Abstract
Purpose
Intestinal metaplasia plays a crucial role in the risk stratification of gastric cancer development. The objective of the study was to develop a prediction model for Operative Link on Gastric Intestinal Metaplasia (OLGIM) Stage III-IV.
Methods
We analyzed 7945 high-risk gastric cancer individuals from 115 hospitals who underwent questionnaires and gastroscope. The participants were assigned to either the development or validation cohort randomly. Demographics and clinical characteristics were obtained. The outcome measurement was OLGIM III-IV. Univariate logistic regression was used for feature selection and multivariate logistic analysis was performed to develop the nomogram. Area under the curves, calibration plots, decision curve and clinical impact analysis were used to assess the performance of the nomogram.
Results
4600 individuals and 3345 individuals were included in the development and validation cohort, of which 124 and 86 individuals were diagnosed with OLGIM III-IV, respectively. Parameters in the training validation cohort matched well and there was no significant difference between two cohorts. A nomogram model for predicting OLGIM Stage III-IV consisted of 4 significantly associated variables, including age, gender, PG I and G-17 (AUC 0.723 and 0.700 for the 2 cohorts). The nomogram demonstrated excellent performance in the calibration curve. Decision curve and clinical impact analysis suggested clinical benefit of the prediction model.
Conclusions
This reliable individualized nomogram might contribute to more accurate management for patients with OLGIM III-IV. Therefore, we suggest that this study be used as an incentive to promote the application.
Keywords: Intestinal metaplasia, Operative link on gastritis intestinal metaplasia stage III-IV, Pepsinogen I, Gastrin-17, Nomogram model
I. Introduction Background
Gastric cancer (GC) is one of the most common cancers and stands as the third leading cause of cancer-related mortality worldwide [1,2]. The diagnosis of GC relies on histological confirmation through endoscopic biopsy. Risk factors for GC include Helicobacter pylori infection, age, smoking, alcohol, high salt intake, and inadequate consumption of fruit and vegetables [3]. Regrettably, the survival rate for patients with gastric cancer remains poor, largely attributed to late-stage diagnoses. The 5-year survival rate among GC patients remains below 50 %, in contrast to the over 90 % survival rate observed in cases of early GC [4,5]. Therefore, the key problem is to improve early detection rate of GC, which suggests the importance of surveillance for patients with high risk of GC [6,7].
In the progression towards the development of intestinal-type gastric adenocarcinoma, the histopathological stages evolve from normal gastric epithelium to chronic gastritis, atrophic gastritis (AG), intestinal metaplasia (IM), dysplasia, and finally GC [6]. Notably, atrophy, intestinal metaplasia, and dysplasia are widely recognized as precancerous lesions [8]. The operative link on gastric intestinal metaplasia assessment (OLGIM) is a grading standard for risk stratification of IM [9]. It ranges from Stage 0 to IV and the OLGIM stage III/IV has a significant association with the development of GC [[10], [11], [12]]. Endoscopic screening programs for GC have shown potential in reducing the risk of GC-related mortality [7,13]. However, it's important to acknowledge that the cost-effectiveness of endoscopic screening may not be justified in regions with medium or low risk for GC unless it is selectively applied to individuals with a high risk of developing gastric cancer. The recommended approach is to adopt a risk stratification strategy, which means repeated endoscopic surveillance every 2–3 years for high-risk patients (OLGIM III-IV) and every 5 years for those with intermediate risk (OLGIM II) [11,14]. However, the application of endoscopic screening is not cost effective in regions with medium/low risk for GC, but it can be improved if it is limited to specific individuals with high risk of GC [15,16]. The widespread utilization of gastroscopy is hindered by the insufficient availability of proficient endoscopists and endoscopic facilities in Asia [17]. In light of this challenge, non-invasive screening methods for early gastric cancer have been previously proposed [18].
Helicobacter pylori (Hp) is the most important established risk factor for GC and is considered as a Class 1 carcinogen of GC [19,20]. GIM prevalence increases with HP infection and HP eradication can reduce the risk of developing gastric adenocarcinoma [6]. Pepsinogen (PG) I and II are produced by the chief cells and mucous neck cells of the stomach, whereas Gastrin-17 (G-17) is a protein specifically secreted by antral G cells [[21], [22], [23]]. As the mucosa of the gastric fundus gland diminishes, the level of PG I gradually decreases, while the level of PG II remains relatively stable [24]. Furthermore, the level of G-17 becomes significantly low in cases of gastric antral atrophy due to the reduced presence of G cells [25]. Serum PGs detection has been widely accepted as they closely related to the occurrence of gastric atrophy and intestinal metaplasia. The serum PG I level was found to be significantly lower in individuals with intestinal metaplasia (IM) at OLGIM stage >2 compared to those with IM at OLGIM stage <2 [16]. In comparison to patients with a PG I/II ratio ≤3 and/or OGLIM stage III-IV, none of the patients with PG I/II ratio >3 and OLGIM stage 0-II developed high-grade adenoma/dysplasia or invasive neoplasia during the follow-up period [12]. It was reported that a risk stratification model combining serum gastrin G-17, PG I levels and patient age was used to predict advanced OLGIM stages [16]. However, there are still no studies based on large samples. Therefore, our study aimed to provide a nomogram for predicting OLGIM stage III-IV among high-risk Chinese gastric cancer population.
