate LACK DNA adsorption to CCM, ethidium bromide was added to the complex, particles were washed and observed under a fluorescence microscopy (Fig. 4B). Unlike naked CCM that showed no fluorescence of ethidium bromide when exposed to UV light, LACK DNA/CCM showed a high red fluorescence. LACK DNA was stably adsorbed, as no DNA was quantifiable in the supernatants for at least 72 h after suspension in PBS (not shown). These results also show that the glyceraldehyde used for chitosan crosslinking did not prevent the electrostatic binding of DNA, stable enough to resist washing with buffer at pH 5.5, like that of nasal lumen.
Fig. 4.
Uptake of LACK DNA/CCM by the nasal mucosa. Mice (n = 3) were given a single i.n. dose of 30 µg of LACK DNA/1.2 mg CCM in 20 µL of PBS (10 µL in each nostril). After 15 min, the nasal mucosa was analyzed in 4 µm - histological sections stained with Masson's trichrome. A) Longitudinal section of the nose of a representative animal is seen (25 x). B-C) Higher magnification (400 x) of inlets in A. Arrows show microparticles stained red in the nasal epithelium. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
CCM is an efficient LACK DNA mucosal delivery system
To assess the ability of CCM to deliver LACK DNA at the nasal mucosa, the adhesion and interaction of LACK DNA/CCM with the epithelium was investigated in vivo. After 15 min of i.n. instillation with 1.2 mg of LACK DNA / CCM in 20 µL of PBS (10 µL in each nostril), mice were euthanized and microparticle interaction with the nasal epithelium was assessed in histological sections stained with Masson’s trichrome. Fig. 4A shows a mouse nose section with sinuses and central septum with the localization of inlets B and C, magnified in Fig. 4B and 4C, respectively. Red LACK DNA/CCM microparticles are clearly seen adhering and traversing the mucosal epithelium within only 15 min, demonstrating their rapid mucoadhesiveness and penetrating ability.
Efficacy of LACK DNA/CCM as mucosal vaccine against leishmaniasis
We have previously demonstrated the partial efficacy of naked LACK DNA i.n. vaccination against CL (L. amazonensis) [7] and VL (L. infantum) [9], [10]. To investigate whether the strong mucoadhesiveness and penetrating ability of LACK DNA/CCM could enhance vaccine efficacy, mice were pre-vaccinated here with LACK DNA / CCM and naked LACK DNA as in those studies. Controls received naked CCM or PBS vehicle in equivalent amounts. One week after the boost dose animals were subcutaneously challenged in the footpad with L. amazonensis. Vaccine efficacy was monitored both by the growth of lesions through infection and parasite load at the end of the experiment. Fig. 5A shows that pre-vaccination with LACK DNA/CCM significantly (p < 0.01) delayed lesion growth as compared with naked DNA, which at a lesser extent was also delayed (p < 0.05) in relation to PBS controls. Naked CCM was inert. Consistent with their slower lesion growth, animals that were pre-vaccinated with LACK DNA/CCM developed significantly (p < 0.001) lower parasite burdens than non-vaccinated controls and animals given naked LACK DNA (Fig. 5B).
Fig. 5.
Efficacy of LACK DNA/CCM against cutaneous leishmaniasis. BALB/c mice were given two i.n. instillations with 30 μg of LACK DNA or 30 μg LACK DNA/1.2 mg CCM in 20 µL of PBS (10 µL / nostril) with a 7-day interval. Controls received PBS alone or naked CCM in equivalent amounts. Seven days after the booster, animals were infected with 105L. amazonensis promastigotes/footpad, as indicated. A) Lesion sizes were measured at the indicated days. B) The parasite loads were evaluated by LDA on day 107. Arithmetic means ± SD (n = 5). * p < 0.05 and ## p < 0.01 in relation to PBS; *** p < 0.01 in relation to both PBS and CCM; # # # p < 0.01.
