Abstract
Gastric carcinomas are among the most common cancers in Germany, with approximately 18,000 new cases per year.
About 10 years ago, based on results of the Trastuzumab for gastric cancer (ToGA) trial, the addition of the monoclonal antibody trastuzumab to a platinum-fluoropyrimidine chemotherapy backbone became the standard-of-care 1st-line therapy for human epidermal growth factor receptor 2 (HER2)-positive gastric cancers. Only patients with primary HER2 gene amplification benefit from this therapy. Thus, accurate HER2 gene amplification detection is predictive and critical for therapy selection. As a gold standard the HER2 status is currently determined in tumor tissue specimens using immune histochemistry and fluorescent in situ hybridisation. However, HER2 amplification is detectable in only about 20 % of gastric carcinomas. The recent approval of an antibody-drug conjugate Trastuzumab deruxtecan (T-DXd) and the establishment of a new subgroup of HER2-low tumors due to the bystander effect associated with T-DXd increases the relevance of precise HER2 diagnostics.
Aim of this analysis was to determine the HER2 amplification status from circulating DNA fragments in blood using a HER2 Copy Number Variation assay to establish a minimal invasive approach.
For the present study, a digital droplet PCR-based method was validated relative to established tissue-based methods. Furthermore and most importantly, the changes of HER2 status during therapy were investigated in seven patients indicating that the changes of HER2 status and number of HER2 copies detected in blood can reflect on therapy efficiency and uncover treatment resistance.
1. Introduction
Gastric cancer belongs to the most frequent malignant tumors in Germany with 18000 cases per year (https://seer.cancer.gov/statfacts/html/stomach.html) and nearly 40 % of the patients are younger than 65 years and employed at diagnosis.
For about 10 years, standard-of-care treatment for HER2-positive advanced gastric cancer includes the application of anti-HER2 antibodies (trastuzumab). Human epidermal growth factor receptor 2, also called ERBB2, encoded by ERBB2 on chromosome 17, belongs to the human epidermal growth factor receptor (EGFR) family, which has an extracellular domain (ECD), a transmembrane domain and an intracellular tyrosine kinase domain. Binding of special ligands to the ECD activates signal transduction pathways important for tumor cell differentiation, growth, apoptosis, migration. Since HER2 lacks ligand-binding activity, it dimerizes with EGFR family members, activating numerous downstream signaling pathways that leads to cell transformation [1,2].
Although non-small cell lung cancer, colon, biliary track cancer, urothelial cancer and pancreatic cancers overexpress HER2 protein and/or show gene amplification in diverse proportions of cases, is most prominent in breast and gastric cancers [3]. HER2 protein overexpression is associated with poorly prognosis leading to targeted drug development [4].
The specific HER2 receptor inhibitors reduce tumor cell growth by blocking the oncogenic growth factor receptor HER2. The effectivity of anti-HER2 drugs depends on the overexpression of the HER2 receptor, mostly as a result of gene amplification. Anti-HER2 receptor therapy is not recommended for HER2-negative gastric cancer [5].
The HER2 status testing algorithm involves immunohistochemistry (IHC) followed by fluorescence in situ hybridization (FISH) for moderately HER2 expressing IHC results (IHC2+) [6]. As an alternative to gold standard FISH technology, chromatogenic in situ hybridization (CISH) technology and its variants can be used. In 304 breast cancer specimens, matches of >96 % were measured between FISH and CISH [2,7].
Currently, about 20 % of gastric cancer patients are found to overexpress HER2 with a high likelihood to benefit from treatment with an anti-HER2 antibody therapy [8].
The anti-HER2 antibody trastuzumab is a humanized recombinant monoclonal antibody that specifically binds to HER2 ECD (part IV), suppresses the intracellular signaling and also due to a reduction of HER2 receptors expression. Resistance to trastuzumab appears to be due to tumor heterogeneity. Furthermore, cell surface proteins like mucins, reduce the interaction of HER2 receptors with trastuzumab [9]. Finally, the drug's inhibitory effect is blocked. To overcome resistance problem, various drugs and treatments are developed such as the antibodies pertuzumab, zanidatamab, pargetuximab, tyrosine kinase inhibitors lapatinib and tucatinib as well as the antibody-drug conjugates (ADC) like trastuzumab emtansine and trastuzumab deruxtecan.
In this context, the new ADC trastuzumab deruxtecan is particularly worth mentioning. It consists of a humanized, monoclonal, anti-HER2 antibody bound to a topoisomerase I inhibitor via a peptide-based linker demonstrated an excellent clinical efficacy of trastuzumab deruxtecan (T-DXd) in HER2-positive advanced gastric adenocarcinoma [10,11]. T-DXd targets the same antigen as trastuzumab but demonstrates increased benefit by delivery of the cytotoxic payload. It is partly due to the bystander anti-tumor effect of T-DXd, which occurs when the cytotoxic payload is released into the tumor cells, diffuses across membranes (due to the high membrane permeability of the payload), and then gets into and destroys neighboring tumor cells [12,13]. This property is especially useful for tumors with heterogenous expression of the targeted antigen such as HER2-positive gastric cancer [14]. These data resulted in the establishment of a new subgroup of HER2-low tumors, which could be treated with HER2-targeted agents. HER2-low cohorts are defined as patients with HER2 IHC 2+/FISH− and IHC 1+ disease.
The proportion of patients with HER2-low gastric cancer is estimated to range between 5 % and 19 % [10,15]. Results of the patients in exploratory cohorts of phase II DESTINY-Gastric01 study showed an overall response rate of 26.3 % (n = 5/19) and 9.5 % (n = 2/21) in these respective subgroups.