2. Methods
2.1. Study design and participants
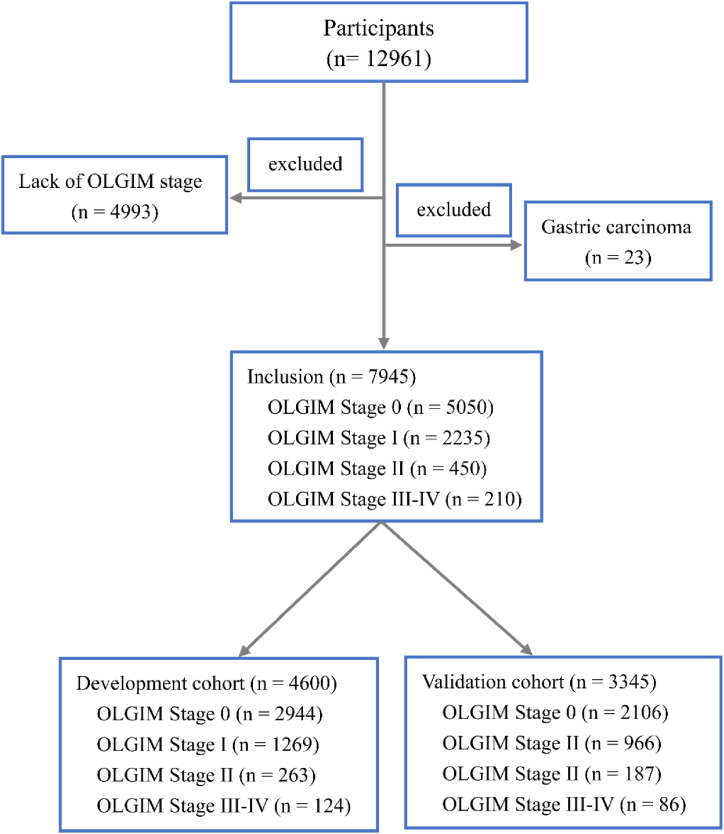
With approval from the Ethics Committees of 115 hospitals in China, we aimed to conduct a multicenter cross-sectional study among the high-risk population of GC The exclusion criteria were as follows, (1) the presence of dysphagia, emesis, abdominal pain, abdominal mass, unintentional weight loss of more than 4.5 kg over the past six months, (2) previous Hp eradication, proton pump inhibitors or H2 blocker intake within the two weeks, (3) pregnancy, cancer history and endoscopy examination within 1 year. The selection process for individuals is presented in Fig. 1.
Fig. 1.
Flowchart. OLGIM, Operative Link on Gastric Intestinal Metaplasia.
2.2. Data collection
We collected the demographic and clinical characteristics of the participants, such as age, gender, body mass index, family history, smoking, alcohol drinking, diet habit (high salt, red meat, white meat, green vegetables, fresh fruits). Laboratory parameters (PG I, PG II, G-17, anti-Hp IgG antibody, Carcinoembryonic antigen (CEA)) were tested by professional staff (PGI, PGII, G-17 and Helicobacter pylori antibody ELISA kits; biohit, Helsinki, Finland; CEA, electrochemiluminescence immunoassay by Roche cobas 8000 modular analyzer series). Furthermore, all subjects underwent a gastroscopy examination by skilled endoscopists, with biopsy specimens collected from gastric antrum, angle, body, and lesion area (at least three for each subject) in each hospital. The diagnosis fulfilled the classification for the latest Sydney system [26]. Two independent, blinded pathologists examined specimens.
2.3. Variable definitions
We defined PGs and G-17 cut-off values as follows. We divided original PGs and G-17 in the development cohort into 20 parts and obtained 20 cut-off values. Then we combined the cut-off value categories with similar prevalence of OLGIM stage III-IV. Finally, PG I, PG II and G-17 were divided into three categories and PG I/II ratio was divided into two categories. CEA was difined as negative (≤5 ng/mL) or positive (>5 ng/mL). Body mass index (BMI) (<18.5, 18.5–23.9, >23.9 kg/m2), age (40–49, 50–59, 60–69 and > 69 years) were also classified into three and four categories, respectively. Regular intake of certain food was defined as eating such food more than three times a week.