LACK DNA/CCM vaccination promoted Th1-type immune responses
The possibility that vaccination efficacy was associated with skewed Th1 responses was investigated. Indeed, increased T-bet concomitant with decreased GATA-3 and Foxp3 gene expression was observed in the lesion-draining lymph nodes of vaccinated mice, as compared with non-vaccinated mice in the critical early days of infection (day 3, Fig. 6 A) measured three days after challenge Early Th1 polarization resulted in long lasting capacity of LACK DNA/CCM to produce IFN-γ in comparison to naked LACK DNA (day 107, Fig. 6 B). These results indicate that in some way, LACK DNA / CCM may be more effective than naked LACK DNA in stimulating a protective immunity.
Fig. 6.
Peripheral Th1 response expansion by vaccination. Mice were fully vaccinated and infected with L. amazonensis, as for Fig. 5. A) After 3 days of infection, gene expression of transcription factors T-bet, GATA-3 and FoxP3 was evaluated in the draining lymph nodes by RT qPCR. GAPDH expression was used as endogenous control and results were expressed as relative quantities (RQ) using the PBS-vaccinated group for normalization. B) After 107 of infection, IFN-γ was measured by ELISA in the infected footpad lysates. Results are representative of 3 different experiments. Means ± SEM (n = 5). **p < 0.05 (vs. LACK DNA), ***p < 0.01 (vs. non-vaccinated PBS).
Discussion
In the last two decades, micro- and nanoparticles of chitosan have been described as interesting carriers for gene delivery [27]. They can increase mucosal DNA delivery by protecting plasmids from the action of DNases in the mucosa; by adhering to the mucosa and slowly releasing the DNA, increasing its absorption; and /or improving the capture of particulate antigens by microfold cells (M cells) and, subsequently, by antigen-presenting cells in the lamina propria [28]. In this work, we attempted to improve the efficacy of LACK DNA vaccine by adsorption onto CCM, and attest its safety to the nasal mucosa.
The extend of d,l-glyceraldehyde cross-linking might determine the number of free amino groups and the positively charged surface of CCM [29]. We observed that our crosslinking process using 10-fold less d,l-glyceraldehyde than used previously [24] did not interfere with the ability of chitosan to bind LACK DNA. An adsorption rate of 100 % was achieved by using 1.2 mg of LACK DNA and 50 mg of CCM, as compared with other studies where only 78 % and 38 % of plasmid DNA adsorption was obtained using similar ratios of plasmid DNA with CCM [30], or polyethylene glycol microparticles [31], respectively.
The histological analysis showed that LACK DNA/CCM attached and entered the nasal mucosal epithelium probably by interactions with the negatively charged mucus mucins [32] followed either by transportation by M cells [33] or paracellular route through tight junctions [34], [35]. No sign of local irritancy or cell infiltration was observed with CCM as opposing to the traditional crosslinking agent glutaraldehyde that is highly cytotoxic [36].
Also, no changes in systemic toxicity parameters were observed, as serum levels of AST, ALT and creatinine and bronchoalveolar cell infiltration remained unchanged after simulation of vaccination schedule with CCM. There is a concern regarding the use of chitosan in individuals allergic to shellfish, since it is obtained through the deacetylation of chitin present in the crustacean exoskeleton. However, topical chitosan bandages proved to be safe in allergic patients [37]. Likewise, no cutaneous sensitization was induced in mice following repeated nasal administration of CCM.
Although intranasal vaccination with LACK DNA alone result in skewed Th1-type immune response in the lesion-draining lymph nodes, this can be pronounced with LACK DNA /CCM. CCM mucosal adjuvanticity apparently operates in a way different from solid lipid nanoparticles (SLN) loaded with retinoic acid that were used to improve the nasal efficacy of LaAg. In that case, the adjuvant effect of SLN was associated with increased Treg cells at the site of infection [38].
This work demonstrated that CCM can be used as a safe i.n. delivery system for LACK DNA vaccine against leishmaniasis. The potential clinical use of CCM as a safe mucoadhesive DNA carrier vaccine for leishmaniasis and other diseases is strenghetened by the recent licensing by the government of India of the world́s first DNA-based for use in people aged 12 years or older against COVID-19, the ZyCov-D [39]. According to the World Health Organization’s ‘COVID-19 Vaccine Tracker and Landscape’, as per today 15 other DNA vaccines, 8 i.n. and 4 oral vaccines are also in clinical development against the novel coronavirus, which may benefit from CCM as a safe mucosal DNA carrier described here.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Vicente Larraga (Centro de Investigaciones Biológicas, Madrid, Spain) for kindly donating the LACK DNA plasmid. This work was funded by CNPQ-Brazil # 312121/2017-2 and FAPERJ-Brazil # E-26/010.002426/2019.
