Abstract
Background
The purpose of this study was to compare the efficacy and safety of utidelone plus capecitabine for advanced first-line versus second-line or above therapy in metastatic breast cancer patients who had previously received anthracycline and taxane. At the same time, we compared the efficacy of utidelone plus capecitabine and vinorelbine plus cisplatin in advanced first-line treatment of metastatic breast cancer.
Patients and methods
A retrospective cohort of 11 patients with metastatic breast cancer previously treated with anthracycline and taxane (including neoadjuvant and adjuvant therapies) for advanced first-line with utidelone plus capecitabine, 32 patients treated with second-line or above, and 60 patients with vinorelbine plus cisplatin between October 2011 and August 2022 was collected. The first and second groups were treated with utidelone plus capecitabine, and the third group was treated with vinorelbine plus cisplatin. The primary endpoint was progression-free survival (PFS), and secondary endpoints were overall survival (OS), objective response rate (ORR), and treatment safety.
Results
By 03/31/2023, median PFS reached 11.70 months (95 % CI 0.093–0.141) in utidelone plus capecitabine group in the advanced first-line therapy, compared to 5.60 months (95 % CI 0.025–0.079) in the second-line or above therapy [HR 0.42, (95 % CI 0.226–0.787), P = 0.0077]. In utidelone plus capecitabine, the median OS was not reached in the advanced first-line therapy, with a mean overall survival of 23.16 months (95 % CI 0.198–0.265); whereas the median OS in the second-line or above therapy was 19.50 months (95 % CI 0.083–0.307), with a mean overall survival of 16.89 months (95 % CI 0.136–0.202) [HR 0.26, (95 % CI 0.098–0.678), P = 0.0495]. The ORR for advanced first-line therapy was 27.27 % (95%CI 0.060, 0.610) compared with 15.63 % (95%CI 0.053, 0.328) for second-line or above. In advanced first-line therapy, utidelone plus capecitabine was superior to vinorelbine plus cisplatin with a median PFS of 6.12 months (95 % CI 0.051–0.072) [HR 0.49, (95 % CI 0.286–0.839), P = 0.0291]. Compared with utidelone plus capecitabine, the median OS in vinorelbine plus cisplatin advanced first-line therapy group was 35.37 months (95 % CI 0.258–0.449), and the mean overall survival was 40.79 months (95 % CI 0.315–0.501) [HR 0.54, (95 % CI 0.188–1.568), P = 0.2587]. The ORR for vinorelbine plus cisplatin was 18.33 % (95 % CI 0.095, 0.304). The most common adverse events in our study were neurological toxicity, hand-foot syndrome, hematological toxicity, gastrointestinal toxicity, and hepatic and renal function abnormalities. There were no deaths due to adverse effects during the utidelone plus capecitabine treatment period.
Conclusions
In MBC, advanced first-line therapy with utidelone plus capecitabine resulted in more favorable PFS, OS, and ORR than second-line or above therapy. In advanced first-line therapy, utidelone plus capecitabine had superior PFS, and ORR compared with vinorelbine plus cisplatin. This study concludes that utidelone plus capecitabine is a more valuable chemotherapy option in advanced first-line MBC.
Keywords: Metastatic breast cancer, Utidelone, Capecitabine, Vinorelbine, First-line therapy
Highlights
-
•
This is a first report of a study of utidelone plus capecitabine in the advanced first-line therapy of metastatic breast cancer.
-
•
This study highlights the importance of utidelone plus capecitabine in advanced first-line metastatic breast cancer.
-
•
These results will serve as a great resource for further study, offering more pertinent information on the applicability and effectiveness of advanced metastatic breast cancer chemotherapy.
Introduction
Breast cancer is the most common malignant tumor in women, and by 2023, Breast cancer incidence is 2.97 million, accounting for 31 % of female cancers [1]. The treatment of breast cancer is a comprehensive process, in which chemotherapy plays an important role. Chemotherapy resistance is an influential factor leading to recurrence and metastasis of breast cancer [2]. With the increasing standardization of breast cancer treatment, the vast majority of patients have been treated with anthracycline and taxane during the adjuvant phase, and a part of them eventually progressed to advanced metastatic breast cancer (MBC), which is highly susceptible to cross-resistance and multidrug resistance at the follow-up phase of therapy [3]. At present, the choice of effective and safe follow-up chemotherapeutic regimen for patients with MBC who have received anthracycline and taxane chemotherapy remains a thorny clinical problem.
There is no standard treatment for advanced MBC according to molecular typing, but chemotherapy is still the basis of subtype MBC treatment. In first-line as well as later lines palliative treatment of MBC, drugs such as capecitabine, vinorelbine, gemcitabine, platinum, eribulin, and utidelone can be considered as single agents or in combination regimens [[4], [5], [6], [7]]. However, these chemotherapeutic agents have limited survival benefits and most studies [[8], [9], [10]] showed no significant difference in overall survival between chemotherapy regimens. Hu XC et al. showed that gemcitabine/cisplatin (GP) was superior to gemcitabine/paclitaxel (GT) for first-line treatment of metastatic triple-negative breast cancer (mTNBC) in terms of PFS (GP: 7.7 months, GT: 6.5 months); however, there was no significant difference in OS [11]. Another study showed that nab-paclitaxel plus carboplatin significantly prolonged the median PFS compared with nab-paclitaxel plus gemcitabine or gemcitabine plus carboplatin in first-line therapy for mTNBC (8.3 months vs. 5.5 months, 6.0 months); while there was no difference in OS [12]. The median PFS of vinorelbine combined with capecitabine in the backline therapy of MBC was 6.2 months, with an OS of 35.47 months, and it was considered an active and well-tolerated treatment option [13]. A real-world study [14] showed that the median OS of first- and second-line therapy with eribulin in patients with endocrine-resistant advanced or metastatic breast cancer was 2.25 years (95 % CI 1.07–2.68) and 1.75 years (95 % CI 1.28–2.45), respectively. Based on these results, it is worthwhile to further explore and study a more effective first-line therapy for MBC patients based on prolonged survival.
Utidelone (UTD1) is an original Chinese genetically engineered modified analogue of epothilone [15]. Due to the advantages of stronger anti-tumor activity, broader anti-tumor spectrum, and the fact that tumor cells resistant to taxanes are still effective, it was approved by China Food and Drug Administration (CFDA) in 2021 in combination with capecitabine for the treatment of patients with recurrent or metastatic breast cancer who have previously received at least one anthracycline or taxane drug. A phase III clinical trial [16] demonstrated that patients treated with utidelone in combination with capecitabine had significantly better PFS than patients treated with capecitabine monotherapy. The final analysis of OS updated in 2020 [17] also showed that utidelone combined with capecitabine significantly improved patient OS compared to capecitabine alone. Vinorelbine is a semisynthetic vinblastine that is not cross-resistant to anthracycline and taxanes. With cisplatin acting on multiple targets, it has synergistic antitumor activity and has better efficacy and safety in MBC after anthracycline and taxane treatment [18,19]. Although there were many chemotherapy regimens for MBC, the efficacy and safety of first-line and above therapy have been studied and compared. However, no studies have been reported on the efficacy and safety of first-line versus second-line or above therapy with utidelone combined with capecitabine in MBC that has received prior anthracycline and taxane; and on the evaluation and comparison of utidelone combined with capecitabine versus vinorelbine combined with cisplatin in late-stage first-line therapy.
The aim of this study is to investigate the efficacy and safety of the late-stage first-line therapy group of utidelone combined with capecitabine versus the second-line or above therapy group in MBC that has received prior anthracycline and taxanes. Meanwhile, to compare the efficacy of utidelone plus capecitabine (UX) versus vinorelbine plus cisplatin (NP) for advanced first-line therapy of MBC via anthracyclines and taxanes. To find out the place of utidelone in combination with capecitabine in the first-line therapy of advanced MBC, and to find a more preferred chemotherapy regimen to provide more effective clinical benefit for patients with MBC.
