Abstract
Purpose
Current reports of adenoid cystic carcinoma of the head and neck (ACC) are all case reports, and there is no basilar summary of its imaging findings. This study aims to summarise ACC's computed tomography (CT) and magnetic resonance imaging (MRI) findings to improve radiologists' knowledge of this disease.
Methods
We collected clinical and imaging data of patients with ACC during the last decade, and two radiologists retrospectively analysed the imaging characteristics.
Results
Of the 16 patients included, six were able to self-perceive bulkiness, and 11 had regional pain. Tumour morphology was regular in six cases, with clear borders in 11 cases, invasion of the surrounding bony mass in 12 cases, and invasion of peripheral nerves in 15 cases. CT mostly shows an irregular soft-tissue density mass with mild-to-moderate enhancement after contrast medium administration. On MRI, the ACC showed isointense or hypointense signals on T1-weighted images (T1WI) and hyperintense or slightly hyperintense signals on T2-weighted images (T2WI). All signals were markedly enhanced after gadolinium enhancement.
Conclusions
ACC often has an irregular morphology, sometimes with a cystic component, enhancement on enhancement scans, easy destruction of adjacent bone, and invasion of peripheral nerves. The diagnosis should be considered when these features are encountered in clinical practice.
Keywords: Adenoid cystic carcinoma, CT, MRI, Case series
1. Introduction
Adenoid cystic carcinoma of the head and neck (ACC) is a rare cancer that accounts for 1 % of all head and neck cancers [1]. It occurs mainly in the salivary glands and is the second most common malignant tumour of the salivary glands after mucoepidermoid carcinoma, followed by the lacrimal glands. Furthermore, ACC can occur in the external auditory canal, skull base, nasal cavity, and other head and neck locations [2]. Notably, ACC exhibits malignant features, such as invasion, recurrence, and metastases [3], and the current primary treatments are surgery and postoperative radiotherapy. Therefore, preoperative imaging is essential to determine the extent of tumour involvement and perineural invasion, and we need to summarise the imaging features of ACC [4]. However, due to the rarity of this disease, most imaging studies are only case reports and do not summarise its imaging characteristics. In this study, we collected cases of ACC diagnosed pathologically in our hospital between May 2014 and June 2022 and focused on imaging manifestations.
2. Methods
2.1. Study participants
This study was performed in strict compliance with the Declaration of Helsinki (2013 edition). This study was approved by the hospital ethics committee (No.2022-L052). The inclusion criteria were as follows: 1) pathologically confirmed ACC; 2) underwent computed tomography (CT) and/or magnetic resonance imaging (MRI) examination, and the image was clear without artefacts; and 3) full clinical data available. We collected relevant cases from the Affiliated Hospital of Nantong University between May 2014 and June 2022 and included 16 patients with complete clinical and imaging data. Of these, 9 underwent CT alone, 1 underwent MRI alone, and 6 underwent both CT and MRI.
2.2. Image acquisition
All imaging data were collected according to the standard criteria. The slice thickness was set at 2 mm for the temporal bone, 3 mm for the orbit, 4 mm for the neck, 4 mm for the parotid gland, and 3 mm for the sinus, depending on the examination site, using a Siemens SOMATOM force dual-source CT scan. MRI examination using a Ge discovery MR750 3.0T scanner, transverse, coronal, and sagittal scans were performed, and the slice thickness also differed according to the site: 3 mm for the orbit, 5.5 mm for the skull, 3 mm for the nasopharynx, 5.5 mm for the sinuses, and 2.5 mm for the pituitary gland.
2.3. Image analysis
All imaging data were independently evaluated by radiologists with 5 and 15 years of experience, and differences were resolved through consultation. The location, morphology, size, borders, homogeneity, degree of enhancement, presence or absence of bony destruction, and invasion of peripheral nerves were determined using plain CT, contrast-enhanced CT, and MRI.
2.4. Statistical analysis
Data analysis was performed using the SPSS software (version 24.0; IBM Corp., Armonk, NY, USA). Because this was an observational study, statistical tests were not performed.