Contributor Information
Eduardo F. Pinto, Email: eduardo@ioc.fiocruz.br.
Izabella P.S. Bezerra, Email: izabella@biof.ufrj.br.
Daniel C.O. Gomes, Email: DGomes@ndi.ufes.br.
Ana Maria B. Martinez, Email: anamartinez@hucff.ufrj.br.
Maria Inês Ré, Email: mariare@mines-albi.fr.
Herbert L. de Matos Guedes, Email: herbert@micro.ufrj.br, herbert@ioc.fiocruz.br.
Bartira Rossi-Bergmann, Email: bartira@biof.ufrj.br.
References
- 1.Carrion C., Robles N., Sola-Morales O., Aymerich M., Ruiz Postigo J.A. Mobile health strategies to tackle skin neglected tropical diseases with recommendations from innovative experiences: Systematic review. JMIR Mhealth Uhealth. 2020;8(12):e22478. doi: 10.2196/22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ponte-Sucre A., Gamarro F., Dujardin J.C., Barrett M.P., López-Vélez R., García-Hernández R., et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017;11(12):e0006052. doi: 10.1371/journal.pntd.0006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iborra S., Solana J.C., Requena J.M., Soto M. Vaccine candidates against leishmania under current research. Expert Rev Vaccines. 2018;17(4):323–334. doi: 10.1080/14760584.2018.1459191. [DOI] [PubMed] [Google Scholar]
- 4.Carneiro M.B., Lopes M.E., Hohman L.S., Romano A., David B.A., Kratofil R., et al. Th1-Th2 Cross-Regulation Controls Early Leishmania Infection in the Skin by Modulating the Size of the Permissive Monocytic Host Cell Reservoir. Cell Host Microbe. 2020;27:752–768.e7. doi: 10.1016/j.chom.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Campos-Neto A. What about Th1/Th2 in cutaneous leishmaniasis vaccine discovery? Braz J Med Biol Res. 2005 Jul;38(7):979–984. doi: 10.1590/s0100-879x2005000700001. [DOI] [PubMed] [Google Scholar]
- 6.Julia V., Rassoulzadegan M., Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science. 1996;274(5286):421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 7.Pinto E.F., Pinheiro R.O., Rayol A., Larraga V., Rossi-Bergmann B. Intranasal vaccination against cutaneous leishmaniasis with a particulated leishmanial antigen or DNA encoding LACK. Infect Immun. 2004:4521–4527. doi: 10.1128/IAI.72.8.4521-4527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinheiro R.O., Pinto E.F., de Matos Guedes H.L., Filho O.A., de Mattos K.A., Saraiva E.M., et al. Protection against cutaneous leishmaniasis by intranasal vaccination with lipophosphoglycan. Vaccine. 2007;25(14):2716–2722. doi: 10.1016/j.vaccine.2006.05.093. [DOI] [PubMed] [Google Scholar]
- 9.Gomes D.C.O., Pinto E.F., Melo L.D.B., Lima W.P., Larraga V., Lopes U.G., et al. Intranasal delivery of naked DNA encoding the LACK antigen leads to protective immunity against visceral leishmaniasis in mice. Vaccine. 2007;25:2168–2172. doi: 10.1016/j.vaccine.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 10.Gomes D.C.O., Souza B.L.S.C., de Matos Guedes H.L., Lopes U.G., Rossi-Bergmann B. Intranasal immunization with LACK-DNA promotes protective immunity in hamsters challenged with Leishmania chagasi. Parasitology. 2011;138(14):1892–1897. doi: 10.1017/S0031182011001417. [DOI] [PubMed] [Google Scholar]
- 11.de Matos Guedes H.L., da Silva Costa B.L., Chaves S.P., de Oliveira Gomes D.C., Nosanchuk J.D., De Simone S.G., et al. Intranasal vaccination with extracellular serine proteases of Leishmania amazonensis confers protective immunity to BALB/c mice against infection. Parasit Vectors. 2014;7:448. doi: 10.1186/1756-3305-7-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leal J.M., Mosquini M., Covre L.P., Stagmiller N.P., Rodrigues R.R., Christensen D., et al. Intranasal vaccination with killed Leishmania amazonensis promastigotes antigen (LaAg) associated with CAF01 adjuvant induces partial protection in BALB/c mice challenged with Leishmania (infantum) chagasi. Parasitology. 2015;142(13):1640–1646. doi: 10.1017/S0031182015001250. [DOI] [PubMed] [Google Scholar]