Material and methods
Research subjects and entry criteria
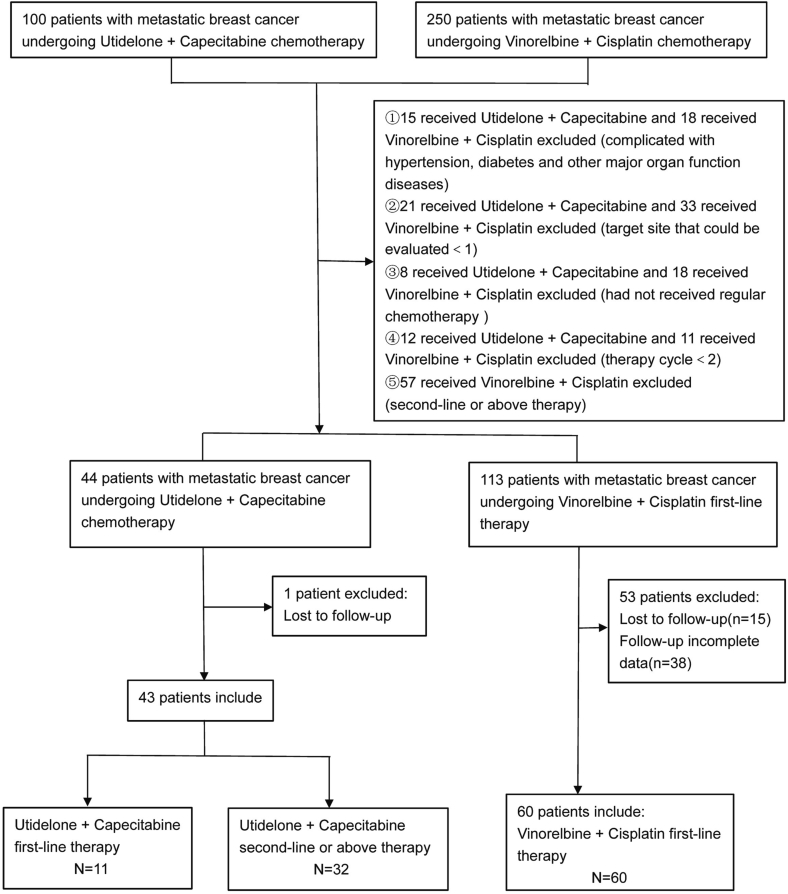
The study retrospectively collected clinical data on metastatic breast cancer patients treated with utidelone plus capecitabine or vinorelbine plus cisplatin at Designated Specialist Hospitals (Yunnan Cancer Hospital, Guangxi Medical University Cancer Hospital, Chongqing Cancer Hospital, The First Affiliated Hospital of Kunming Medical University, The Third People's Hospital of Yunnan Province) between October 2011 and August 2022 following progression of anthracycline and taxane. The study ultimately included 11 patients in the first-line therapy treated with utidelone in combination with capetabine, 32 patients in the second-line or above, and 60 patients in the first-line therapy treated with vinorelbine combined with cisplatin(Fig. 1). All study subjects were female, aged 22–70 years, with an average age of (49 ± 8.60) years. Baseline characteristics such as sex, age, BMI, and body weight were compared among the three groups of subjects, with no statistically significant difference (P > 0.05) and were comparable.
Fig. 1.
Flow chart of patient enrollment.
Case diagnosis criteria: All patients with metastatic breast cancer underwent imaging such as physical examination, whole blood examination, blood biochemical examination, CT scan or MRI examination at Designated Specialist Hospitals. Tissue biopsy or lumpectomy was performed after evaluation by breast surgeons at Designated Specialist Hospitals. The histopathologic specimens were diagnosed as breast cancer by >2 doctors in the pathology department of Designated Specialist Hospitals. In addition, the results of pathological immunohistochemistry and Fish (Fluorescence in Situ Hybridization) gene test in all patients with metastatic breast cancer were obtained directly by pathologist or pathologic consultation in Designated Specialist Hospitals. Advanced first-line therapy was defined as patients with prior anthracycline and taxane use, first diagnosis of metastatic breast cancer, and first treatment with utidelone plus capecitabine or vinorelbine plus cisplatin.
Inclusion criteria: (1) histologically or cytologically confirmed metastatic breast cancer; (2) Patients who have progressed to treatment with utidelone plus capecitabine or vinorelbine plus cisplatin after previous treatment with anthracycline and taxane (including neoadjuvant and adjuvant therapies); (3) at least one target site that could be evaluated; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; (5) Disorders of the Nervous system that are less than grade 2 on the National Cancer Institute (NCI) Common Toxicity Criteria (CTC) version 4.03.
Exclusion criteria: (1) In breastfeeding period; (2) Chemotherapy cycles <2; (3) Combination of other primary tumors; (4) Combined with major organ function diseases such as hypertension and diabetes; (5) patients did not receive regular chemotherapy in hospital; (6) Missing patient clinical data and follow-up data do not satisfy the data analysis.
Ethical statement: The study complied with the Declaration of Helsinki (as revised in 2013) and was approved by institutional committee board of the Third Affiliated Hospital of Kunming Medical University (Yunnan Cancer Hospital) (No. KYLX2023–107). All patients obtained informed consent.
Study design and treatment
The clinicopathological data of 103 patients with metastatic breast cancer were collected, including height, weight, age, Menopause, tumor size, clinical stage, lymph node metastasis, number of Metastasis site, Primary tumor excised, etc. Among them, there were 11 patients in the first-line therapy group of utidelone combined with capecitabine, 32 patients in the second-line therapy group and above, and 60 patients in the first-line therapy group of vincristine combined with cisplatin. Each treatment group received per each 21-day treatment cycle: The group treated with utidelone plus capecitabine received utidelone (30 mg/m2 IV once daily days 1–5) plus capecitabine (1000 mg/m2 orally twice daily on days 1–14). The group treated with vincristine plus cisplatin received vincristine (25 mg/m2 IV once daily days 1 and 8) plus cisplatin (75 mg/m2 IV once daily days 1–3). All patients continued chemotherapy until progression, intolerable toxicity, or discontinuation of treatment was indicated. Dosage reductions during chemotherapy were allowed to control toxicity.
Patient evaluation included physical examination, whole blood examination, blood biochemistry, CT scan and MRI. Blood samples were taken and evaluated at baseline, followed by physical examination, whole blood examination, and blood biochemical examination once a week, and enhanced CT and MRI every two cycles. All patients underwent radiographic evaluation of tumor remission every two treatment cycles until they developed disease progression or unacceptable toxicity and stopped treatment or died. Assessment of tumor remission was performed using enhanced CT and MRI. The researchers contacted each patient by telephone before the final OS analysis to confirm their survival status and collect survival information from each patient.
Side effects were collected from medical records and laboratory tests during treatment. Adverse events are classified according to the Version 4.03 Common Terminology Criteria for Adverse Events (CTCAE). Routine hematologic and blood biochemical tests were performed once a week between the 3rd and 5th day of each treatment cycle until the treatment was discontinued. In addition, patients' vital signs were evaluated periodically during treatment, and electrocardiograms, echocardiography, and physical examinations were performed to observe for any abnormalities and significant changes from baseline. All assessments and examinations were performed at baseline and at the end of each treatment cycle during treatment.
Progression-Free survival (PFS), overall survival (OS), efficacy and safety of different treatment groups were compared based on the data collected; Meanwhile, the prognostic factors of metastatic breast cancer among different treatment groups were analyzed.
End of study
The primary endpoint was progression-free survival (PFS), defined as progression from the first day of chemotherapy to the onset of disease based on version 1.1 of the Solid Tumor Response Assessment Criteria (RECIST) [20].
The secondary endpoint was overall survival (OS), defined as the time from the first day of chemotherapy to death due to any cause. Objective response rate (ORR), defined as the proportion of complete response (CR) and partial response (PR) in all patients and treatment safety. The progression-free survival (PFS) and overall survival (OS) of the study population were calculated using the Kaplan-Meier product-limit method.