2.4. Statistical analysis
We randomly divided the cohort into development (4600 individuals) and validation (3345 individuals) datasets. We used the chi-square test to preliminarily evaluate the differences among no IM, OLGIM I, OLGIM II and OLGIM III-IV in development cohort. The endpoints of our study were OLGIM stage III–IV. To determine the independent predictors of OLGIM III-IV, we performed a logistic regression analysis with a stepwise backward selection of variables. Variables with significance in univariate analysis entered the multivariate regression models. A nomogram interpreting the logistic regression model were accordingly established for predicting OLGIM III-IV probabilities. We conducted internal validation to evaluate model performance. The discriminative ability was assessed by calculating the area under curve (AUC) of the receiver operating characteristic (ROC) curves. The AUC differences between two cohorts were compared by Delong test. Calibration plots were performed to assess the accuracy of prediction by bootstrap validation with 1000 repetitions. We also assessed the p-value of the unreliability statistics, average and maximum errors between the predictions and observations from the calibration curve. Decision curve analysis (DCA) and clinical impact curve (CIC) were performed to determine the nomogram's clinical effectiveness. All statistical analyses were performed using IBM SPSS statistics version 22.0 (SPSS, Armonk, NY, USA) and R software version 4.04 (www.r-project.org). R packages including pROC, rms, rmda, regplot, regplot, ResourceSelection, etc were used. Differences were considered significant at P < 0.05. Two-sided P < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
As presented in Fig. 1, a total of 12961 individuals were included in our study, in which, 5016 individuals were excluded. 4600 participants were randomly selected as a development cohort and the rest were selected as a validation cohort. There was no significant difference between development and validation cohort (Table 3). The development cohort consisted of 2944 individuals without intestinal metaplasia (IM), 1269 individuals with OLGIM stage I, 263 individuals with OLGIM stage II and 124 individuals with OLGIM stage III–IV. The validation cohort included 2106 individuals without IM, 966 individuals with OLGIM stage I, 187 individuals with OLGIM stage II, and 86 individuals with OLGIM stage III–IV. The demographics and clinical features of individuals with OLGIM III-IV were compared with those with no IM, OLGIM stage I, II in the derivation cohort (Table 1). Age, gender, Hp, PG I, PG I/II ratio, family history, smoking, high salt diet, green vegetables, fresh fruits were all significantly different among participants without IM, with OLGIM stage I, stage II and stage III–IV.
Table 3.
Demographics and clinical characteristics of the development and validation cohorts.
| Development cohort (N = 4600) | Validation cohort (N = 3345) | P value | |
|---|---|---|---|
| Age, years | 0.149 | ||
| 40–49 | 1271 | 910 | |
| 50–59 | 1593 | 1105 | |
| 60–69 | 1283 | 955 | |
| >69 | 453 | 375 | |
| Gender | 0.703 | ||
| Female | 2366 | 1706 | |
| Male | 2234 | 1639 | |
| Body mass index | 0.608 | ||
| ≤18.5 | 268 | 203 | |
| 18.5–23.9 | 2809 | 2006 | |
| >23.9 | 1523 | 1136 | |
| Helicobacter pylori | 0.619 | ||
| Negative | 2629 | 1893 | |
| Positive | 1971 | 1452 | |
| CEA | 0.451 | ||
| Negative | 4444 | 3221 | |
| Positive | 156 | 124 | |
| PG I, ng/mL | 0.773 | ||
| ≤42.16 | 230 | 159 | |
| 42.16–56.51 | 231 | 177 | |
| >56.51 | 4139 | 3009 | |
| PG II, ng/mL | 0.825 | ||
| ≤5.47 | 1152 | 826 | |
| 5.47–9.80 | 1383 | 994 | |
| >9.80 | 2065 | 1525 | |
| PG I/II ratio | 0.657 | ||
| ≥5.25 | 4142 | 3022 | |
| <5.25 | 458 | 323 | |
| G-17, pmol/L | 0.107 | ||
| ≤1.00 | 931 | 621 | |
| 1.00–7.98 | 2289 | 1733 | |
| >7.98 | 1380 | 991 | |
| Family history | 0.803 | ||
| No | 3993 | 2910 | |
| Yes | 607 | 435 | |
| Smoking | 0.394 | ||
| No | 3527 | 2592 | |
| Yes | 1073 | 753 | |
| Alcohol drinking | 0.624 | ||
| No | 3789 | 2741 | |
| Yes | 811 | 604 | |
| High salt diet | 0.727 | ||
| Occasional | 2657 | 1919 | |
| Regular | 1943 | 1426 | |
| Red meat | 0.130 | ||
| Occasional | 1887 | 1429 | |
| Regular | 2713 | 1916 | |
| White meat | 0.766 | ||
| Occasional | 2541 | 1859 | |
| Regular | 2059 | 1486 | |
| Green vegetables | 0.205 | ||
| Occasional | 916 | 628 | |
| Regular | 3684 | 2717 | |
| Fresh fruits | 0.376 | ||
| Occasional | 2150 | 1597 | |
| Regular | 2450 | 1748 | |
| OLGIM Stage, n (%) | 0.649 | ||
| No IM | 2944 | 2106 | |
| I | 1269 | 966 | |
| II | 263 | 187 | |
| III-IV | 124 | 86 |
CEA, carcinoembryonic antigen; PG, pepsinogen; G-17, gastrin-17; IM, intestinal metaplasia; OLGIM, Operative Link on Gastritis Intestinal Metaplasia.