3. Results
Among the 16 included patients, the male-to-female ratio was 1:1, and the age of the patients ranged from 16 to 76 years (mean age, 57 years). The locations of the ACC differed. The tumours were located in the external auditory canal, lacrimal gland, pharynx, palate, skull base, and maxillary sinus in one case; the sphenoid sinus, submandibular gland, and parotid gland in two cases; and the nasal cavity in four cases. Self-perceived bulkiness was present in 6 patients and localised pain in 11 patients. Tumour morphology was regular in 6 cases, with clear borders in 11 cases. The ACC appeared as a regular or irregular soft-tissue mass on CT that is iso - or slightly hypodense. Among them, 6 cases had homogeneous density, and 10 cases had uneven density. Calcification was observed in 0/16 cases. Enhancement examinations generally showed moderate enhancement, and when the tumour was larger, it showed obvious heterogeneous enhancement. The bone window revealed an invasion of the surrounding bone in 12 patients (Fig. 1). In the ACC of the paranasal sinuses, the anteroposterior diameter of the pterygopalatine fossa was widened in six cases, and the greater palatine foramen was invaded in four cases (Fig. 2). The masses were isointense or hypointense on T1-weighted images (T1WI) in seven cases, hyperintense or slightly hyperintense on T2-weighted images (T2WI) in five cases, isointense on T2WI in one case, and hypointense on T2WI in one case. An obvious hyperintense cystic change was observed in the middle of the lesions in four cases. All patients exhibited significant enhancement on post-gadolinium enhancement scans (Fig. 3). Table 1, Table 2 show the clinical data and imaging findings of the 16 patients.
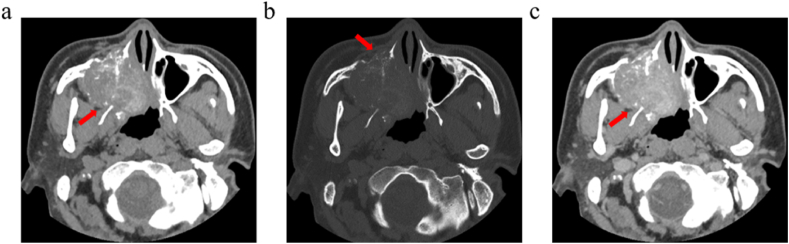
Fig. 1.
Male, 40 years, ACC of the right maxillary sinus, nasal cavity. (a) CT plain scan showed a mass-like soft tissue density mass in the right maxillary sinus with a patchy dense shadow inside, which protruded into the sphenoid sinus and nasal cavity. (b) The bone window showed bone destruction and resorption adjacent to the orbital floor, sinus wall, nasal septum, and hard palate. (c) The enhancement scan showed mild heterogeneous enhancement. (Soft tissue window: window width 450 Hu, window level 35 Hu; bone window: window width 1800 Hu, window level 500 Hu).
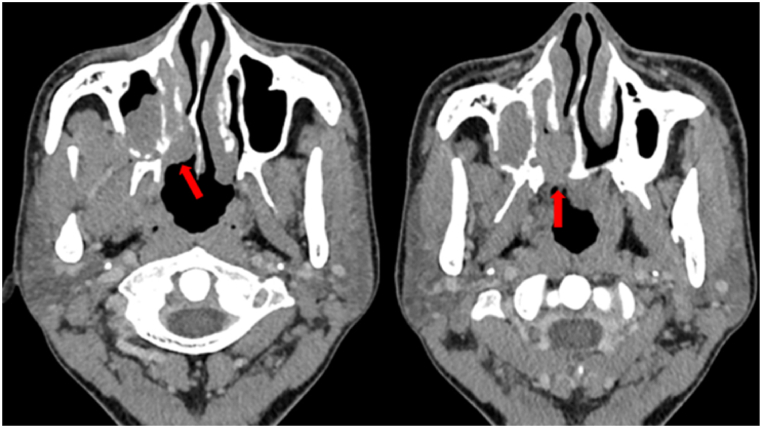
Fig. 2.
Male, 40 years, ACC of the right maxillary sinus and nasal cavity. The tumour infiltrated along the pterygopalatine fossa and greater palatine foramen. (Soft tissue window: window width 350 Hu, window level 50 Hu).