Statistical analysis
IBM SPSS Statistics 26.0 and GraphPad Prism 8.0.2 were used for all statistical analyses, and P values <0.05 were considered statistically significant. Categorical variables were expressed as their respective percentages and analyzed by chi square test or Fisher exact test. The efficacy and safety variables were summarized using descriptive statistics. For ORR, 95 % confidence intervals were calculated using the Clopper Pearson method. For PFS and OS, we used the Kaplan-Meier method for survival analysis and estimated the median and 95 % confidence interval. A log-rank test was carried out for treatment comparison at a twosided alpha level of 0.05. The risk ratio (HR) and 95 % confidence interval were calculated using single-factor and multi-factor Cox proportional risk model. Univariate Cox model was used to test the combined prognostic value of breast cancer, while the clinical significance of the variables were considered together and then included in the multifactorial Cox model.
Results
Patient characteristics
Ultimately, 11 patients in the first-line therapy group of utidelone plus capecitabine, 32 patients in the second-line or above therapy group and 60 patients in the first-line therapy group of vincristine plus cisplatin were included. The baseline demographics and clinical characteristics of all patients were summarized in Table 1. All patients were metastatic breast cancer, Chinese women, aged 22 to 70 years, and had previously received anthracycline and taxane. P value was obtained by Pearson chi-square test or Fisher exact method. In utidelone plus capecitabine, there was no significant difference in baseline clinical characteristics between the first-line therapy and the second-line or above therapy (P > 0.05). However, In the first-line therapy group of utidelone combined with capecitabine, compared with vinorelbine combined with cisplatin, there were statistical differences between the groups P < 0.05 distant metastasis (P = 0.001) and Primary tumor excised (P = 0.035). We conclude that there is a difference in distant metastasis and primary tumor excised at baseline (Table 1).
Table 1.
Study subject demographics.
| Demographic characteristic | Group 1 |
Group 2 |
||||
|---|---|---|---|---|---|---|
| UX first-line therapy (n = 11) | UX second-line or above therapy (n = 32) | P-value | UX first-line therapy (n = 11) | NP first-line therapy (n = 60) | P-value | |
| Age (year), % | ||||||
| ≤50 | 6(54.55 %) | 17(53.13 %) | 0.935 | 6(54.55 %) | 36(60.00 %) | 0.735 |
| >50 | 5(45.45 %) | 15(46.87 %) | 5(45.45 %) | 24(40.00 %) | ||
| Menopause, % | ||||||
| Yes | 5(45.45 %) | 18(56.25 %) | 0.536 | 5(45.45 %) | 26(43.33 %) | >0.999 |
| No | 6(54.55 %) | 14(43.75 %) | 6(54.55 %) | 34(56.67 %) | ||
| BMI (kg/m2),% | ||||||
| ≤22 | 6(54.55 %) | 19(59.38 %) | 0.779 | 6(54.55 %) | 16(26.67 %) | 0.084 |
| >22 | 5(45.45 %) | 13(40.62 %) | 5(45.45 %) | 44(73.33 %) | ||
| T, % | ||||||
| ≤2 | 1(9.09 %) | 7(21.87 %) | 0.656 | 1(9.09 %) | 20(33.33 %) | 0.156 |
| >2 | 10(90.91 %) | 25(78.13 %) | 10(90.91 %) | 40(66.67 %) | ||
| N, % | ||||||
| Yes | 6(54.55 %) | 19(59.38 %) | 0.779 | 6(54.55 %) | 44(73.33 %) | 0.209 |
| No | 5(45.45 %) | 13(40.62 %) | 5(45.45 %) | 16(26.67 %) | ||
| M, % | ||||||
| No | 7(63.64 %) | 21(65.62 %) | 0.905 | 7(63.64 %) | 57(95.00 %) | 0.001 |
| Yes | 4(36.36 %) | 11(34.38 %) | 4(36.36 %) | 3(5.00 %) | ||
| ER, % | ||||||
| + | 6(54.55 %) | 14(43.75 %) | 0.536 | 6(54.55 %) | 40(66.67 %) | 0.439 |
| − | 5(45.45 %) | 18(56.25 %) | 5(45.45 %) | 20(33.33 %) | ||
| PR, % | ||||||
| + | 7(63.64 %) | 13(40.62 %) | 0.187 | 7(63.64 %) | 38(63.33 %) | 0.985 |
| − | 4(36.36 %) | 19(59.38 %) | 4(36.36 %) | 22(36.67 %) | ||
| HER2, % | ||||||
| + | 1(9.09 %) | 12(37.50 %) | 0.129 | 1(9.09 %) | 17(28.33 %) | 0.269 |
| − | 10(90.91 %) | 20(62.50 %) | 10(90.91 %) | 43(71.67 %) | ||
| Ki67, % | ||||||
| <14 | 1(9.09 %) | 6(18.75 %) | 0.656 | 1(9.09 %) | 11(18.33 %) | 0.676 |
| ≥14 | 10(90.91 %) | 26(81.25 %) | 10(90.91 %) | 49(81.67 %) | ||
| Metastasis site, % | ||||||
| ≤2 | 8(72.73 %) | 13(40.62 %) | 0.066 | 8(72.73 %) | 47(78.33 %) | 0.682 |
| >2 | 3(27.27 %) | 19(59.38 %) | 3(27.27 %) | 13(21.67 %) | ||
| Primary tumor excised, % | ||||||
| Yes | 8(72.73 %) | 23(71.88 %) | 0.957 | 8(72.73 %) | 56(93.33 %) | 0.035 |
| No | 3(27.27 %) | 9(28.12 %) | 3(27.27 %) | 4(6.67 %) | ||
| Received radiation therapy, % | ||||||
| Yes | 6(54.55 %) | 14(43.75 %) | 0.536 | 6(54.55 %) | 27(45.00 %) | 0.560 |
| No | 5(45.45 %) | 18(56.25 %) | 5(45.45 %) | 33(55.00 %) | ||
| Received targeted therapy, % | ||||||
| Yes | 3(27.27 %) | 11(34.38 %) | >0.999 | 3(27.27 %) | 10(16.67 %) | 0.411 |
| No | 8(72.73 %) | 21(65.62 %) | 8(72.73 %) | 50(83.33 %) | ||
| Received endocrine therapy, % | ||||||
| Yes | 4(36.36 %) | 11(34.38 %) | >0.999 | 4(36.36 %) | 24(40.00 %) | >0.999 |
| No | 7(63.64 %) | 21(65.62 %) | 7(63.64 %) | 36(60.00 %) | ||
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type 2; UX (Utidelone plus Capecitabine); NP (Vinorelbine plus Cisplatin).
ER and PR status was defined with the cutoff value of 1 % positive tumor cells.
HER2-positive was defined as scored 3+ by immunohistochemistry; for scores 2+, fluorescence in situ hybridization was performed to determine HER2 positivity; and 0 and 1+ were regarded as HER2-negative.