Furthermore, in contrast to breast cancer tissues, gastric tumor tissues are thought to be intratumorally more heterogenous, leading to a relatively low HER2 detection rate of 20 % of the initially analyzed tumors. It is estimated that this intratumoral heterogeneity leads to false results in about 23 % of the cases [8,16,17].
In 2013, a sensitive approach to detect HER2 gene amplifications was published for breast cancer and gastric cancer cells [[18], [19], [20]]. Here, the circulating tumor DNA (ctDNA) released into blood was used to analyze the gene amplification status by digital droplet PCR based method (ddPCR). Gene amplification is measured using the copy number of the HER2 gene versus reference genes found in liquid biopsies [[21], [22], [23]]. Overall, the ddPCR technology is an excellent tool serving this task enabling specific and very fast measurement of the absolute amount of DNA copies [24]. Secondly, the ctDNA reflects the most up-to-date status of the tumor genome to overcome the intratumoral heterogeneity not visible in tissue analyses [25,26]. In gastric cancers, the concordance between IHC/FISH analyses of the tissue and ddPCR based amplification detection was about 91 % [23,27]. Wang et al. used NGS to determine the HER2 amplification status and analyzed 56 samples. The relative low concordance rate of about 60 % between tissue and liquid biopsy specimens were shown. It is important to consider that sequencing methods are more expensive, require more ctDNA, and are more time-consuming compared to the ddPCR based method.
The aim of our study was the determination of HER2 amplification status of gastric cancer patients in cell free DNA from plasma, in which blood samples were collected next to diagnoses and during treatment. The samples were analyzed retrospectively. For this purpose, ddPCR-based measurements were used and the concordance of the ddPCR based method was reevaluated in relation to the established methods in tumor tissue and respect to the established method from Shoda et al. [21,22]. Furthermore, the cut-off for the assessment of HER2 status was validated with control samples from non-cancer patients.
Liquid biopsy samples from 20 gastric cancer patients, 12 with and 8 without HER2 amplification, and samples from 22 non-cancer patients were analyzed. In total, more than 450 HER2 analyses of 72 blood collection time points were performed.
2. Materials and methods
2.1. Patient recruitment and blood collection
All patients were treated in the university hospital Knappschaftskrankenhaus Bochum GmbH. Patients' informed written consent was obtained prior to sample collection, analysis and usage of clinical data. The ethical committee of the Ruhr University Bochum, Germany, approved collection and analyses of samples for this study (permission number: 16–5962, date 28/02/2017). Peripheral blood samples were collected in K2-EDTA Vacutainer tubes (Becton Dickenson). Subsequently, plasma samples were prepared within 4 h, by centrifugation and stored as described [28].
2.2. HER2 analyses of tissue specimens
The HER2 status of patients were determined using standard procedure of IHC and FISH according to the TOGA rules using tumor tissue samples in routine pathological practice. In brief, for IHC the antibody Clone CB11 (Leica/Novocastra) was used on Leica Bond III automated staining machines according to the instructions of the manufacturer. For FISH, the Zytolight SPEC ERBB2/CEN 17 Dual Color Probe (Zytovision, Berlin, Germany) was used (CEN17: centromere chromosome 17). FISH analysis was performed if samples were scored as IHC score 2+; HER2 amplification by FISH was defined as a HER2/CEN 17 ratio 2 or more.
2.3. Isolation of DNA
Standard plasma volumes for ctDNA isolation by QIAamp circulating nucleic acid kit (Qiagen, Hilden, Germany) were 3 ml for ddPCR following the manufacturer's protocol (Qiagen, Hilden, Germany). The ctDNA isolation was done as described [28]. The ctDNA was analyzed by Bioanalyzer 2100 DNA D1000 chips (Agilent Technologies Inc. Santa Clara, USA) according to manufacturer's instructions.
The genomic DNA of peripheral blood cells of the patients was additionally purified using Qiagen DSP Blood isolation kit for total nucleic acid isolation. Formalin-fixed and paraffin-embedded tissue sections derived from primary tumor tissues were used for DNA isolation by Promega MAXwell 16 FFPE Plus lev DNA purification kit (As1135) with the Promega Maxwell instrument AS2000 or by Promega Reliaprep kit (Promega GmbH, Mannheim, Germany) according to the procedures established for routine clinical use (Institute for Pathology of the Ruhr University Bochum, Germany). To exclude that the detected HER2 amplification originated and got passed on from blood cells we isolated the cellular DNA from blood cells and compared the copy numbers to the values detected in plasma.
2.4. ddPCR assays, CNV calculation and determination of the cut-off level
HER2 gene FAM-labeled detection assay purchased from Bio-Rad was performed in combination with HEX-labeled reference gene detection assays as described previously [20,23,29]. In brief, the CNV of the target gene HER2 was determined by calculation of the ratio of the HER2 concentration (copies/μl) to the reference concentration (copies/μl), times the number of copies of reference in the human genome. The concentration of HER2 was determined 4–6 times simultaneously in relation to the respective reference gene. Information about reference genes and details of the ddPCR assays are summarized in supplementary information (SI Figures S1-S3). The ddPCR approach was established using the gDNA from cell lines of CAPAN1, CFPAC and KATO III, all with HER2 amplification and from cell line HCT116 as a cell line without HER2 amplification as given by Cosmic database (COSMIC https://cancer.sanger.ac.uk/cosmic). A comparison of data and accuracy of ddPCR HER2 analysis was given in supplementary information (Figs. S1–S3). The ddPCR based CNV of HER2 was detectable with a maximal coefficient of variation of 0.1 and an inter-assay variability of 0.3.