Table 1.
Comparison of demographics and clinical characteristics among individuals with no IM, OLGIM stage I, stage II, and stage III–IV in the training sample.
| No IM (n = 2944) | stage I (n = 1269) | stage II (n = 263) | stage III–IV (n = 124) | P value | |
|---|---|---|---|---|---|
| Age, years | <0.001 | ||||
| 40–49 | 903 | 302 | 50 | 16 | |
| 50–59 | 1041 | 444 | 81 | 27 | |
| 60–69 | 733 | 393 | 101 | 56 | |
| >69 | 267 | 130 | 31 | 25 | |
| Gender | 0.021 | ||||
| Female | 1549 | 642 | 125 | 50 | |
| Male | 1395 | 627 | 138 | 74 | |
| Body mass index | 0.271 | ||||
| ≤18.5 | 160 | 90 | 14 | 4 | |
| 18.5–23.9 | 1793 | 770 | 163 | 83 | |
| >23.9 | 991 | 409 | 86 | 37 | |
| Helicobacter pylori | |||||
| Negative | 1738 | 676 | 141 | 74 | 0.003 |
| Positive | 1206 | 593 | 122 | 50 | |
| CEA | 0.387 | ||||
| Negative | 2847 | 1229 | 250 | 118 | |
| Positive | 97 | 40 | 13 | 6 | |
| PG I, ng/mL | <0.001 | ||||
| ≤42.16 | 126 | 75 | 16 | 11 | |
| 42.16–56.51 | 126 | 79 | 15 | 12 | |
| >56.51 | 2692 | 1115 | 232 | 101 | |
| PG II, ng/mL | 0.108 | ||||
| ≤5.47 | 158 | 51 | 18 | 10 | |
| 5.47–9.80 | 875 | 381 | 88 | 41 | |
| >9.80 | 1911 | 837 | 157 | 73 | |
| PG I/II ratio | 0.045 | ||||
| ≥5.25 | 2676 | 1126 | 234 | 106 | |
| <5.25 | 268 | 143 | 29 | 18 | |
| G-17, pmol/L | 0.165 | ||||
| ≤1.00 | 1611 | 713 | 155 | 70 | |
| 1.00–7.98 | 419 | 185 | 33 | 26 | |
| >7.98 | 914 | 371 | 75 | 28 | |
| Family history | 0.001 | ||||
| No | 2592 | 1073 | 216 | 112 | |
| Yes | 352 | 196 | 47 | 12 | |
| Smoking | 0.016 | ||||
| No | 2289 | 965 | 185 | 88 | |
| Yes | 655 | 304 | 78 | 36 | |
| Alcohol drinking | 0.255 | ||||
| No | 2429 | 1055 | 206 | 99 | |
| Yes | 515 | 214 | 57 | 25 | |
| High salt diet | 0.045 | ||||
| Occasional | 1740 | 703 | 139 | 75 | |
| Regular | 1204 | 566 | 124 | 49 | |
| Red meat | 0.910 | ||||
| Occasional | 1201 | 526 | 106 | 54 | |
| Regular | 1743 | 743 | 157 | 70 | |
| White meat | 0.319 | ||||
| Occasional | 1614 | 703 | 159 | 65 | |
| Regular | 1330 | 566 | 104 | 59 | |
| Green vegetables | 0.040 | ||||
| Occasional | 591 | 230 | 62 | 33 | |
| Regular | 2353 | 1039 | 201 | 91 | |
| Fresh fruits | 0.033 | ||||
| Occasional | 1365 | 582 | 146 | 57 | |
| Regula0072 | 1579 | 687 | 117 | 67 |
IM, intestinal metaplasia; OLGIM, Operative Link on Gastritis Intestinal Metaplasia; CEA, carcinoembryonic antigen; PG, pepsinogen; G-17, gastrin-17. Fresh fruit = Occasional; Regular.