Clinical efficacy and prognosis analysis
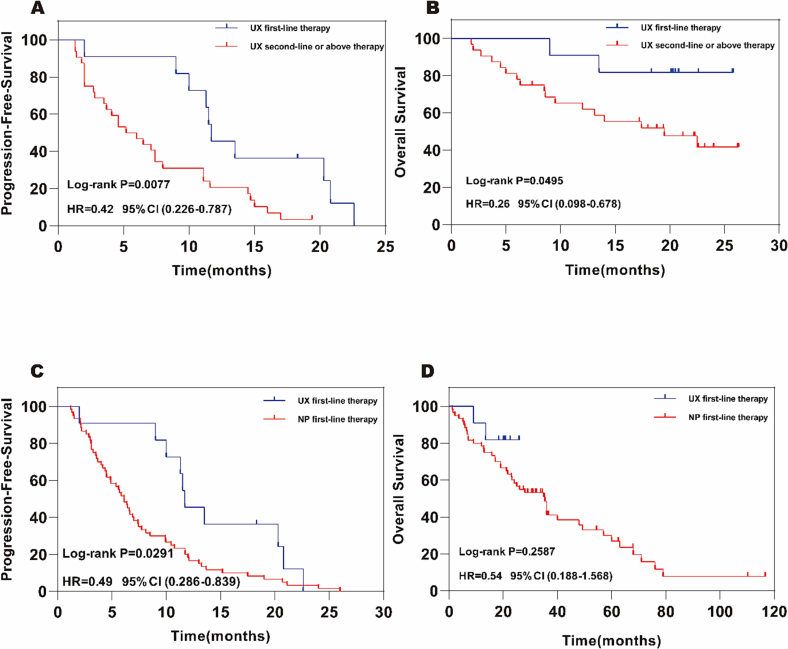
The cut-off date for this research was 31 March 2023, a total of 40 progression-free survival events occurred in the utidelone plus capecitabine first-line versus second-line or above group. There were 70 progression-free survival events in the group treated with utidelone plus capecitabine versus vincristine combined with cisplatin. There were 10 cases in the utidelone plus capecitabine (UX) first-line therapy group, 30cases in the second-line or above therapy group, and 60 cases in the vinorelbine plus cisplatin (NP) first-line therapy group. Follow-up time of the group treated with UX was from January 2021 to March 2023, while the group treated with NP was from October 2011 to March 2023. Based on the researchers' assessment, median PFS was 11.70 months (95 % CI 0.093–0.141) in the UX first-line therapy group, and 5.60 months (95 % CI 0.025–0.079) in the second-line or above therapy group [HR 0.42, (95 % CI 0.226–0.787), log-rank P = 0.0077; Fig. 2A]. P = 0.0077 < 0.05, so PFS was statistically significant in the two groups. In addition, the median PFS in the group of UX for advanced first-line therapy was better than that in the group of NP for 6.12 months (95 % CI 0.051–0.072) [HR 0.49, (95 % CI 0.286–0.839), log-rank P = 0.0291; Fig. 2C]. P = 0.0291 < 0.05, PFS difference between the two groups were statistically significant.
Fig. 2.
Kaplan-Meier survival estimates for PFS,OS.
CI confidence interval, UX (Utidelone plus Capecitabine), NP (Vinorelbine plus Cisplatin).
A Progression-Free-Survival in the UX first-line vs second-line or above therapy population.
B Overall survival in the UX first-line vs second-line or above therapy population.
C Progression-Free-Survival in the UX first-line vs NP first-line therapy population.
D Overall survival in the UX first-line vs NP first-line therapy population.
In the final OS analysis of cut-off date, 2deaths occurred in 11 patients in the first-line therapy group with UX, 17deaths in 32 patients in the second-line or above, and 43 deaths in 60 patients in the first-line treatment group with NP. The mortality rates were 18.18 %, 53.13 % and 71.67 %, respectively (Follow-up time of UX was shorter than that of NP). The median OS was not reached in the UX first-line therapy group, and the mean overall survival was 23.16 months (95 % CI 0.198–0.265). In contrast, the median OS in the second-line or above therapy group was 19.50 months (95 % CI 0.083–0.307), and the mean overall survival was 16.89 months (95 % CI 0.136–0.202) [HR 0.26, (95 % CI 0.098–0.678), log-rank P = 0.0495; Fig. 2B]. Compared with the late first-line therapy group of UX, the median OS of the late first-line therapy group of NP was 35.37 months (95 % CI 0.258–0.449), and the mean overall survival was 40.79 months (95 % CI 0.315–0.501) [HR 0.54, (95 % CI 0.188–1.568), log- rank P = 0.2587; Fig. 2D].
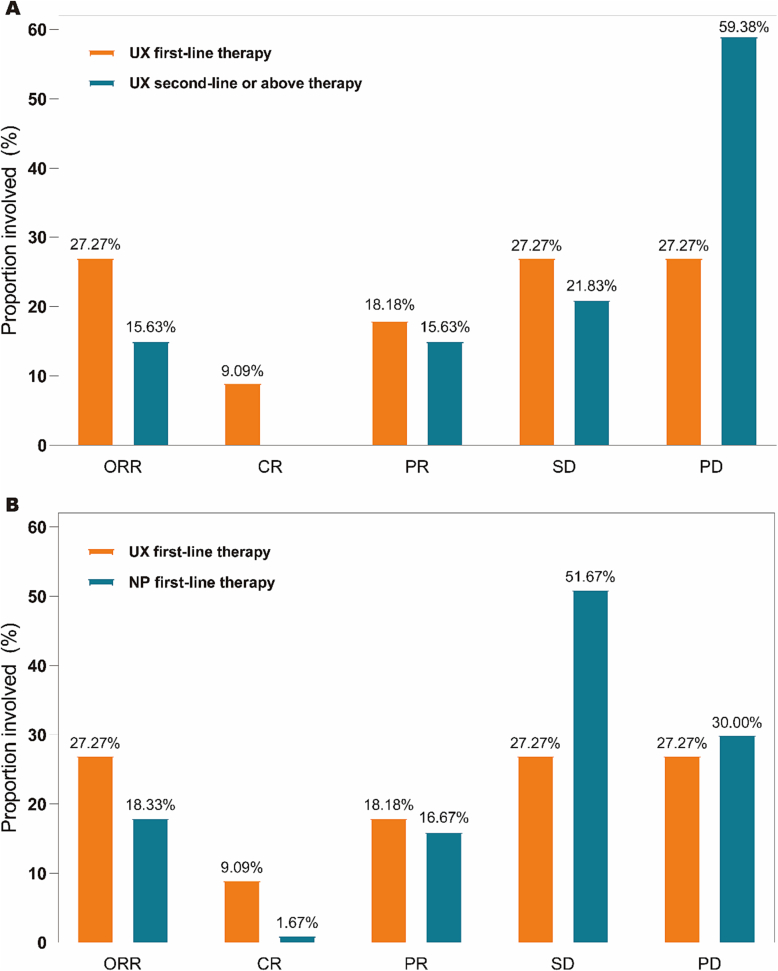
Of the 11 patients in the late first-line therapy group of UX, 9 were evaluated and 2 were considered unavailable (One patient did not undergo enhanced CT and MRI for physical reasons when completing the second cycle of chemotherapy, and one did not undergo enhanced CT and MRI for economic reasons). At the end of the study, a total of 1 complete response (CR), 2 partial response (PR), 3 stable disease, and 3 progressive disease (PD) were observed (Table 2), with an ORR of 27.27 % (95 % CI 0.060,0.610). Of the 32 patients treated with UX for advanced second line or higher, one had a severe new crown fever without enhanced CT or MRI. Therefore, a total of 31 subjects were evaluated. At the end of the study, a total of 0 CR, 5 PR, 7 SD, and 19 PD were observed (Table 2) with an ORR of 15.63 % (95%CI 0.053,0.328). ORR (UX late first-line vs. second-line or above therapy groups) P = 0.401 > 0.05, the difference was not statistically significant (Table 2). In the NP first-line therapy group, 60 patients completed at least 2 cycles of chemotherapy and underwent regular imaging examinations, so the efficacy could be evaluated. By the end of the study, a total of 1 CR, 10 PR, 31 SD, and 18 PD were observed (Table 2), with an ORR of 18.33 % (95 % CI 0.095,0.304). ORR (UX late first line vs. NP late first line) P = 0.444 > 0.05, the difference was not statistically significant (Table 2). The efficacy of the study population was analyzed based on the investigator's assessment showing that the proportion of patients achieving ORR, CR, PR, and SD in the first-line therapy group of UX was higher than the proportion of patients in the second-line or above (Fig. 3A). The proportion of patients who achieved ORR, CR, and PR in the late first-line therapy group of UX was higher than that of patients in the late first-line therapy group of NP; whereas the proportion of patients with SD was lower than that of patients in the late first-line therapy group of NP(Fig. 3B).
Table 2.