Contingency table were analyzed by Graph path prism 4 (9.5.1) using Fisher's exact test (alpha 0.5).
3. Results
3.1. Patients characteristics
The patients'characteristics were summarized in Table 1. We collected blood samples from 20 gastric cancer patients. Twelve patients were assessed as HER2 positive (overexpressed/amplified), of those 9 patients were scored as IHC3+ and 3 patients as IHC2+ with FISH amplification, and 8 patients as negative by tissue IHC and FISH. The dependency of the HER2 status from histological features of the tumor (as reported by Ma et al., 2023 [2]) was not evident in our small group of patients. The patients in the HER2 positive group have 58 % tubular, and 50 % intestinal typed (according to Laurén) adenocarcinoma, and patients in the HER2 negative group had 37 % tubular, and 37 % intestinal typed.
Table 1.
Characteristics of gastric cancer patients.
| ID | age | sex | tissue HER2 IHC score | Initiale TNM stagea | Grading | UICC | type Laurén | Histology adenocarcinoma | response to anti-HER2 chemotherapy |
|---|---|---|---|---|---|---|---|---|---|
| GC11 | 76 | m | 3 + pos | cT3 cN0 M1 (pul) | G2 | IIA | intestinal | moderately differentiated tubular | Responder, sec. resist |
| GC13 | 65 | f | 3 + pos | cT3 N1 Mx (hep, splenic) | G2 | IV | intestinal | moderately differentiated tubular | Responder, sec. resist |
| GC15 | 68 | m | 3 + pos | ypT3 pN1pM1 (lym, per) | G3 | IV | naa | poorly differentiated, Barrett CA | Responder, sec. resist |
| GC02 | 53 | f |
2 + pos, FISH + 6.8 |
uT3 N + M1 (bone metastasis) | G3 | IV | diffuse | poorly differentiated, signet ring cellular | Responder, SD |
| GC20 | 56 | m |
2 + pos, FISH + 6.6 |
uT3 N+ cM0 | G2 | IIB | intestinal | moderately differentiated tubular | Responder, CR |
| GC21 | 60 | m | 3 + pos | uT3 N2 M1 (pul, hep) | G2 | IV | diffuse | AC of the gastroesophageal junction | Responder, sec. resist |
| GC22 | 74 | m | 3 + pos | uTx Nx M1 (pul, hep) | G2 | IV | na | moderately differentiated, gastroesophageal type | Responder, sec. resist |
| GC05 | 72 | m | 3 + pos | uT3 μN + M0 | na | IB | mixed | poorly differentiated | No trastr, SD |
| GC07 | 59 | m | 3 + pos | uT3 μN1 μM1 (hep) | G2/3 | IV | diffuse | poorly differentiated tubular, | Responder, sec. resist |
| GC08 | 63 | m | 3 + pos | uT2 μN2 cM0 | G2 | IIIA | intestinal | well differentiated tubular | Responder, sec. resist |
| GC09 | 74 | m | 3 + pos | cTx cN1 cM (hep, per, splenic) | G1 | IV | intestinal | well differentiated tubular | Responder, sec. resist |
| GC19 | 64 | m |
2 + pos, FISH + 2.3 |
cT3 N0 M0 | G3 | IIA | intestinal | poorly differentiated tubular | Primary resistant |
| GC01 | 63 | m | neg | uT3 μN + M0 | G1 | IIB | intestinal | well differentiated tubular | No trastruzumab |
| GC03 | 66 | m | neg | uT3 μN + M0 | G2 | IIIB | na | AC of the lower third of the oesophagus (AEG) | No trastruzumab |
| GC04 | 32 | f | neg | uT3 μN + M0 | G3 | IIB | diffuse | poorly differentiated, signet ring cellular | No trastruzumab |
| GC14 | 66 | f | neg | uT3 μN + M0 | G2 | IIIB | na | moderately differentiated tubular AC of gastric antrum | No trastruzumab |
| GC16 | 58 | m | neg | uT3+ μN + M0 | G2 | IIIA | na | moderately differentiated tubular | No trastruzumab |
| GC17 | 61 | m | neg | uTx N0 M1 (hep) | G3 | IIIC | diffuse | poorly differentiated | No trastruzumab |
| GC18 | 60 | m | neg | uT3 μN + cM0 | G3 | IIB | intestinal | poorly differentiated | No trastruzumab |
| GC10 | 52 | m | na | pT3 pN1 cM0 L1 V0 Pn1 R0 | G3 | IIIA | na | poorly differentiated, distal oesophageal type | No trastruzumab |
na, not assessed or data missing, HER2 amplification was assessed by FISH >2.4, IHC score according to TOGA criteria, *Hep - hepatic, pul - pulmonal, per – peritoneal, f – female, m – male, AC adenocarcinoma, responder, sec resist – secondary resistant. The non-cancer group of patients includes patients with gastrointestinal symptoms without signs of malignancy.
3.2. HER2 amplification status in tissue: comparison of CNV detection methods IHC/FISH versus ddPCR
As no tissue was available from one patient, the comparison between IHC/FISH results and ddPCR CNV measurement was performed on 11 of 12 GC patients. The ddPCR-based copy number variation assay (CNV), described in Material & Methods section, can detect HER2 gene amplification in 10 of 11 samples in genomic DNA from tumor tissue (Table 2). The one tCNV not consistent with the IHC3+ IHC result derived from a patient with an intestinally typed tubular adenocarcinoma. For this purpose, the cut-off of 2.4 established by Shoda et al., 2015 was used [21]. The results of ddPCR based HER2 CNV detection using tissue gDNA (tCNV) were in 91 % agreement with the IHC/FISH analyses of the tissue material (p = 0.0006*, likelihood ratio 2.8). The Spearman's coefficients were summarized in Supplementary Fig. S4A. All reference genes had a strong concordance to each other (Spearman coeffient >0.88, supplementary file Fig. S4).