3.2. Nomogram construction and validation
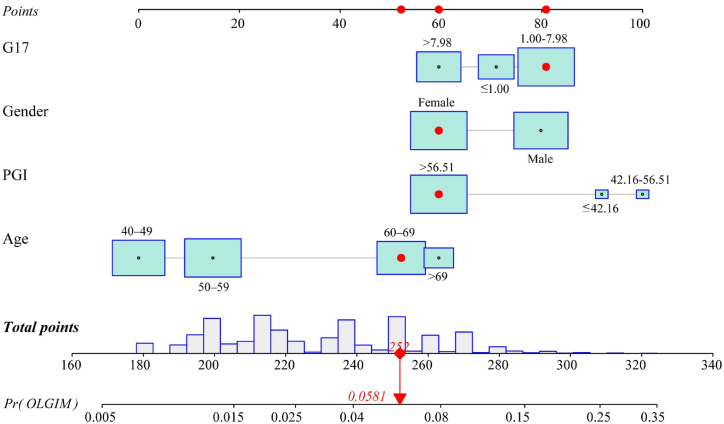
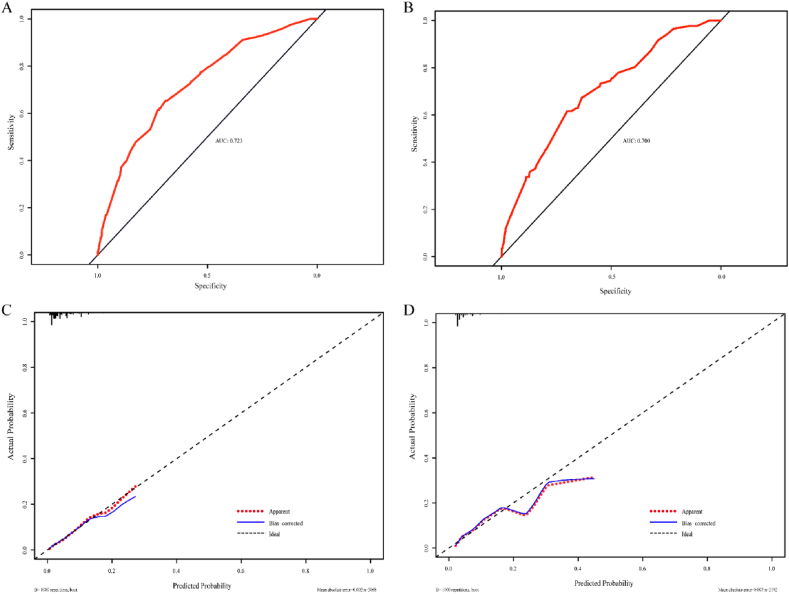
In the development cohort, univariate and multivariate logistic regression analysis were used to determine the risk factors correlated with participants with OLGIM stage III-IV. In the univariate regression analysis, there were statistical differences in variables (age, gender, PG I, PG I/II ratio, G-17) were significantly associated with OLGIM III-IV (Table 2). We then used multivariate logistic regression analysis to screen independent factors establish the predictive nomogram. Four variables were included in the model for OLGIM III-IV after a backward, stepwise selection procedure. According to the screened variables, we constructed a nomogram for OLGIM stage III-IV (Fig. 2). The estimated OLGIM III-IV probability can be obtained by adding the points of each variable, finding the corresponding point on the total points axis and drawing a vertical line downward from that point. The higher the total score calculated from the nomograms, the higher the likelihood of OLGIM III-IV. The performance evaluation was conducted by using the ROC curve and calibration plot, and the results were shown in Fig. 3 and Table 4. The apparent AUC for the score on the development cohort was 0.723, and 0.700 for validation cohort (Fig. 3A and B), which indicated that the model is sufficiently accurate. The calibration curves of the nomogram were close to the ideal curves (Fig. 3C and D). As shown in Table 4, the calibration was good and there was no significant maximum and average differences between the prediction probabilities and the observation frequencies in the development sample. In the validation cohort, the calibration was also acceptable, with average and maximum errors of 0.6 % and 2.3 %, respectively. The results of the Hosmer-Lemeshow test (χ2 = 4.54, p = 0.81 for the development cohort; χ2 = 7.96, p = 0.44 for the validation cohort) also indicated that the model was well-fitted.
Table 2.