Study subject end of treatment objective response rates.
| Group 1 |
Group 2 |
|||||
|---|---|---|---|---|---|---|
| UX first-line therapy (n = 11) | UX second-line or above therapy (n = 32) | P-value | UX first-line therapy (n = 11) | NP first-line therapy (n = 60) | P-value | |
| Best response, a(n) | ||||||
| CR | 1 | 0 | 1 | 1 | ||
| PR | 2 | 5 | 2 | 10 | ||
| SD | 3 | 7 | 3 | 31 | ||
| PD | 3 | 19 | 3 | 18 | ||
| NA | 2 | 1 | 2 | 0 | ||
| Total | 11 | 32 | 11 | 60 | ||
| ORR,b % | 27.27 | 15.63 | 0.401 | 27.27 | 18.33 | 0.444 |
| 95%Clc | 0.060,0.610 | 0.053,0.328 | 0.060,0.610 | 0.095,0.304 | ||
CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR objective response rate, CI confidence interval, UX (Utidelone plus Capecitabine); NP (Vinorelbine plus Cisplatin).
Assessed according to RECIST1.1.
ORR = (CR + PR)/total × 100 %.
Calculated using Clopper Pearson method.
Fig. 3.
ORR,CR,PR,SD,PD ratio of the different treatment groups.
CR complete response, PR partial response, SD stable disease, PD progressive disease, ORR objective response rate, UX (Utidelone plus Capecitabine), NP (Vinorelbine plus Cisplatin).
A ORR,CR,PR,SD,PD ratio of the UX first-line vs second-line or above therapy.
B ORR,CR,PR,SD,PD ratio of the UX first-line vs NP first-line therapy.
In order to investigate whether there were differences in the efficacy of different treatment groups of breast cancer between the CR/PR and SD/PD groups. X2 test and Fisher exact method were used to analyze the efficacy of UX in 9 patients of first-line therapy, 31 patients of second-line therapy and above, and 60 patients of NP. The results showed that p-values were > 0.05. It can be considered that the difference between the late first-line therapy of UX and the second-line or above therapy groups was not statistically significant between the two groups in terms of clinical efficacy CR/PR and SD/PD; the difference between the late first-line therapy of UX and the late first-line therapy group of NP was not statistically significant in terms of CR/PR and SD/PD in terms of clinical efficacy (Table 3). Table 4 shows the efficacy of UX based on the subtypes of breast cancer. In advanced first-line, there was a statistical difference in efficacy between UX and NP in the HER2-, HER2- ER+ PR+ subtypes (P = 0.030, 0.004), with no significant difference seen in other subtypes at present (Table 4). These results must be interpreted with caution because the sample sizes of the subgroups are so small.
Table 3.
The efficacy of different treatment groups between CR/PR and SD/PD.
| Group 1 |
Group 2 |
|||||
|---|---|---|---|---|---|---|
| UX first-line therapy (n = 9) | UX second-line or above therapy (n = 31) | P-value | UX first-line therapy (n = 9) | NP first-line therapy n = 60 | P-value | |
| CR/PR | 3 | 5 | 0.348 | 3 | 11 | 0.373 |
| SD/PD | 6 | 26 | 6 | 49 | ||
CR complete response, PR partial response, SD stable disease, PD progressive disease, UX (Utidelone plus Capecitabine); NP (Vinorelbine plus Cisplatin).
Table 4.
The efficacy of utidelone plus capecitabine based on the subtypes of breast cancer.
| Molecular subtype | Group 1 |
Group 2 |
||||
|---|---|---|---|---|---|---|
| UX first-line therapy | UX second-line or above therapy | P-value | UX first-line therapy | NP first-line therapy | P-value | |
| HER2+ | ||||||
| CR/PR | 1 | 2 | 0.231 | 1 | 4 | 0.278 |
| SD/PD | 0 | 10 | 0 | 13 | ||
| NA | / | / | / | / | ||
| HER2- | ||||||
| CR/PR | 2 | 3 | 0.396 | 2 | 6 | 0.030 |
| SD/PD | 6 | 16 | 6 | 37 | ||
| NA | 2 | 1 | 2 | 0 | ||
| HER2- ER- PR- | ||||||
| CR/PR | 1 | 1 | 0.580 | 1 | 2 | >0.999 |
| SD/PD | 2 | 8 | 2 | 7 | ||
| NA | 0 | 1 | / | / | ||
| HER2- ER+ PR+ | ||||||
| CR/PR | 1 | 2 | 0.109 | 1 | 4 | 0.004 |
| SD/PD | 1 | 6 | 1 | 24 | ||
| NA | 2 | 0 | 2 | 0 | ||
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type 2; UX (Utidelone plus Capecitabine); NP (Vinorelbine plus Cisplatin), CR complete response, PR partial response, SD stable disease, PD progressive disease, NA not available.
ER and PR status was defined with the cutoff value of 1 % positive tumor cells.
HER2-positive was defined as scored 3+ by immunohistochemistry; for scores 2+, fluorescence in situ hybridization was performed to determine HER2 positivity; and 0 and 1+ were regarded as HER2-negative.
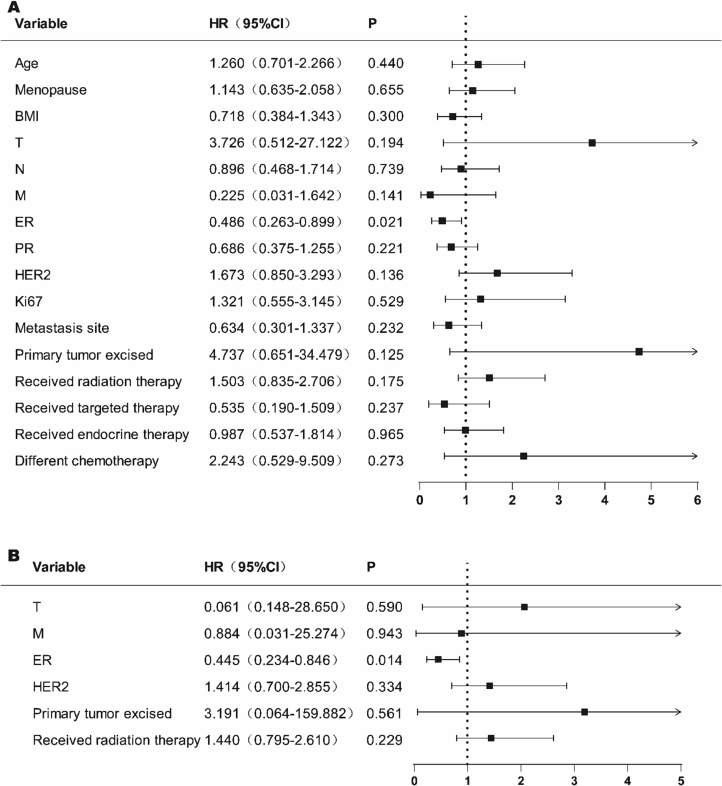
To explore the prognostic factors in patients with breast cancer between the first-line therapy group treated with UX and those treated with second-line or above. We first screened the variables by single-factor COX regression analysis (Fig. 4A). Among the variables considered, age (P = 0.048), menopause (P = 0.008), M (P = 0.049), ER (P = 0.004), PR (P = 0.008) were significantly correlated with the prognosis of breast cancer patients (Fig. 4A). The clinical significance of the variables was also considered, and variables with P < 0.1 were included in the COX multifactorial regression analysis to comprehensively analyze the effects of multifactorial interactions on breast cancer prognosis. The results of multifactorial COX regression analysis showed that: ER (P = 0.006) and primary tumor excised (P = 0.049) were statistically significant at P < 0.05 in both groups, and had a significant and independent correlation with breast cancer prognosis (Fig. 4B).
Fig. 4.
The univariate and multivariate logistic regression analysis in the UX first-line vs second-line or above therapy.
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type 2; UX (Utidelone plus Capecitabine).
A Univariate Cox model analysis for OS.
B Multivariate Cox model analysis for OS.