- 13.Da Silva-Couto L., Ribeiro-Romão R.P., Saavedra A.F., Costa B.L.S., Moreira O.C., Gomes-Silva A., et al. Intranasal vaccination with leishmanial antigen protects golden hamster (Mesocricetus auratus) against Leishmania (Viannia) braziliensis infection. PLoS Negl Trop Dis. 2015;9(1):e3439. doi: 10.1371/journal.pntd.0003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratti J.E., Ramos T.D., Pereira J.C., da Fonseca-Martins A.M., Maciel-Oliveira D., Oliveira-Silva G., et al. Efficacy of intranasal LaAg vaccine against Leishmania amazonensis infection in partially resistant C57Bl/6 mice. Parasit Vectors. 2016;9(1):534. doi: 10.1186/s13071-016-1822-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pereira Silva Bezerra I., Amaral Abib M., Rossi-Bergmann B. Intranasal but not subcutaneous vaccination with LaAg allows rapid expansion of protective immunity against cutaneous leishmaniasis. Vaccine. 2018;36(18):2480–2486. doi: 10.1016/j.vaccine.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Pratti J.E.S., da Fonseca Martins A.M., da Silva J.P., Ramos T.D., Pereira J.C., Firmino-Cruz L., et al. The role of TLR9 on Leishmania amazonensis infection and its influence on intranasal LaAg vaccine efficacy. PLoS Negl Trop Dis. 2019;13(2):e0007146. doi: 10.1371/journal.pntd.0007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto E.F., de Mello C.M., Rossi-Bergmann B. Interferon-gamma-inducing oral vaccination with Leishmania amazonensis antigens protects BALB/c and C57BL/6 mice against cutaneous leishmaniasis. Vaccine. 2003;21(25–26):3534–3541. doi: 10.1016/s0264-410x(03)00427-4. [DOI] [PubMed] [Google Scholar]
- 18.Pinheiro R.O., Pinto E.F., Lopes J.R., Guedes H.L., Fentanes R.F., Rossi-Bergmann B. TGF-beta-associated enhanced susceptibility to leishmaniasis following intramuscular vaccination of mice with Leishmania amazonensis antigens. Microbes Infect. 2005;7(13):1317–1323. doi: 10.1016/j.micinf.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Marques-da-Silva E.A., Coelho E.A., Gomes D.C., Vilela M.C., Masioli C.Z., Tavares C.A., et al. Intramuscular immunization with p36(LACK) DNA vaccine induces IFN-gamma production but does not protect BALB/c mice against Leishmania chagasi intravenous challenge. Parasitol Res. 2005;98(1):67–74. doi: 10.1007/s00436-005-0008-8. [DOI] [PubMed] [Google Scholar]
- 20.Bookstaver M.L., Tsai S.J., Bromberg J.S., Jewell C.M. Improving Vaccine and Immunotherapy Design Using Biomaterials. Trends Immunol. 2018;39(2):135–150. doi: 10.1016/j.it.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buschmann M.D., Merzouki A., Lavertu M., Thibault M., Jean M., Darras V. Chitosans for delivery of nucleic acids. Adv Drug Deliv Rev. 2013;65(9):1234–1270. doi: 10.1016/j.addr.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar A., Vimal A., Kumar A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int J Biol Macromol. 2016;91:615–622. doi: 10.1016/j.ijbiomac.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 23.Kravanja G., Primožič M., Knez Ž., Leitgeb M. Chitosan-based (Nano)materials for Novel Biomedical Applications. Molecules. 2019;24(10):1960. doi: 10.3390/molecules24101960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira B.F., Santana M.H.A., Ré M.I. Spray-dried chitosan microspheres cross-linked with d, l-glyceraldehyde as a potential drug delivery system: preparation and characterization. Braz J Chem Eng. 2005;22(03):353–360. doi: 10.1590/S0104-66322005000300004. [DOI] [Google Scholar]