Table 2.
ddPCR based CNV measurement: comparison tissue DNA versus ctDNA.
| tissue gDNA Baseline |
ctDNA first sample |
|||||||
|---|---|---|---|---|---|---|---|---|
| pat | Patho HER2 status (TOGA) | Mean tCNV∗1 | tCNV Ref_Chr.17 | gDNA HER2 status | T0 vs B0 (weeks) | Mean pCNV∗1 | pCNV Ref_Chr.17 | ctDNA HER2 status |
| GC11 | Her2 pos | 1.75 ± 0.13 | 2.21 | neg | 58 | 1.9 ± 0.3 | 2.26 | neg |
| GC13 | Her2 pos | 3.96 ± 0.6 | 3.15 | pos | 30 | 2.2 ± 0.4 | 1.94 | neg |
| GC15 | Her2 pos | 16.8 ± 4.7 | 16.3 | pos | 2 | 1.9 ± 0.4 | 1.53 | neg |
| GC2 | Her2 pos | 11.5 ± 4.1 | 11.5 | pos | 1 | 6.2 ± 1.3 | 7.4 | pos |
| GC20 | Her2 pos | 55.1 ± 12.3 | 33 | pos | 7 | 2.2 ± 0.1 | 2.38 | neg |
| GC21 | Her2 pos | 12.1 ± 1.0 | 11.26 | pos | 3 | 1.9 ± 0.3 | 1.74 | neg |
| GC22 | Her2 pos | 49 ± 5.5 | 23.4 | pos | 0 | 30.3 ± 6.1 | 17.4 | pos |
| GC5 | Her2 pos | 2.9 ± 0.5 | 2.57 | pos | 5 | 2.0 ± 0.4 | 1.9 | neg |
| GC7 | Her2 pos | 3.4 ± 0.6 | 2.35 | pos*2 | 73 | 2.2 ± 0.6 | 1.8 | neg |
| GC8 | Her2 pos | 4.7 ± 0.3 | 2.13 | pos*2 | 60 | 2.1 ± 0.2 | 1.97 | neg |
| GC9 | Her2 pos | 4.2 ± 1.4 | 5.2 | pos | 17 | 1.9 ± 0.3 | 1.74 | neg |
| GC19 | Her2 pos | na | na | No tissue | 12 | 1.9 ± 0.4 | 1.8 | neg |
The ddPCR based analysis of the reference gene located near the HER2 gene on chromosome 17 can successfully distinguish polysomy of chromosome 17 from HER2 gene amplification (patients GC7 and GC8) (Table 2, all data are listed in the supplementary file 2).
Table 2 CNV of HER2 gene were determined from specimens of patients with positive HER2 status using ddPCR assays. tCNV - gDNA from tissue specimens, pCNV – ctDNA from plasma used in ddPCR CNV assays. Sensitivity of the ddPCR-based assay from tissue derived gDNA is 90 % with a specificity of 100 %, likelihood +2.8, p-value 0.0006 (Fisher's test alpha 0.05). If the blood was drawn simultaneously to the tissue sampling, HER2 gene amplification can be detected in the ctDNA from blood. T0 vs B0 = time between tissue removal date T0 and first blood collection date B0. *1ddPCR based CNV-analysis HER2 gene (± standard deviation) on chromosome 17 versus 4 different reference genes (EIF2C1 Chr1, RPP30 Chr10, RPPH1 Chr14 und TERT Chr5), *2 Comparison of CNV calculated by reference on chromosome 17 versus CNV using the reference genes outside chromosome 17 can indicate polysomy of chromosome 17.
3.2.1. Results of CNV using circulating tumor DNA (pCNV)
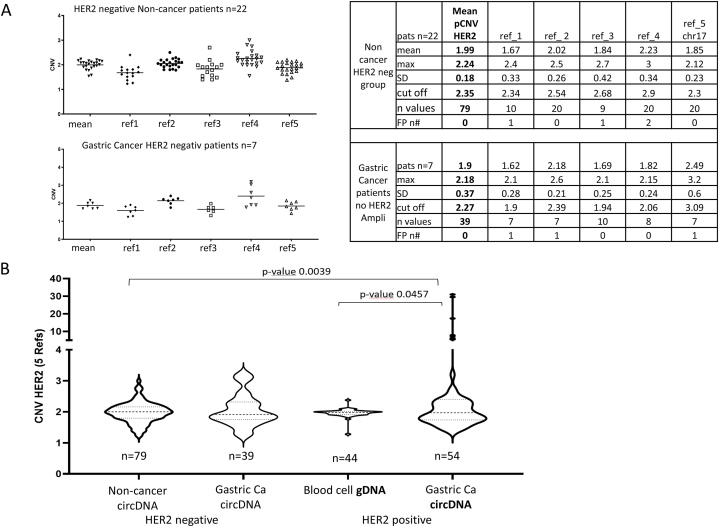
The cut-off value reported by Shoda et al., 2015 [21] was tested using samples from patients without malignant disease and from gastric cancer patients who were classified as HER2-negative based on tissue biopsy analysis (Fig. 1). The pCNV varied dependently from the reference gene used. If only one reference gene used for pCNV determination, some sample would be classified as HER2 amplified in the specimens of non-cancer people (supplementary file Fig. S4D).
Fig. 1.