Meanwhile, we explored the factors influencing the prognosis of breast cancer patients between the two groups of UX and NP in advanced first-line therapy. Variables were screened by single factor COX regression analysis (Fig. 5A). Among the variables considered, ER (P = 0.021) was significantly associated with prognosis in breast cancer patients (Fig. 5A). Considering the clinical significance of the variables, the variables with P < 0.2 were included in COX multivariate regression analysis to comprehensively analyze the impact of multivariate interaction on breast cancer prognosis. Multivariate COX regression analysis showed that ER (P = 0.014) P < 0.05 was statistically significant and correlated with breast cancer prognosis (Fig. 5B).
Fig. 5.
The univariate and multivariate logistic regression analysis in the UX first-line vs NP first-line therapy.
BMI, body mass index; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor type 2.
A Univariate Cox model analysis for OS.
B Multivariate Cox model analysis for OS.
Safety analysis
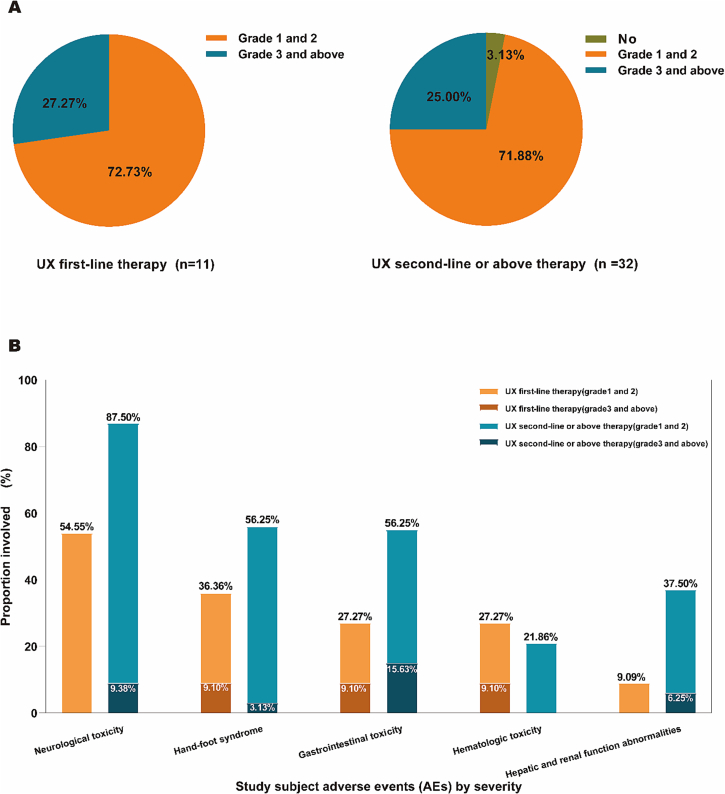
Patients in both the UX first-line and second-line or above treatment groups received more than two cycles of chemotherapy (range: 2 to 11 cycles). During treatment, all patients underwent regular hematology, blood biochemistry, electrocardiogram, echocardiography, and physical examination. The late first-line therapy group (11 patients) of UX had a grade 1–2 adverse event in 8 patients (72.73 %) and any grade 3 and above adverse event in 3 patients (27.27 %); In the first-line therapy group of UX (11 patients), 8 (72.73 %) patients experienced any grade 1–2 adverse events and 3 (27.27 %) patients experienced any grade 3 and above adverse events, whereas in the second-line or above therapy group (32 patients), 23 (71.88 %) patients experienced any grade 1–2 adverse events and 8 (25.00 %), and 1 patient did not experience any discomfort during drug administration (Fig. 6A). We found that there was a more balanced proportion of any adverse events that occurred during the administration of UX first-line compared with the second-line or above therapy group.
Fig. 6.
Treatment-related adverse events of Utidelone plus Capecitabine.
UX (Utidelone plus Capecitabine).
A Any treatment-emergent adverse event.
B Classification of toxic and side effects.
In terms of the grading of toxic side effects, the first-line therapy group (11 patients) of UX showed neurological toxicity grade 1–2 in 6 patients (54.55 %), and there was no grade 3 and above toxicity; hand-foot syndrome grade 1–2 in 3 patients (27.27 %), and grade 3 and above toxicity in 1 patients (9.10 %); gastrointestinal toxicity grade 1–2 in 2 patients (18.18 %), grade 3 and above toxicity was 1 patient (9.10 %); hematologic toxicity grade 1–2 was 2 patients (18.18 %), grade 3 and above toxicity was 1 patient (9.10 %); hepatic and renal function abnormalities grade 1–2 was 1 patient (9.09 %), and grade 3 and above toxicity did not occur (Fig. 6B).
In the UX second-line or above therapy group (32 patients), there were 25 patients (78.13 %%) of neurological toxicity grade 1–2, and 3 patients (9.38 %) of grade 3 and above toxicity; 17 patients (53.13 %) of hand-foot syndrome grade 1–2, and 1 patient (3.13 %) of grade 3 and above toxicity; 13 patients (40.63 %) of gastrointestinal toxicity grade 1–2 and 5 patients (15.63 %); and 7 patients (21.88 %) of hematologic toxicity grade 1–2, grade 3 and above toxicity did not occur; hepatic and renal function toxicity grade 1–2 was 10 cases (31.25 %), grade 3 and above toxicity was 2 cases (6.25 %) (Fig. 6B). These results must be interpreted with caution due to the small sample size.
In conclusion, based on this study, MBC previously treated with anthracyclines and taxane, there was a significant benefit of UX in the first-line versus the second-line or above therapy groups, with a significant prolongation of the median PFS (11.70 months vs. 5.60 months) and a statistically significant difference of P = 0.0077, as well as a statistically significant difference in the mean overall survival (23.16 months vs. 16.89 months) of P = 0.0495, a statistically significant difference. And surprisingly, a significant benefit was found in first-line application of UX versus NP in MBC after progression by anthracycline and taxane therapy, with a significant prolongation of the median PFS (11.70 months vs. 6.12 months) and a statistically significant difference of P = 0.0291, and the mean overall survival (23.16 months vs. 40.79 months) of P = 0.2587, the difference is not yet statistically significant and needs to be further analyzed.
Discussion
This is a very valuable multicenter real-world study. We report, for the first time, the clinical efficacy of utidelone combined with capecitabine for the treatment of advanced MBC in the first versus the second line and the comparative efficacy of utidelone combined with capecitabine versus vinorelbine combined with cisplatin for advanced first-line therapy. We found that patients who received utidelone in combination with capecitabine as advanced first-line therapy had longer PFS and OS compared with the second-line or above therapy group. More interestingly, utidelone combined with capecitabine had a longer PFS compared to vinorelbine combined with cisplatin in the first-line therapy of advanced MBC.
In advanced metastatic breast cancer (MBC), tumors are susceptible to previously treated anthracyclines and taxanes, and the choice of a follow-up chemotherapy regimen is a major headache for clinicians. Previous clinical and experimental studies have shown that combination chemotherapy is more effective than chemotherapy alone [[21], [22], [23]]. Combination chemotherapies typically involve drugs with multiple mechanisms of action and synergy without overlaying chemotherapy-related toxicity. Patients with advanced metastatic breast cancer who were adjuvant to anthracycline and taxanes have the option of utidelone plus capecitabine and vinorelbine plus cisplatin in combination with chemotherapy [16,17,24,25]. In a Phase III clinical trial [17], the median OS of utidelone in combination with capecitabine was significantly better than that of patients treated with capecitabine alone [HR 0.75, 95 % CI (0.59–0.94), P = 0.0142]. Du F et al. showed [26] that PFS was higher in patients with vinorelbine plus cisplatin (NP) than in patients with vinorelbine plus capecitabine (NX) (5.3 months vs. 3.0 months P = 0.023).
In this study, patients with MBC who received first-line therapy with utidelone in combination with capecitabine had significantly longer PFS than patients treated with second-line or above therapy. OS was analyzed using our available data and there were significant differences between the two groups, with OS in the first-line treatment group. In addition, during exploration, we were surprised to find that MBC patients treated with utidelone in combination with capecitabine in advanced first-line therapy had significantly longer PFS than patients treated with vinorelbine plus cisplatin. Analysis of overall survival data is immature, and differences in OS have not been statistically significant using the available data. However, the OS we obtained was affected by many confounding factors due to the different treatment strategies used by patients after disease progression. Therefore, further research is needed to identify predictive factors that can help guide physicians in choosing the most appropriate medications for individual patients. We did not perform subgroup analyses in this study due to the small sample size, but the survival curves showed a trend toward separation, and it is possible that meaningful final results will emerge after expanding the database and increasing the follow-up time. In this study, the results of utidelone plus capecitabine were consistent with those of Zhang et al. [16,17], and that of vinorelbine plus cisplatin with Wang [27], Li [28] et al.