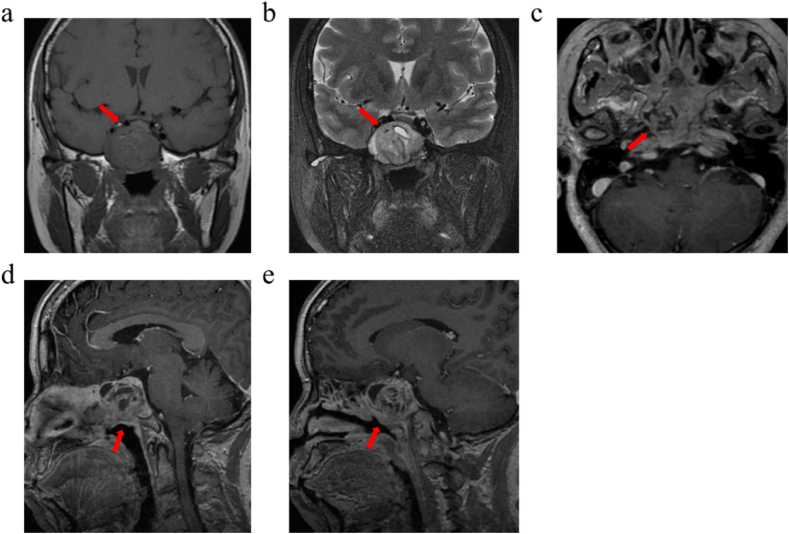
Fig. 3.
Female, 54 years, sphenoid ACC. (a) The tumour showed a mixed isointensity on T1WI. (b) Slightly high mixed signal on T2WI, including a small cystic high signal. The tumour showed cribriform changes on T2WI. (c) The transverse view shows a tumour involving bilateral pterygopalatine fossa. (d、e) On contrast-enhanced scan, the tumour was significantly enhanced unevenly, and cystic components were not enhanced.
Table 1.
Basic conditions and clinical presentation of included patients.
| No. | Gender | Age | Location | Symptoms |
|---|---|---|---|---|
| 1 | Male | 64 | External auditory canal | Intra-aural occlusion in the right ear with hearing loss for 5 years |
| 2 | Female | 16 | Lacrimal gland | Left orbital mass for 3 months |
| 3 | Female | 68 | Sphenoid sinus | Dizzy headache with decreased vision in left eye for 2 weeks |
| 4 | Male | 62 | Pharynx | Pharyngeal discomfort for 3 years |
| 5 | Male | 38 | Sinus and nasal cavity | Right palate mass for 2 weeks |
| 6 | Female | 59 | Sinus and nasal cavity | Left nasal mass for 2 years |
| 7 | Female | 45 | Submandibular gland | Left submandibular repeated pain for 1 month |
| 8 | Female | 65 | Parotid gland | Left ear pain and tinnitus for 1 year |
| 9 | Male | 76 | Paranasal sinus | Right maxillofacial numbness for 2 months |
| 10 | Female | 63 | Submandibular gland | Painless right mandibular mass for 2 years |
| 11 | Male | 63 | Sinus and nasal cavity | Right-sided facial itching with paroxysmal pain for 4 months |
| 12 | Male | 40 | Sinus and nasal cavity | Right side nasal obstruction with rhinorrhoea for 10 days |
| 13 | Female | 54 | Sinus and nasal cavity | Left side nasal obstruction with recurrent bleeding for 1 week |
| 14 | Male | 68 | Parotid gland | Post auricular mass on the left side for 1 year |
| 15 | Male | 70 | Sinus and nasal cavity | Right-sided nasal obstruction for 1 year |
| 16 | Female | 58 | Sphenoid sinus | Ear swelling with nasal congestion for 2 years, aggravated for over 2 months |
Table 2.