Uni- and multivariate analyses of individuals for predicting OLGIM stage III-IV in the training sample.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95 % CI) | P Value | OR (95 % CI) | P Value | |
| Age, years | – | – | – | – |
| 40–49 | – | – | – | – |
| 50–59 | 1.464 (0.784–2.734) | 0.232 | 1.556 (0.830–2.914) | 0.168 |
| 60–69 | 4.312 (2.453–7.579) | <0.001 | 4.770 (2.699–8.431) | <0.001 |
| >69 | 5.284 (2.780–10.044) | <0.001 | 5.967 (3.109–11.450) | <0.001 |
| Gender | – | – | – | – |
| Female | – | – | – | – |
| Male | 1.643 (1.140–2.370) | 0.008 | 1.836 (1.259–2.677) | 0.002 |
| Body mass index | – | – | – | – |
| ≤18.5 | – | – | – | – |
| 18.5–23.9 | 1.852 (0.670–5.116) | 0.235 | – | – |
| >23.9 | 1.493 (0.525–4.247) | 0.452 | – | – |
| Helicobacter pylori | – | – | – | – |
| Negative | – | – | – | – |
| Positive | 0.974 (0.675–1.404) | 0.887 | – | – |
| CEA | – | – | – | – |
| Negative | – | – | – | – |
| Positive | 1.492 (0.641–3.474) | 0.353 | – | – |
| PG I, ng/mL | – | – | – | – |
| >56.51 | – | – | – | – |
| 42.16–56.51 | 2.537 (1.359–4.739) | 0.003 | 3.363 (1.749–6.464) | <0.001 |
| ≤42.16 | 2.308 (1.208–4.408) | 0.011 | 2.637 (1.348–5.162) | 0.005 |
| PG II, ng/mL | – | – | – | – |
| >9.80 | – | – | – | – |
| 5.47–9.80 | 0.833 (0.531–1.308) | 0.428 | – | – |
| ≤5.47 | 1.350 (0.887–2.055) | 0.162 | – | – |
| PG I/II ratio | – | – | – | – |
| ≥5.25 | – | – | – | – |
| <5.25 | 1.696 (1.013–2.838) | 0.045 | – | – |
| G-17, pmol/L | – | – | – | – |
| >7.98 | – | – | – | – |
| 1.00–7.98 | 1.662 (1.068–2.588) | 0.024 | 1.894 (1.200–2.989) | 0.006 |
| ≤1.00 | 1.218 (0.690–2.149) | 0.496 | 1.407 (0.784–2.526) | 0.253 |
| Family history | – | – | – | – |
| No | – | – | – | – |
| Yes | 0.789 (0.431–1.446) | 0.443 | – | – |
| Smoking | – | – | – | – |
| No | – | – | – | – |
| Yes | 1.430 (0.961–2.127) | 0.078 | – | – |
| Alcohol drinking | – | – | – | – |
| No | – | – | – | – |
| Yes | 1.191 (0.760–1.866) | 0.445 | – | – |
| High salt diet | – | – | – | – |
| Occasional | – | – | – | – |
| Regular | 0.944 (0.654–1.363) | 0.759 | – | – |
| Red meat | – | – | – | – |
| Occasional | – | – | – | – |
| Regular | 0.893 (0.622–1.283) | 0.541 | – | – |
| White meat | – | – | – | – |
| Occasional | – | – | – | – |
| Regular | 1.102 (0.769–1.579) | 0.598 | – | – |
| Green vegetables | – | – | – | – |
| Occasional | – | – | – | – |
| Regular | 0.693 (0.460–1.042) | 0.078 | – | – |
| Fresh fruits | – | – | – | – |
| Occasional | – | – | – | – |
| Regular0020 | 1.016 (0.709–1.457) | 0.931 | – | – |
OLGIM, Operative Link on Gastritis Intestinal Metaplasia; OR, odds ratio; CI, confidence interval; CEA, carcinoembryonic antigen; PG, pepsinogen; G-17, gastrin-17. Fresh fruit = Occasional; Regular.
Fig. 2.
Nomogram predicting individuals with OLGIM stage III-IV. Each clinical variable has a specific number of points (0–100). The sum of points of each variable was correlated with the probability of OLGIM stage III-IV. The figure presents an example which explains the use of the nomogram. The score of a female participant aged 60–69, with G17 value 1.00–7.98 and PG I value more than 56.51, was 252, which is corresponded to a 5.81 % probability of OLGIM stage III-IV. PG, pepsinogen; G17, gastrin-17.
Fig. 3.
The receiver operating characteristic (ROC) curves and calibration curves for the nomogram. (A) ROC curve for the nomogram in the development cohort. (B) ROC curve for the nomogram in the validation cohort. (C) Calibration curve for the nomogram in the development cohort. (D) Calibration curve for the nomogram in the validation cohort.
Table 4.
Validation of the nomogram.
| Training sample (n = 3068) | Validation sample (n = 2192) | ||
|---|---|---|---|
| Discrimination | AUC | 0.723 | 0.700 |
| P-valuea | 0.522 | ||
| Calibration | P-value of unreliability index | 0.989 | 0.413 |
| E aver | 0.002 | 0.006 | |
| E max | 0.018 | 0.023 | |
For AUC comparison. AUC, area under the ROC curve; E, difference in predicted probability and observed frequencies; E aver, average error. Emax, maximal error.