Re-Validation of cut-off CNV depending on reference genes and patient groups: A CNV is dependent on the reference gene used for CNV determination. The cut off at 2.4 given by Shoda et al., 2015 can be used for diagnosis if mean CNV values from 4 to 5 reference genes are used for HER2. CNV-HER2 values calculated with one reference gene, on the other hand, lead to false positive classifications. B CNV values per patient group determined with 5 reference genes were summarized by violin plots. Blood cell-derived genomic DNA from gastric cancer patients with HER2 amplification can be used as a negative control to classify ctDNA-based CNV values (for details see supplementary information). (SD – standard deviation, FP n# - number of false positive detected patients).
In the small group analyzed herein, the cut off of 2.4 given by Shoda group can discriminate the groups without false positive results, if the results of 4–5 reference genes were used.
In order to exclude that changes of hematopoietic cells influence the pCNV, the cellular gDNA from blood cells of corresponding plasma samples was additionally measured by ddPCR from 6 HER2 positive patients. In the 6 specimens of HER2 positive patients, the cellular gDNA derived CNV was below the cut off of 2.4 (Supplementary Fig. S5), suggesting that the cellular gDNA can be used as an individual control to the pCNV measurement.
Due to the high individual variability of reference gene copies in patient samples, only the measurement of HER2 gene copies in relation to several different reference genes can be used to determine the mean CNV. Narrowing down HER2 CNV determination based on only 1–2 reference genes would lead to frequent false positive results (Fig. 1). The measurements of the patient collectives confirmed the established cut-off value of 2.4 in gastric carcinoma and the concordance values obtained in 60 patients by Shoda et al., 2017 (72–93 %) [22].
Thus, the ddPCR-based detection method for HER2 amplification in gastric carcinoma from ctDNA in blood is qualified to monitor the changes during treatment.
The good agreement between IHC/FISH and tissue based CNV determination (tCNV) (Table 2) was not confirmed regarding the pCNV from specimens at the first sampling time. In two cases the HER2 amplification can be accurately measured using circulating tumor DNA (ctDNA) from plasma, resulting in a sensitivity and specificity of 0.2 and 1, with an estimated likelihood ratio of 20 (p-value 0.48; supplementary file Fig. S4C). In one case the pCNV confirmed the negative tCNV result.
Blood samples of the two accurately detected cases (GC2 and GC22, Table 2) were obtained at nearly the same time (±1 day) as cancer tissue sampling. In all other cases the blood collection took place after the start of therapy or after surgery. The tissue of the patient GC2 was described as a signet-ring cell tumor of diffuse type according to Laurén with moderate HER2 expression (IHC2+) and HER2 amplification detected by FISH. The tissue of the 2nd pCNV HER2 positive patient showed high HER2 expression (IHC3+) and was typed as gastroesophageal moderate differentiated tumor tissue.
In seven patients with tissue HER2 positive status, pCNV was measured during therapy. After initiation of therapy, no amplification of the HER2 gene was detectable in any patient sample (Table 2, Fig. 2). The amount of ctDNA was determined by absolute HER2 gene copies measured in ddPCR and additionally by Bioanalyzer measurements (Fig. 2, Supplementary Fig. S6). The course of therapy is reflected in changes in pCNV and in the amount of HER2 ctDNA in the blood (Fig. 2A). When the tumor burden is high during disease progression, both HER2-ctDNA levels and CNV levels increase. In patients with complete remission or stable disease, HER2 ctDNA levels remain low and pCNV level remain constant (Fig. 2B).
Fig. 2.
HER2 CNV monitoring of gastric cancer patient during treatment with initial HER2 amplification. Monitoring of HER2 CNV (red) from HER2 copy number relative to 4–5 reference gene copies and absolute amount of HER2 ctDNA (blue) were measured at different time points during therapy. A HER2 CNV and ctDNA HER2 cop/μl decrease with response to initial therapy in a stage IV patient. Response to therapy is reflected by the decrease in CNV and absolute levels of ctDNA. After a stable phase of disease, the ctDNA-HER2 amount and HER2-CNV increase again with disease progression (left). The ctDNA bioanalyzer show increased amount of ctDNA at 165 bp at the beginning of therapy and during progression (right). B HER2-CNV monitoring of 6 patients: HER2-CNV and HER2 ctDNA remain consistently low in patients with stable disease or complete remission; with relapse and progression, HER2 ctDNA levels increase. CR complete remission, PD progression disease, SD stable disease, ED diagnosis of gastric cancer.
This work is a preliminary experience based on a limited series of cases (summarized in Supplementary Fig. S7).
4. Discussion
Gastric cancer is a heterogenous group of diseases with different responsiveness to treatments and therapeutic decisions should consider the molecular tumor profile. The benefit of trastuzumab treatment is limited to gastric cancer with HER2 overexpression status of IHC 3+ or moderate overexpression (IHC 2+) with HER2 gene amplification (FISH positive) [30].
A main prerequisite for the accurate molecular characterization of the tumor is the selection of the optimal analysis method. The TOGA criteria summarizing the properties for detection of HER2 amplifications in tissues are based on two technologies, FISH and IHC. Both analysis methods are time-consuming, resource-intensive. The chromatogenic in situ hybridization (CISH) technology and its variants was developed to reduce the costs. Both FISH and CISH are approved by FDA and gave concordant results in over 98 % of breast cancer specimens [7]. However, sometimes IHC/FISH analyses gave false results. The use of different fixatives and the storage time of specimens influenced the detectability of HER2 [31]. Particularly, for gastric cancer tumors with IHC 2+, the rate of HER2 gene amplification in tumor tissue specimens was only 38–56 %, probably due to intratumoral heterogeneity. The discordance of HER2 gene amplification results among surgically resected and biopsy specimens underscores this feature [[31], [32], [33], [34]].