Imaging findings of the included patients.
| No. | Radiological manifestations |
||||||
|---|---|---|---|---|---|---|---|
| Size (mm) |
Boundary | Morphology | Density | Signal | Enhancement | Bone invasion | |
| 1 | 28*18 | Not well-defined | Regular | Homogeneous soft tissue density | – | Moderate enhancement | Yes |
| 2 | 30*23 | Well-defined | Regular | Soft tissue density, internal fluid density | Iso-hypointense on T1WI, hyperintense and linear hypointense on T2WI | Obvious enhancement | Yes |
| 3 | 45*45 | Well-defined | Irregular | Soft tissue density, internal fluid density | Hypointense on T1WI, hyperintense on T2WI | Obvious enhancement | Yes |
| 4 | 28*22 | Not well-defined | Irregular | Homogeneous soft tissue density | – | Mild enhancement | No |
| 5 | 39*30 | Not well-defined | Irregular | Homogeneous soft tissue density | – | Mild enhancement | Yes |
| 6 | 27*16 | Well-defined | Irregular | – | Slightly hypointense on T1WI, slightly hyperintense on T2WI | Obvious enhancement | Yes |
| 7 | 57*44 | Not well-defined | Irregular | Soft tissue density, internal fluid density | – | Moderate enhancement | No |
| 8 | 44*26 | Not well-defined | Irregular | Soft tissue density, internal fluid density | – | Obvious enhancement | Yes |
| 9 | 40*31 | Well-defined | Regular | Homogeneous soft tissue density | Isointense on T1WI, hypointense on T2WI | Obvious enhancement | Yes |
| 10 | 22*20 | Well-defined | Regular | Homogeneous soft tissue density | – | Moderate enhancement | No |
| 11 | 51*31 | Well-defined | Irregular | Soft tissue density, internal multiple hyperdensities | Isointense on T1WI, isointense on T2WI | Obvious enhancement | Yes |
| 12 | 56*45 | Well-defined | Irregular | Soft tissue density, internal multiple hyperdensities | Isointense on T1WI, iso-hyperintense on T2WI | Mild enhancement | Yes |
| 13 | 35*33 | Well-defined | Irregular | Homogeneous soft tissue density | – | Moderate enhancement | Yes |
| 14 | 32*28 | Well-defined | Regular | Slightly higher density | – | – | No |
| 15 | 50*40 | Well-defined | Irregular | Slightly higher density | – | – | Yes |
| 16 | 41*36 | Well-defined | Regular | Soft tissue density, internal fluid density | Mixed signal on T1WI, mixed signal on T2WI | Obvious enhancement | Yes |
4. Discussion
The ACC is composed of epithelial and myoepithelial cells containing round or ovoid cystic lacunae of various sizes [5]. ACC generally arises in the 3rd to 9th decades, with an average of the 5th, without a clear sex predisposition [6]. Our patient initially presented with a painless mass, with subsequent progressive enlargement of the mass and symptoms of pain, compression, and nerve invasion. Currently, radical resection is the mainstay of treatment for ACC, allowing for acceptable initial tumour survival [7,8]. However, despite its slow growth rate, the tumour is highly invasive, and the extent of invasion tends to extend beyond that observed macroscopically at the time of surgery, often leading to local recurrence and distant metastasis [9]. Therefore, an accurate preoperative imaging diagnosis is needed to determine the nature of the tumour and the degree of surrounding invasion early.
The extremely thin collimation and high spatial resolution of CT scanning are the theoretical bases of multiplanar imaging and optimal three-dimensional post-processing, which gives maxillofacial CT imaging higher image quality [10]. In our 16 patients, most tumours occurred in the nasal cavity and paranasal sinuses, consistent with Li et al.'s previous study [11]. In the nasal cavity and paranasal sinuses, 50 % of patients had nasal congestion symptoms, which is lower than the 70 % reported in the literature, whereas 87.5 % of patients had head and face pain, significantly higher than the 15 % reported in the literature. A CT scan of the ACC can appear as a mass-like irregular soft tissue mass with some uneven density and some visible fluid density, suggesting a mucinous component within the tumour. Some may have patchy hyperdensity, which indicates invasion of the mass into the surrounding bony mass and resorptive destruction of the bony mass. When the tumour is small, it often shows poor contrast with the surrounding normal tissue. When the tumour is large, its internal density is generally uneven, and an irregular sheet-like low-density shadow can be observed. On contrast-enhanced CT, the solid component shows mild-to-moderate heterogeneous enhancement, whereas the cystic component does not. These results are consistent with those previously reported [5,12]. CT can clearly show changes in adjacent bone erosion and destruction; however, it is often unable to accurately determine the boundary of the tumour and the depth of invasion. Consistent with previous studies, our 16 patients exhibited varying degrees [11].