Zhang P et al. reported that the ORR of utidelone in combination with capecitabine was 42.4 % [29]. In this study, the ORR in the first-line therapy group of utidelone combined with capecitabine was 27.27 % (95 % CI 0.060,0.610), while that in the second-line or above therapy group was 15.63 % (95 % CI 0.053,0.328); the difference between the two groups was not statistically significant (P = 0.401). The ORR of the utidelone combined with capecitabine first-line treatment group in this study was favorable compared with the 16 % - 25 % remission achieved by the various regimens used in MBC after prior treatment with anthracycline and taxane drugs [[30], [31], [32]]. He K et al. reported that 37.8 % of ORR in her2-negative advanced MBC first-line therapy was composed of vinorelbine plus cisplatin [33]. The ORR in our study was 18.33 % (95 % CI 0.095, 0.304) for the late first-line therapy with vinorelbine combined with cisplatin, and there was no statistically significant difference in utidelone combined with capecitabine compared with vinorelbine combined with cisplatin (P = 0.444).
In the OS factor analysis of metastatic breast cancer, Cox proportional risk model was used to exclude the influence of confounding factors. In first-line therapy with utidelone in combination with capecitabine versus second-line or above, ER positivity (HR 0.180, P = 0.006) was significantly associated with better OS, whereas primary tumor excised (HR 4.760, P = 0.049) was significantly associated with poorer OS. ER positivity (HR 0.445, P = 0.014) was significantly associated with better OS in first-line therapy with utidelone combined with capecitabine versus vinorelbine combined with cisplatin, which is consistent with the report of Wang et al. [19].
In the study of utidelone /capecitabine combination or monotherapy, adverse events associated with utidelone were generally mild to moderate and were considered clinically manageable [29]. The most common adverse events in our study were peripheral neuropathy, hand-foot syndrome, hematological toxicity, gastrointestinal toxicity, and hepatorenal toxicity, similar to those reported in previous studies [16]. The proportion of any adverse events occurring during dosing for first-line therapy with utidelone in combination with capecitabine versus second-line or above therapy was similar between the two groups. Among the specific toxic side effects, the incidence of neurologic toxicity, hand-foot syndrome, gastrointestinal toxicity, and hepatic and renal toxicity was higher in the second-line or above therapy group than in the first-line therapy group; and hematological toxicity was lower in the second-line or above therapy group than in the first-line therapy group. In these adverse events, recovery was observed by delayed dosing, dose reduction, or symptomatic adjuvant therapy (mecobalamin /vitamins, etc.), usually within 14 days. No deaths caused by utidelone /capecitabine were found in our study. No comparisons were made between the utidelone /capecitabine-treated group and the vinorelbine/ cisplatin -treated group because the vinorelbine combined with cisplatin group could not be followed up for adverse events. Overall, utidelone /capecitabine was considered well tolerated in this study.
Of course, as with other retrospective studies, we cannot completely rule out selection bias. Although the main factors affecting efficacy were included, other factors reported to affect efficacy, such as tumor type and tumor grade, were not collected in this study. Due to the sample size limitations and incompletely unavoidable selection bias of this study, future studies will need to assess efficacy and tolerability in larger patient populations.
In conclusion, this study is the first to demonstrate that in MBC with disease progression after anthracycline and taxanes, utidelone in combination with capecitabine as advanced first-line therapy results in more favorable PFS, OS, and ORR than second-line or above therapy. PFS and ORR are superior to those of vinorelbine and cisplatin in advanced first-line therapy. We suggest that utidelone in combination with capecitabine is a more valuable salvage therapy for advanced first-line MBC.
Funding
The present study was supported by the National Natural Science Foundation of China (grant no. 81960542, 82360614 and 81960517), Science and Technology Project of Yunnan Provincial Science and Technology Department (grant no. 202001AU070053, 202001AU070093 and 202201AY070001-169), Yunnan Health Training Project of High Level Talents (grant no. H-2019075), Beijing Science And Technology Innovation Medical Development Foundation (grant no. KC2021-JK-0044-6) and Wu Jieping Medical Foundation (grant no. 320.6750.2022-19-58).
Ethics approval and consent to participate
The author is responsible for all aspects of the work to ensure that issues related to the accuracy and completeness of any part of the work are properly investigated and resolved. Subjects have given their written informed consent and that the study protocol was approved by the institute's committee on human research. Study approval statement: This study complies with the Declaration of Helsinki (revised in 2013) and was approved by the ethics committee of the Third Affiliated Hospital of Kunming Medical University (Yunnan Cancer Hospital) (No. KYLX2023-107). Written informed consent was obtained from participants to participate in the study.
Patient consent for publication
Not applicable.
CRediT authorship contribution statement
Conceived and designed the study: RG, KZ, QSZ, SCT. Data collection and analysis: PPB, XW, RL, XQL, SRW, JWZ, XT, FZ, QM, YZ, BYT, XQX. Generated figures: XT, FZ, QM, YZ. Wrote the manuscript: PPB, XW, RL, XQL, SRW. Revised and edited the manuscript: SCT, QSZ, KZ, RG. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no competing interests.
Acknowledgements
We are grateful to all of the reviewers for their comments.
Contributor Information
Rong Guo, Email: guorong2320@126.com.
Kai Zheng, Email: zhengkai1003@yeah.net.
Shaoqiang Zhou, Email: 610943791@qq.com.
Shicong Tang, Email: tang_shicong@126.com.
Data availability
The datasets used during the current study are available from the corresponding author on reasonable request.