3.3. Clinical value of the nomogram
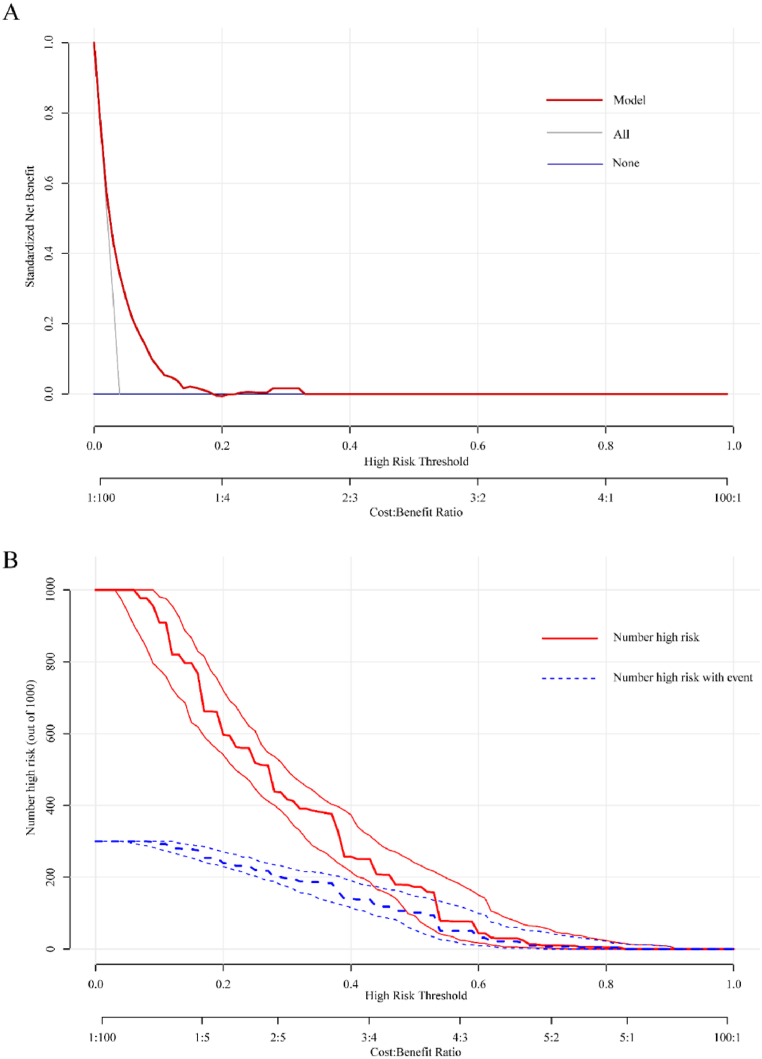
According to the DCA of the model for OLGIM III-IV, this nomogram improved clinical risk prediction against threshold probabilities of OLGIM III-IV (Fig. 4A). As for clinical applicability of risk prediction OLGIM III-IV nomogram, CIC analysis was conducted (Fig. 4B). CIC demonstrated that the nomogram had a better overall net benefit within the wide and practical threshold probabilities ranges. Therefore, the model had significant predictive value.
Fig. 4.
Decision curve analysis (DCA) and clinical impact curve analysis (CICA) of the nomogram. (A) DCA for the nomogram. The y-axis measures the net benefit. The red line represents the nomogram. The blue line represents the assumption that no participants had OLGIM III-IV, and the grey line represents the assumption that all participants had OLGIM III-IV. (B) CICA for the nomogram. The red curve is the number of participants characterized as positive (high risk) by the nomogram model under each threshold probability; the blue curve represents the number of true positives at each threshold probability. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The number of newly diagnosed cases of GC remains high and is expected to persistently rise among younger patients, highlighting the ongoing significant public health challenge worldwide [27]. Extensive gastric IM can be diffuse throughout both the gastric antrum and body mucosa, which is one of the important risk factors for GC and deserves regular follow-up [11,28]. Staging systems, operative link for gastritis assessment (OLGA) and OLGIM, have been proposed for the stratification of GC risk [10]. OLGA or OLGIM stage III-IV was associated with an increased GC risk [10,14]. Nevertheless, implementing endoscopic screening for high-risk GC individuals is often not cost-effective. While screening strategies have proven successful for early detection in Japan and South Korea, they may not be feasible in regions with low to intermediate incidence rates or in countries with large populations, potentially leading to delayed diagnoses for many patients [2,13,29]. For the high-risk population, non-invasive test would reduce the burden and related costs of endoscopic surveillance [11]. In this multicenter study with a large sample size, we developed a novel prediction model for OGLIM stage III-IV. This is the first model to utilize large datasets for predicting high-risk IM patients.
GC often exhibits familial aggregation, with individuals with a family history of GC facing a twofold increased risk of developing the disease [30]. It was reported that the family history of precancerous changes and GC was associated with non-cardia GC [31]. IM increases with age and high-risk OLGIM stages are relatively rare in individuals under the age of 40 [32]. In our study, a significant higher age was observed among individuals in OLGIM stage III-IV. Smoking is a well-known risk factor associated with GC and increases the risk of early gastric neoplasia among high-risk IM patients [14]. Age >70 years was associated with a 9-fold higher probability of developing gastric epithelial neoplastic lesions [33]. Dietary habits have been considered as a GC essential risk factor [34]. GC is more prevalent in men, and IM tends to be more severe in males than in females [1,35]. In this study, we found age, male, family history, smoking, high salt diet, lack of green vegetables and fresh fruits were different among OLGIM stage. Furthermore, logistic regression analysis showed that age and gender were significantly related to OLGIM stage III-IV.