The histopathological subtypes of ACC are tubular, cribriform, and solid. The tubular type is a tube-like structure composed of two layers of cells: epithelial cells in the inner layer and myoepithelial cells in the outer layer. The cribriform type is the most common type. The tumor cells are arranged in a cribriform meshwork, containing mucoid substances. Solid-type tumour cells have a laminar distribution; only a few tubules or sieve pores are visible. Affected by the type of tumour pathology, the imaging features of MRI are more discriminatory than those of CT. On T2WI, hypointense lesions correspond to the solid type and have a worse prognosis, whereas hyperintense lesions correspond to the cribriform or tubular type [13]. In our included patients, the masses were all isointense or hypointense on T1WI, whereas on T2WI, hypointense, isointense, slightly hyperintense, or hyperintense masses were observed, with isointensity/hyperintensity being the most common, with distinct hyperintense cystic change observed in the middle of the lesion. All MRI scans were markedly enhanced. Among these, the cribriform type is the most characteristic, with multiple cystic signal shadows.
The ACC is characterised by neurotropic growth. Nerve invasion is mainly observed in the trigeminal nerve, facial nerve, and their branches. Specifically, the tumour grows along the nerve and extends considerably from the main tumour mass. The affected nerves are anterogradely or retrogradely thickened, with abnormal strengthening and muscle denervation [14]. The pterygopalatine fossa is an important component of brain communication, leading to different parts passing through multiple foramina, with important nerves passing through them. ACC should be suspected if changes in the palatal foramen magnum or pterygopalatine fossa are observed in space-occupying palate or maxillary sinus lesions, suggesting nerve invasion. Of our patients, 87.5 % had peripheral nerve invasion, slightly higher than the 70 % reported by Li et al.
Common head and neck tumours should be distinguished from diseases at different sites. ACC arising in the parotid, submandibular, and sublingual glands must be differentiated from pleomorphic adenomas [15]. Pleomorphic adenoma is the most common salivary gland tumour, with a predilection for all ages, most commonly 30–60 years of age, and female predominance. The tumour boundary was clear, and there was no bony destruction. Furthermore, the tumour is surrounded by an intact capsule. Approximately half of pleomorphic adenomas are spherical or lobulated masses, whereas most other salivary gland tumours are not lobulated [16]. Pleomorphic adenoma shows low density on CT, high signal on T2WI, and low signal edge, which reflects that pleomorphic adenoma contains a rich myxocartilage-like matrix, which is the characteristic feature of pleomorphic adenoma. The enhancement scans tended to continuously and gradually enhance, and the ADC values of DWI were significantly higher than those of other salivary gland tumours [17].
ACC occurring in the sinuses and nasal cavities must be differentiated from squamous carcinomas and lymphomas. The most common non-epithelial malignancy occurring in the nasal cavity is lymphoma and epithelial tumour is squamous cell carcinoma. Both occur more commonly in middle-aged and older males and experience nonspecific symptoms such as nasal obstruction, localised pain, epistaxis, unilateral facial or nasal swelling, rhinorrhoea, and headache. On imaging, there was no obvious difference between the two presentations [18].
Lymphomas are relatively large, more homogeneous in texture, often undergo adjacent osseous remodelling, and less frequently invade nerves. If a lymphoma is accompanied by cervical lymph node invasion, differentiation from ACC is favourable. Squamous cell carcinoma is often heterogeneous in texture due to intratumoural necrosis or haemorrhage and can present with significant bone destruction and nerve invasion [19]. ACC arising in minor salivary glands at sites such as the oral palate, tongue, and buccal mucosa must be differentiated from other malignancies of epithelial origin. Other malignant tumours of oral epithelial origin, such as squamous carcinomas, often show signs of epithelial ulceration and lymph node metastasis; however, nerve invasion is rare.