Analysis of ctDNA from blood (liquid biopsies) instead of tissue specimens can be advantageous to prevent from false results. For liquid biopsy samples the ddPCR approach can be used to detect the HER2 amplification [18,23,27]. In principle, liquid biopsy based HER2 detection can overcome the dependence on the quality of the tissue biopsy, which in turn is influenced both by the marked heterogeneity in gastric cancer and by the type of fixation and the age of the sample.
The preliminary data presented here, support a cut-off level of HER2 CNV amplification at > 2.4, in particular, if it was used in relation to at least 4–5 reference genes. This value is relatively low for example in contrast to the CNV cut-off level estimated by whole genome sequencing approaches given with >4 [27,35]. These recently used methods to determine the CNV status of HER2 need more resources as the ddPCR based technology. The ddPCR pCNV detection on 60 gastric cancer and 30 “healthy” patients had shown a sensitivity and specificity of 0.73 and 0.93 (p-value 0.001) using preoperative samples [22]. The data on 29 gastric cancer patients with preoperative corresponding liquid biopsy samples gave concordance between tCNV and pCNV of 96.6 % using a cut off of 2.4 [36].
The liquid biopsy based pCNV determination is very strongly dependent on the time of blood collection in relation to the therapy [22,31]. All three samples collected at baseline confirmed the tissue based CNV determination, and showed two HER2 positive and one HER2 negative results. 84 % of blood samples in the small group of cases presented here were collected after treatment initiation and had pCNV below the cut off of 2.4. The loss of HER2 positivity during treatment of gastric cancer was described also in tissue samples and it was associated with resistance to trastuzumab therapy regimen [31,34].
The potential of ddPCR based HER2 amplification measurement from blood, as previously described for breast, colon, and gastric tumors, was also confirmed in the gastric cancer samples presented herein. The HER2 amplification status may change due to clonal growth and intratumoral selection during the progression of the tumor. Newer approaches demonstrated that 54 % of treated gastric cancer patients altered the initially measured HER2 status during therapy [22,32]. The systematic comparison of molecular analyses of tissue-derived versus liquid biopsy-derived DNA collected simultaneously from 23 patients revealed that molecular changes were found more frequently in liquid biopsy samples than in tissue biopsies, 87 % versus 48 % respectively [35]. In addition, diverse HER2 expression in metastatic lesions and primary tumors [27,37] together with HER2 expression levels changing during treatment, reinforce the need for testing subsequent to diagnosis during therapy and at disease progression.
The present data on therapy progression of gastric cancer patients show that, as in other tumor entities, tumor cells with HER2 amplification are effectively reduced under targeted therapy. Tumor cells with HER2 amplification are no longer measurable in liquid biopsy as a sign of efficient therapy. Conversely, disease progression can lead to a redetection of tumor cell DNA, i.e., detection of HER2 amplification from blood. In particular, the approach can help to map the success of a (also neoadjuvant) therapy.
As a minimally invasive procedure the liquid biopsy based HER2-CNV detection method is more suitable to monitor the molecular changes during therapy as tissue biopsies. The liquid biopsy approach eliminates the need for hospitalization associated with side-effect-prone tissue removal. The procedure is risk-reducing for patients and particularly resource-saving due to the short time required for analysis (only one working day from ctDNA isolation to the result) and the relatively low material cost. The clinical application of the method, particularly for the evaluation of therapy success, is therefore desirable. Furthermore, there is a considerable socioeconomic aspect: since approximately 40 % of gastric cancer patients are younger than 65 years at the time of diagnosis and are thus capable of working, the non-invasive examination procedure preserves the ability to work of many of those affected and results in fewer costs due to absences from work.
In view of the more effective HER2-targeting therapies that are coming into clinical use like Trastuzumab deruxtecan (T-DXd) and the need to differentiate the new subgroup of HER2-low, there is an increasing need for more precise and sensitive HER2 testing methods to identify patients who might benefit from anti-HER2 targeted treatment.
5. Conclusion
The data presented here may serve as a basis for clinical studies that help to assess a prognostic or predictive value of the analytical procedure in particular in respect to the new treatment options like T-Dxd which was approved from FDA for treatment of advanced HER2-positive gastric cancer in January 2021. In breast cancer, HER2-low expressing tumors represent a subgroup benefiting from T-Dxd. The definition of the HER2-low status includes either tumors with an IHC score 1+ or with an IHC score 2+ but without HER2 amplification evidence. The liquid biopsy based ddPCR approach with its robustness and performer independency can be advantageous to define the subgroup of HER2-low expressing gastric tumors.
Studies analyzing HER2 expression ctDNA analyses will help to enlighten the connection between HER2 status and for example T-DXd efficacy and the bystander antitumor effect, probably resulting in more accurate patient selection and precise prediction of response.
Basically, the determination of HER2 positivity is one of the crucial challenges considering the development of HER2-targeted therapies for gastric cancer. Importantly, the semi-quantitative determination of HER2 expression in IHC and the HER2 amplification detection, could not provide a dichotomous distribution of HER2 status, but rather there is a continuous spectrum with cut-off point to be determined. The non-invasive determination of the current valid HER2 status via liquid biopsy would significantly increase the specificity of the subgroup definition of patients for different anti-HER2 drug and would thus represent a milestone in the development of targeted therapies.
The minimal invasive and easy to use approach of pCNV detection by liquid biopsy would open the possibility to follow the dynamic changes of HER2 expression. This would have important implications for anti-HER2 treatments and open the way to detecting residual resistance and avoid the burden on patients during tissue biopsy.