References
- 1.Siegel R.L., Miller K.D., Wagle N.S., Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763. [DOI] [PubMed] [Google Scholar]
- 2.Settleman J. Cancer: bet on drug resistance. Nature. 2016;529(7586):289–290. doi: 10.1038/nature16863. [DOI] [PubMed] [Google Scholar]
- 3.Lee D.W., Keam B., Lee K.S., Ahn J.H., Sohn J., Ahn J.S., et al. A phase II trial of S-1 and oxaliplatin in patients with metastatic breast cancer previously treated with anthracycline and taxane (KCSG-BR07-03) Cancer Res Treat. 2023;55(2):523–530. doi: 10.4143/crt.2022.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardoso F., Paluch-Shimon S., Senkus E., Curigliano G., Aapro M.S., André F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2022;20(6):691–722. doi: 10.6004/jnccn.2022.0030. [DOI] [PubMed] [Google Scholar]
- 6.Li J., Jiang Z. Chinese Society of Clinical Oncology Breast Cancer (CSCO BC) guidelines in 2022: stratification and classification. Cancer Biol Med. 2022;19(6):769–773. doi: 10.20892/j.issn.2095-3941.2022.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Shaughnessy J., Schwartzberg L., Danso M.A., Miller K.D., Rugo H.S., Neubauer M., et al. Phase III study of iniparib plus gemcitabine and carboplatin versus gemcitabine and carboplatin in patients with metastatic triple-negative breast cancer. J Clin Oncol. 2014;32(34):3840–3847. doi: 10.1200/JCO.2014.55.2984. [DOI] [PubMed] [Google Scholar]
- 8.Yuan P., Hu X., Sun T., Li W., Zhang Q., Cui S., et al. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: a randomised clinical trial. Eur J Cancer. 2019;112:57–65. doi: 10.1016/j.ejca.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Rugo H.S., Roche H., Thomas E., Chung H.C., Lerzo G.L., Vasyutin I., et al. Efficacy and safety of ixabepilone and capecitabine in patients with advanced triple-negative breast cancer: a pooled analysis from two large phase III, randomized clinical trials. Clin Breast Cancer. 2018;18(6):489–497. doi: 10.1016/j.clbc.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 10.Li S., Meng W., Zhang J., Xie X., Hao C., Jia Y., et al. A randomized controlled phase II trial of vinorelbine plus capecitabine versus docetaxel plus capecitabine in anthracycline-pretreated women with metastatic breast cancer. J Cancer Res Ther. 2020;16(5):1069–1076. doi: 10.4103/jcrt.JCRT_792_19. [DOI] [PubMed] [Google Scholar]
- 11.Hu X.C., Zhang J., Xu B.H., Cai L., Ragaz J., Wang Z.H., et al. Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16(4):436–446. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 12.Yardley D.A., Coleman R., Conte P., Cortes J., Brufsky A., Shtivelband M., et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol. 2018;29(8):1763–1770. doi: 10.1093/annonc/mdy201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres A., Ramdial J.L., Aguirre L.E., Mahtani R., Vogel C.L. Vinorelbine plus Capecitabine (Vinocap): a retrospective analysis in heavily pretreated HER2 negative metastatic breast cancer patients. Breast Cancer Res Treat. 2019;176(2):253–260. doi: 10.1007/s10549-019-05203-1. [DOI] [PubMed] [Google Scholar]
- 14.Kikawa Y., Kotake T., Tsuyuki S., Kang Y., Takahara S., Fujimoto Y., et al. Effectiveness of eribulin as first-line or second-line chemotherapy for HER2-negative hormone-resistant advanced or metastatic breast cancer: findings from the multi-institutional, prospective, observational KBCRN A001: E-SPEC study. Breast Cancer. 2022;29(5):796–807. doi: 10.1007/s12282-022-01357-x. [DOI] [PubMed] [Google Scholar]
- 15.Villegas C., González-Chavarría I., Burgos V., Iturra-Beiza H., Ulrich H., Paz C. Epothilones as natural compounds for novel anticancer drugs development. Int J Mol Sci. 2023;24(7):6063. doi: 10.3390/ijms24076063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang P., Sun T., Zhang Q., Yuan Z., Jiang Z., Wang X.J., et al. Utidelone plus capecitabine versus capecitabine alone for heavily pretreated metastatic breast cancer refractory to anthracyclines and taxanes: a multicentre, open-label, superiority, phase 3, randomised controlled trial. Lancet Oncol. 2017;18(3):371–383. doi: 10.1016/S1470-2045(17)30088-8. [DOI] [PubMed] [Google Scholar]
- 17.Xu B., Sun T., Zhang Q., Zhang P., Yuan Z., Jiang Z., et al. Efficacy of utidelone plus capecitabine versus capecitabine for heavily pretreated, anthracycline- and taxane-refractory metastatic breast cancer: final analysis of overall survival in a phase III randomised controlled trial. Ann Oncol. 2021;32(2):218–228. doi: 10.1016/j.annonc.2020.10.600. [DOI] [PubMed] [Google Scholar]
- 18.Martin M., Campone M., Bondarenko I., Sakaeva D., Krishnamurthy S., Roman L., et al. Randomised phase III trial of vinflunine plus capecitabine versus capecitabine alone in patients with advanced breast cancer previously treated with an anthracycline and resistant to taxane. Ann Oncol. 2018;29(5):1195–1202. doi: 10.1093/annonc/mdy063. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z., Xu L., Wang H., Li Z., Lu L., Li X., et al. Lobaplatin-based regimens outperform cisplatin for metastatic breast cancer after anthracyclines and taxanes treatment. Saudi J Biol Sci. 2018;25(5):909–916. doi: 10.1016/j.sjbs.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Yu Y.F., Wang Y., Fu T.P., Chen K., Liu J.Q., Yao H.R. Trastuzumab combined with doublet or single-agent chemotherapy as first-line therapy for HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2018;168(2):337–348. doi: 10.1007/s10549-017-4592-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan Y., Yost S.E., Cui Y., Ruel C., Murga M., Tang A., et al. Phase I trial of ipatasertib plus carboplatin, carboplatin/paclitaxel, or capecitabine and atezolizumab in metastatic triple-negative breast cancer [published online ahead of print, 2023 Apr 6] Oncologist. 2023;oyad026 doi: 10.1093/oncolo/oyad026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang B., Sun T., Zhao Y., Wang S., Zhang J., Wang Z., et al. A randomized phase 3 trial of gemcitabine or nab-paclitaxel combined with cisPlatin as first-line treatment in patients with metastatic triple-negative breast cancer. Nat Commun. 2022;13(1):4025. doi: 10.1038/s41467-022-31704-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sipos O., Tovey H., Quist J., Haider S., Nowinski S., Gazinska P., et al. Assessment of structural chromosomal instability phenotypes as biomarkers of carboplatin response in triple negative breast cancer: the TNT trial. Ann Oncol. 2021;32(1):58–65. doi: 10.1016/j.annonc.2020.10.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodler E.T., Kurland B.F., Griffin M., Gralow J.R., Porter P., Yeh R.F., et al. Phase I study of veliparib (ABT-888) combined with cisplatin and vinorelbine in advanced triple-negative breast cancer and/or BRCA mutation-associated breast cancer. Clin Cancer Res. 2016;22(12):2855–2864. doi: 10.1158/1078-0432.CCR-15-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Du F., Yuan P., Luo Y., Wang J., Ma F., Cai R., et al. Efficacy and toxicity of vinorelbine (NVB)-based regimens in patients with metastatic triple negative breast cancer (mTNBC) pretreated with anthracyclines and taxanes. Zhonghua Zhong Liu Za Zhi. 2015;37(10):788–792. [PubMed] [Google Scholar]
- 27.Wang J., Zheng R., Wang Z., Yang Y., Wang M., Zou W. Efficacy and safety of vinorelbine plus cisplatin vs. gemcitabine plus cisplatin for treatment of metastatic triple-negative breast cancer after failure with anthracyclines and taxanes. Med Sci Monit. 2017;23:4657–4664. doi: 10.12659/MSM.905300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M., Fan Y., Li Q., Zhang P., Yuan P., Ma F., et al. Vinorelbine plus platinum in patients with metastatic triple negative breast cancer and prior anthracycline and Taxane treatment. Medicine (Baltimore) 2015;94(43) doi: 10.1097/MD.0000000000001928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P., Tong Z., Tian F., Wang Y., Yang J., Li W., et al. Phase II trial of utidelone as monotherapy or in combination with capecitabine in heavily pretreated metastatic breast cancer patients. J Hematol Oncol. 2016;9(1):68. doi: 10.1186/s13045-016-0297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang H., Li H., Song G., Di L., Shao B., Yan Y., et al. Pegylated liposomal doxorubicin (Duomeisu®) monotherapy in patients with HER2-negative metastatic breast cancer heavily pretreated with anthracycline and taxanes: a single-arm, phase II study. Breast Cancer Res Treat. 2023;199(1):67–79. doi: 10.1007/s10549-023-06894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang R., Chen Y., Liu X., Gui X., Zhu A., Jiang H., et al. Efficacy of apatinib 250 mg combined with chemotherapy in patients with pretreated advanced breast cancer in a real-world setting. Front Oncol. 2023;13:1076469. doi: 10.3389/fonc.2023.1076469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yardley D.A., Reeves J., Dees E.C., Osborne C., Paul D., Ademuyiwa F., et al. Ramucirumab with Eribulin versus eribulin in locally recurrent or metastatic breast cancer previously treated with anthracycline and taxane therapy: a multicenter, randomized, phase II study. Clin Breast Cancer. 2016;16(6):471–479.e1. doi: 10.1016/j.clbc.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 33.He K., Wang X., Guan X., Yu Q., Ma Q., Liu Z., et al. Vinorelbine plus gemcitabine or cisplatin as first-line treatment of HER2-negative advanced breast cancer. Anticancer Res. 2017;37(10):5647–5653. doi: 10.21873/anticanres.12000. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the current study are available from the corresponding author on reasonable request.