In addition to the commonly used CT and MRI imaging, PET/CT plays an important role in differentiating between primary and secondary ACC and diagnosing distant metastasis of ACC [[20], [21], [22]]. 18F-FDG PET/CT combined with clinicopathological characteristic evaluation helps distinguish between primary and secondary lung ACC and provides guidance for clinical decision-making. In addition, the prostate-specific membrane antigen (PSMA) is expressed in most epithelial tumour cells. PSMA PET/CT has a high sensitivity for ACC. PSMA PET plays an important complementary role in staging and evaluating early treatment responses [23].
Because ACC easily infiltrates surrounding tissues and can easily invade nerves, surgery combined with radiotherapy has the best curative effect. Larger tumour tissues can be surgically resected, whereas radiation therapy is often administered as an adjunctive treatment to nerve remnants. Unfortunately, although people often choose a combination therapy of the two methods, local recurrence still often occurs because of the aggressive nature of the tumour. In our patients, 3 recurrences were observed. The overall prognosis of ACC is rather poor [24]. This prompted us to accurately diagnose the disease using preoperative imaging combined with clinical data so as not to delay clinical treatment. This study has some limitations owing to the small number of cases. More relevant cases should be collected in the future for statistical analysis of the imaging findings of ACC to provide a more precise inductive summary.
5. Conclusions
The diagnosis of ACC is supported when an irregular mass occurring in the salivary gland region of the head and neck shows diffuse infiltrative growth with cribriform changes in the middle of the lesion and generally moderate enhancement. The tumour shows drilling growth, spread over long distances along the nerve, adjacent bony destruction, and clinical manifestations of facial pain, numbness, or muscle atrophy with denervation. This is a good way to solve the lag in pathological diagnosis using imaging to judge the nature of the tumour in advance.
Data Availability
All the data generated or analysed during this study are included within the article.
CRediT authorship contribution statement
Yidan Wang: Writing – review & editing, Writing – original draft. Xiaoli Guo: Validation, Supervision. Ke Yu: Validation, Software, Formal analysis. Xiying Shen: Visualization, Data curation. Jia Liu: Visualization, Data curation. Tianye Zhao: Project administration, Methodology. Hongmei Gu: Supervision, Resources, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This research was supported by the fund from Nantong Municipal Social and People's Livelihood Science and Technology Project (MS12020042), and the fund for Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX21_1467).
References
- 1.Coca-Pelaz A., et al. Adenoid cystic carcinoma of the head and neck--An update. Oral Oncol. 2015;51(7):652–661. doi: 10.1016/j.oraloncology.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Bacherini D., et al. Bilateral choroidal metastasis from adenoid cystic carcinoma of the submandibular salivary gland. Eur. J. Ophthalmol. 2022;32(1):NP258–NP263. doi: 10.1177/1120672120970405. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Russo C.A., et al. Radiation therapy for adenoid cystic carcinoma of the head and neck. Cancers. 2021;13(24) doi: 10.3390/cancers13246335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuzaki H., et al. Minor salivary gland tumors in the oral cavity: diagnostic value of dynamic contrast-enhanced MRI. Eur. J. Radiol. 2012;81(10):2684–2691. doi: 10.1016/j.ejrad.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Uraizee I., Cipriani N.A., Ginat D.T. Adenoid cystic carcinoma of the oral cavity: radiology-pathology correlation. Head Neck Pathol. 2018;12(4):562–566. doi: 10.1007/s12105-017-0849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huston B., et al. Adenoid cystic carcinoma of the trachea resulting in fatal asphyxia. J. Forensic Sci. 2017;62(1):244–246. doi: 10.1111/1556-4029.13236. [DOI] [PubMed] [Google Scholar]