- 25.Sousa-Batista A.J., Pacienza-Lima W., Ré M.I., Rossi-Bergmann B. Novel and safe single-dose treatment of cutaneous leishmaniasis with implantable amphotericin B-loaded microparticles. Int J Parasitol Drugs Drug Resist. 2019;11:148–155. doi: 10.1016/j.ijpddr.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Titus R.G., Marchand M., Boon T., Louis J.A. A limiting dilution assay for quantifying Leishmania major in tissues of infected mice. Parasite Immunol. 1985;7(5):545–555. doi: 10.1111/j.1365-3024.1985.tb00098.x. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y., Tan Y.F., Wong Y.S., Liew M.W.J., Venkatraman S. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17(6):381. doi: 10.3390/md17060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Devraj K.M., Ramanjaneyulu K., Hima Bindu N., Ramakrishnamacharya S.V. Preparation and Characterization of Chitosan Microspheres Crossed-linked with 2, 3 - Dihydroxypropanal as a competitive Drug Delivery System. J Pharmacy Research. 2011;4(5):1433–1435. doi: 10.1081/ddc-120018377. [DOI] [Google Scholar]
- 30.Oliveira B.F., Santana M.H.A., Ré M.I. Spray-Dried Chitosan Microspheres as a pDNA Carrier. Drying Technol. 2006;24:373–382. [Google Scholar]
- 31.Chen Z., Cai X., Yang Y., Wu G., Liu Y., Chen F., et al. Promoted transfection efficiency of pDNA polyplexes-loaded biodegradable microparticles containing acid-labile segments and galactose grafts. Pharm Res. 2012;2:471–482. doi: 10.1007/s11095-011-0577-4. [DOI] [PubMed] [Google Scholar]
- 32.Dhawan S., Singla A.K., Sinha V.R. Evaluation of mucoadhesive properties of chitosan microspheres prepared by different methods. AAPS Pharm Sci Tech. 2004;5(4):e67. doi: 10.1208/pt050467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kyd J.M., Cripps A.W. Functional differences between M cells and enterocytes in sampling luminal antigens. Vaccine. 2008;26(49):6221–6224. doi: 10.1016/j.vaccine.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 34.Dodane V., Amin Khan M., Merwin J.R. Effect of chitosan on epithelial permeability and structure. Int J Pharm. 1999;182(1):21–32. doi: 10.1016/s0378-5173(99)00030-7. [DOI] [PubMed] [Google Scholar]
- 35.Vllasaliu D., Exposito-Harris R., Heras A., Casettari A., Garnett M., et al. Tight junction modulation by chitosan nanoparticles: comparison with chitosan solution. Int J Pharm. 2010;400(1–2):183–193. doi: 10.1016/j.ijpharm.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Nair M., Best S.M., Cameron R.E. Crosslinking collagen constructs: Achieving cellular selectivity through modifications of physical and chemical properties. Appl Sci. 2020;10:6911- doi: 10.3390/app10196911. [DOI] [Google Scholar]
- 37.Waibel K.H., Haney B., Moore M., Whisman B., Gomez R. Safety of chitosan bandages in shellfish allergic patients. Mil Med. 2011;176(10):1153–1156. doi: 10.7205/milmed-d-11-00150. [DOI] [PubMed] [Google Scholar]
- 38.Bezerra I.P.S., Costa-Souza B.L.S., Carneiro G., Ferreira L.A.M., de Matos Guedes H.L., Rossi-Bergmann B. Nanoencapsulated retinoic acid as a safe tolerogenic adjuvant for intranasal vaccination against cutaneous leishmaniasis. Vaccine. 2019 Jun 19;37(28):3660–3667. doi: 10.1016/j.vaccine.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 39.Momin T., Kansagra K., Patel H., Sharma S., Sharma B., Patel J., et al. Safety and Immunogenicity of a DNA SARS-CoV-2 vaccine (ZyCoV-D): Results of an open-label, non-randomized phase I part of phase I/II clinical study by intradermal route in healthy subjects in India. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]