Data availability statement
The data associated with the study has been included in the supplementary material file provided with the manuscript.
CRediT authorship contribution statement
Susanne Klein-Scory: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. Swetlana Ladigan-Badura: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. Thomas Mika: Writing – review & editing. Berlinda Verdoodt: Resources, Writing – review & editing. Andrea Tannapfel: Resources, Writing – review & editing. Michael Pohl: Writing – review & editing. Roland Schroers: Supervision, Writing – review & editing. Alexander Baraniskin: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The work was supported by financial funding from Gregorius Agricola foundation Ruhr (Germany) and from Ruhr University Bochum Department of Medicine FoRUM 19-6805. The publication costs were financed by the DFG-publication fond via Ruhr University Bochum. We want to thank Christina Eilert-Micus and Sabine Bachmann for excellent technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21339.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Swain S.M., Shastry M., Hamilton E. Targeting HER2-positive breast cancer: advances and future directions. Nat. Rev. Drug Discov. 2023;22:101–126. doi: 10.1038/s41573-022-00579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma C., Wang X., Guo J., Yang B., Li Y. Challenges and future of HER2-positive gastric cancer therapy. Front. Oncol. 2023;13 doi: 10.3389/fonc.2023.1080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan M., Schwaederle M., Arguello D., Millis S.Z., Gatalica Z., Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roviello G., Catalano M., Iannone L.F., Marano L., Brugia M., Rossi G., et al. vol. 24. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico; 2022. pp. 981–996. (Current Status and Future Perspectives in HER2 Positive Advanced Gastric Cancer). [DOI] [PubMed] [Google Scholar]
- 5.Bang Y.-J., van Cutsem E., Feyereislova A., Chung H.C., Shen L., Sawaki A., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England) 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Boku N. HER2-positive gastric cancer. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2014;17:1–12. doi: 10.1007/s10120-013-0252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mollerup J., Henriksen U., Müller S., Schønau A. Dual color chromogenic in situ hybridization for determination of HER2 status in breast cancer: a large comparative study to current state of the art fluorescence in situ hybridization. BMC Clin. Pathol. 2012;12:3. doi: 10.1186/1472-6890-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Cutsem E., Bang Y.-J., Feng-Yi F., Xu J.M., Lee K.-W., Jiao S.-C., et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Price-Schiavi S.A., Jepson S., Li P., Arango M., Rudland P.S., Yee L., et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int. J. Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 10.Shitara K., Bang Y.-J., Iwasa S., Sugimoto N., Ryu M.-H., Sakai D., et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020;382:2419–2430. doi: 10.1056/NEJMoa2004413. [DOI] [PubMed] [Google Scholar]
- 11.Shitara K., Baba E., Fujitani K., Oki E., Fujii S., Yamaguchi K. Discovery and development of trastuzumab deruxtecan and safety management for patients with HER2-positive gastric cancer. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2021;24:780–789. doi: 10.1007/s10120-021-01196-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azar I., Alkassis S., Fukui J., Alsawah F., Fedak K., Al Hallak M.N., et al. Spotlight on trastuzumab deruxtecan (DS-8201,T-DXd) for HER2 mutation positive non-small cell lung cancer. Lung cancer (auckland, N.Z.) 2021;12:103–114. doi: 10.2147/LCTT.S307324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modi S., Jacot W., Yamashita T., Sohn J., Vidal M., Tokunaga E., et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 2022;387:9–20. doi: 10.1056/NEJMoa2203690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Cutsem E., Di Bartolomeo M., Smyth E., Chau I., Park H., Siena S., et al. LBA55 - primary analysis of a phase II single-arm trial of trastuzumab deruxtecan (T-DXd) in western patients (Pts) with HER2-positive (HER2+) unresectable or metastatic gastric or gastroesophageal junction (GEJ) cancer who progressed on or after a trastuzumab-containing regimen. Ann. Oncol. : official journal of the European Society for Medical Oncology. 2021;32:S1283–S1346. [Google Scholar]
- 15.Yamaguchi K, Bang Y-J, Iwasa S, Sugimoto N, Ryu M-H., Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, Saito K, Kawaguchi Y, Kamio T, Kojima A, Sugihara M, Shitara K. 1422MO Trastuzumab deruxtecan demonstrates efficacy in HER2-POSITIVE and HER2-LOW advanced gastric cancer: findings from a post-hoc exploratory biomarker analysis of DESTINY-Gastric01 study. Ann Oncol. 31, 2020;S4,:S899–900. doi: 10.1016/j.annonc.2020.08.1928. https://www.annalsofoncology.org/article/S0923-7534(20)41924-6/pdf [DOI] [Google Scholar]