- 7.Du Y., Zeng Y. Analysis of postoperative radiotherapy for non-metastatic head and neck adenoid cystic carcinoma based on SEER data. J. Int. Med. Res. 2022;50(8) doi: 10.1177/03000605221115151. 30006052211151 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ayoub N., et al. Management of adenoid cystic carcinoma of the head and neck: experience of the national cancer institute, Egypt. Gulf J Oncolog. 2022;1(39):63–69. [PubMed] [Google Scholar]
- 9.Friedl M., et al. Pretherapeutic serum albumin as an outcome prognosticator in head and neck adenoid-cystic carcinoma. Biomedicines. 2022;10(1) doi: 10.3390/biomedicines10010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazziotti S., et al. Postprocessing in maxillofacial multidetector computed tomography. Can. Assoc. Radiol. J. 2015;66(3):212–222. doi: 10.1016/j.carj.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., et al. Computed tomography and magnetic resonance imaging of adenoid cystic carcinoma in the maxillary sinus: a retrospective study with radiologic-histopathologic correlations. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(1):111–121. doi: 10.1016/j.oooo.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Lasrado S., Moras K., Prabha B.B. A rare case of adenoid cystic carcinoma of the sphenoid sinus presenting with lateral rectus palsy. Indian J. Otolaryngol. Head Neck Surg. 2018;70(3):459–46 1. doi: 10.1007/s12070-018-1370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lahjaouj M., et al. Advanced adenoid cystic carcinoma of maxillary sinus: rare case report and review of literature. Int J Surg Case Rep. 2021;80 doi: 10.1016/j.ijscr.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh F.M., Mak S.Y., Bonington S.C. Patterns of spread of head and neck adenoid cystic carcinoma. Clin. Radiol. 2015;70(6):644–653. doi: 10.1016/j.crad.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Cicero G., et al. Cross-sectional imaging of parotid gland nodules: a brief practical guide. J Clin Imaging Sci. 2018;8:14. doi: 10.4103/jcis.JCIS_8_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda K., et al. The usefulness of MR in establishing the diagnosis of parotid pleomorphic adenoma. AJNR Am J Neuroradiol. 1996;17(3):555–559. [PMC free article] [PubMed] [Google Scholar]
- 17.Kato H., et al. Pleomorphic adenoma of salivary glands: common and uncommon CT and MR imaging features. Jpn. J. Radiol. 2018;36(8):463–471. doi: 10.1007/s11604-018-0747-y. [DOI] [PubMed] [Google Scholar]
- 18.Kim S.H., et al. Differential diagnosis of sinonasal lymphoma and squamous cell carcinoma on CT, MRI, and PET/CT. Otolaryngol. Head Neck Surg. 2018;159(3):494–500. doi: 10.1177/0194599818770621. [DOI] [PubMed] [Google Scholar]
- 19.Kato H., et al. Differentiation of extranodal non-Hodgkins lymphoma from squamous cell carcinoma of the maxillary sinus: a multimodality imaging approach. SpringerPlus. 2015;4:228. doi: 10.1186/s40064-015-0974-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun X., et al. Clinical use of (18)F-FDG PET/CT in the differential diagnosis of patients with primary and secondary adenoid cystic carcinoma of the lung: a retrospective cohort study. Transl. Lung Cancer Res. 2022;11(8):1643–1656. doi: 10.21037/tlcr-22-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Q., et al. 99mTc-MIBI SPECT/CT and FDG PET/CT in isolated bilateral renal metastases from adenoid cystic carcinoma of the maxilla. Clin. Nucl. Med. 2022;47(2):e205–e207. doi: 10.1097/RLU.0000000000003872. [DOI] [PubMed] [Google Scholar]
- 22.Datta Gupta S., et al. Rare brain metastasis in parotid adenoid cystic carcinoma detected on 68Ga-PSMA PET/CT. Clin. Nucl. Med. 2021;46(11):e561–e562. doi: 10.1097/RLU.0000000000003813. [DOI] [PubMed] [Google Scholar]
- 23.Dhiantravan N., Ravi Kumar A.S., Cavanagh K., McDowell L. A role of psma pet/ct in multimodality imaging approach in adenoid cystic carcinoma. J. Med. Imaging. Radiat. Oncol. 2021;65:213–215. doi: 10.1111/1754-9485.13116. [DOI] [PubMed] [Google Scholar]
- 24.van Weert S., et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49(8):824–829. doi: 10.1016/j.oraloncology.2013.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data generated or analysed during this study are included within the article.