- 16.Warneke V.S., Behrens H.-M., Böger C., Becker T., Lordick F., Ebert M.P.A., et al. Her2/neu testing in gastric cancer: evaluating the risk of sampling errors. Ann. Oncol. : official journal of the European Society for Medical Oncology. 2013;24:725–733. doi: 10.1093/annonc/mds528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wakatsuki T., Yamamoto N., Sano T., Chin K., Kawachi H., Takahari D., et al. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J. Gastroenterol. 2018;53:1186–1195. doi: 10.1007/s00535-018-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Murillas I., Lambros M., Turner N.C. Determination of HER2 amplification status on tumour DNA by digital PCR. PLoS One. 2013;8 doi: 10.1371/journal.pone.0083409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gevensleben H., Garcia-Murillas I., Graeser M.K., Schiavon G., Osin P., Parton M., et al. Noninvasive detection of HER2 amplification with plasma DNA digital PCR. Clin. Cancer Res. : an official journal of the American Association for Cancer Research. 2013;19:3276–3284. doi: 10.1158/1078-0432.CCR-12-3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belgrader P., Tanner S.C., Regan J.F., Koehler R., Hindson B.J., Brown A.S. Droplet digital PCR measurement of HER2 copy number alteration in formalin-fixed paraffin-embedded breast carcinoma tissue. Clin. Chem. 2013;59:991–994. doi: 10.1373/clinchem.2012.197855. [DOI] [PubMed] [Google Scholar]
- 21.Shoda K., Masuda K., Ichikawa D., Arita T., Miyakami Y., Watanabe M., et al. HER2 amplification detected in the circulating DNA of patients with gastric cancer: a retrospective pilot study. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2015;18:698–710. doi: 10.1007/s10120-014-0432-5. [DOI] [PubMed] [Google Scholar]
- 22.Shoda K., Ichikawa D., Fujita Y., Masuda K., Hiramoto H., Hamada J., et al. Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer. Gastric Cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2017;20:126–135. doi: 10.1007/s10120-016-0599-z. [DOI] [PubMed] [Google Scholar]
- 23.Kinugasa H., Nouso K., Tanaka T., Miyahara K., Morimoto Y., Dohi C., et al. Droplet digital PCR measurement of HER2 in patients with gastric cancer. Br. J. Cancer. 2015;112:1652–1655. doi: 10.1038/bjc.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Bae Y., Shibayama S., Wang X., Kato M., Dong L. International co-validation on absolute quantification of single nucleotide variants of KRAS by digital PCR. Anal. Bioanal. Chem. 2022;414:5899–5906. doi: 10.1007/s00216-022-04155-8. [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Li B., Liu Z., Gong J., Shao L., Ren J., et al. HER2 copy number of circulating tumour DNA functions as a biomarker to predict and monitor trastuzumab efficacy in advanced gastric cancer. Eur. J. Cancer. 2018;88:92–100. doi: 10.1016/j.ejca.2017.10.032. https://www.sciencedirect.com/science/article/pii/S0959804917313722 Available from: [DOI] [PubMed] [Google Scholar]
- 26.Shen L. Liquid biopsy: a powerful tool to monitor trastuzumab resistance in HER2-positive metastatic gastric cancer. Cancer Commun. 2018;38:72. doi: 10.1186/s40880-018-0344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang D.-S., Liu Z.-X., Lu Y.-X., Bao H., Wu X., Zeng Z.-L., et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut. 2019;68:1152. doi: 10.1136/gutjnl-2018-316522. [DOI] [PubMed] [Google Scholar]
- 28.Klein-Scory S., Maslova M., Pohl M., Eilert-Micus C., Schroers R., Schmiegel W., et al. Significance of liquid biopsy for monitoring and therapy decision of colorectal cancer. Transl. Oncol. 2018;11:213–220. doi: 10.1016/j.tranon.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hindson B.J., Ness K.D., Masquelier D.A., Belgrader P., Heredia N.J., Makarewicz A.J., et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal. Chem. 2011;83:8604–8610. doi: 10.1021/ac202028g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi S.S., Badgwell B.D. Current treatment and recent progress in gastric cancer. CA A Cancer J. Clin. 2021;71:264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saeki H., Oki E., Kashiwada T., Arigami T., Makiyama A., Iwatsuki M., et al. vol. 105. European journal of cancer; 2018. pp. 41–49. (Re-evaluation of HER2 Status in Patients with HER2-Positive Advanced or Recurrent Gastric Cancer Refractory to Trastuzumab (KSCC1604)). (Oxford, England : 1990) [DOI] [PubMed] [Google Scholar]
- 32.Yoshida H., Yamamoto N., Taniguchi H., Oda I., Katai H., Kushima R., et al. Comparison of HER2 status between surgically resected specimens and matched biopsy specimens of gastric intestinal-type adenocarcinoma. Virchows Arch. : Int. J. Pathol. 2014;465:145–154. doi: 10.1007/s00428-014-1597-3. [DOI] [PubMed] [Google Scholar]
- 33.Seo S., Ryu M.-H., Park Y.S., Ahn J.Y., Park Y., Park S.R., et al. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric cancer. official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2019;22:527–535. doi: 10.1007/s10120-018-0891-1. [DOI] [PubMed] [Google Scholar]
- 34.Pietrantonio F., Caporale M., Morano F., Scartozzi M., Gloghini A., Vita F de, et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: implication for further clinical research. Int. J. Cancer. 2016;139:2859–2864. doi: 10.1002/ijc.30408. [DOI] [PubMed] [Google Scholar]
- 35.Parikh A.R., Leshchiner I., Elagina L., Goyal L., Levovitz C., Siravegna G., et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat. Med. 2019;25:1415–1421. doi: 10.1038/s41591-019-0561-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siravegna G., Sartore-Bianchi A., Nagy R.J., Raghav K., Odegaard J.I., Lanman R.B., et al. Plasma HER2 (ERBB2) copy number predicts response to HER2-targeted therapy in metastatic colorectal cancer. Clin. Cancer Res. : an official journal of the American Association for Cancer Research. 2019;25:3046–3053. doi: 10.1158/1078-0432.CCR-18-3389. [DOI] [PubMed] [Google Scholar]
- 37.Peng Z., Zou J., Zhang X., Yang Y., Gao J., Li Y., et al. HER2 discordance between paired primary gastric cancer and metastasis: a meta-analysis. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2015;27:163–171. doi: 10.3978/j.issn.1000-9604.2014.12.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with the study has been included in the supplementary material file provided with the manuscript.