Helicobacter pylori (Hp) is considered as Class 1 carcinogen, and its eradication has been demonstrated to reduce the incidence of GC [20]. Hp is the main etiological factor for atrophic changes in the gastric mucosa. The history of Hp infection was more prevalent in high-risk IM patients [14]. Hp infection is the most common cause of AG [6]. There were significant differences in Hp prevalence among the non-atrophic gastritis, OLGA stage I–II and stage III–IV subgroups [36]. Hp infection was one of the independent risk factors of participants with OLGA any-stage and OLGA stage III–IV [36]. IM, characterized by the replacement of normal gastric mucosa by metaplastic intestinal epithelium, is considered an adaptive response to chronic injury [37]. Hp infection has been reported as a significant factor in AG development, while environmental and host factors play a more crucial role in IM development [38]. Some reports have indicated that Hp eradication can improve AG and IM [39,40]. However, others reported that Hp eradication led to a significant reduction in AG, but not in IM [35,41]. In this study, there were not significant differences for Hp infection among the non-IM, OLGIM stage I, stage II and stage III–IV subgroups. Consequently, it was not identified as a risk factor for predicting OLGIM stage III-IV.
Serum PG measurements have significant value in screening for AG and in predicting GC risk. Serum PG I level adjusted by patient's age can be used in the management of patients at risk for GC, which was with a high predicted probability in individuals with OLGIM stage ≥2 [16]. The concentration of pepsinogen I (PG I) of less than 70 ng/ml and a PG I/II ratio of less than 3.0 are widely accepted as the cutoff points for GC screening in Japan [42]. The cutoff values used in this study were determined based on the characteristics of the study population and should be further validated in additional cohorts. G-17 is almost exclusively secreted by G cells of the digestive tract and its production is regulated by gastric acid secretion. The combination of pepsinogen, G-17 and anti-Hp antibodies serological assays was a reliable tool for AG diagnosis [43]. However, it was also reported that anti-Hp IgG, PG I, PG I/II ratio, and G-17 demonstrated low discrimination for gastric precancerous lesions in a multiethnic population of the United States [44]. In the present study, there were significant differences in PG I and PG I/II ratio among the non-IM, OLGIM stage I, II and III–IV subgroups. Logistic regression analysis showed that PG I, and G-17 were independent risk factors in the model of OLGIM stage III–IV. Meanwhile, it is advisable for high-risk patients to undergo regular gastroscopy and pathological examinations. Additional techniques such as chromoendoscopy or narrow-band imaging (NBI) can also enhance diagnostic accuracy [45,46].
We created a new nomogram to predict OLGIM stage III–IV based on the potential risk factors identified by multivariate logistic regression analysis (age, gender, PG I, G-17). The nomogram serves as a valuable tool for generating personalized risk estimates by incorporating various determinant variables, aligning with our quest for comprehensive clinical models and the realization of personalized healthcare [47]. Our nomogram is capable of providing individualized risk assessments and can serve as an effective screening tool for identifying OLGIM stage III–IV. Based on the ROC curve, calibration plot, decision curve analysis (DCA), and clinical impact curve (CIC), it is evident that this nomogram is not only feasible and reliable but also effective and convenient in clinical practice. Predictive model based on demographic and clinical variables may improve the early diagnosis of individuals with OLGIM stage III-IV. For example, when a patient's nomogram score reaches 252, the patient may have a 5.81 % probability of OLGIM stage III-IV. It is strongly recommended for high-risk individuals to undergo regular gastroscopy screenings. The higher the assigned nomogram risk score, the more urgent and crucial the need for screening becomes.
We acknowledge that our study has certain limitations. Firstly, it is a cross-sectional study, which means we cannot ascertain whether the influence of the selected variables on OLGIM stage changes over time. Secondly, external validation is still necessary to confirm the validity of our model.
5. Conclusion
In this study, we developed and validated a nomogram which could predict OLGIM stage III-IV in high-risk Chinese GC population. Our nomogram might serve as a non-invasive and valuable tool for screening high-risk GC individuals and facilitate the early diagnosis of GC patients.
Ethics approval and consent to participate
The study was approved by the ethics committee of The First Affiliated Hospital of USTC (No.2016-34). All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The informed consent was obtained from all patients for the publication of all their data and/or images included.
Consent for publication
Informed consent was obtained from all patients for being included in the study.
Data availability statement
Data will be made available on request.
CRediT authorship contribution statement
Song Wang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Formal analysis, Data curation, Conceptualization. Meng Qian: Software, Methodology, Investigation. Min Wu: Software, Methodology, Investigation. Shuo Feng: Software, Methodology, Investigation. Kaiguang Zhang: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Methodology, Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all patients and their relatives for their participation in this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e21905.
IM, intestinal metaplasia; OLGIM, Operative Link on Gastritis Intestinal Metaplasia; CEA, carcinoembryonic antigen; PG, pepsinogen; G-17, gastrin-17.
Contributor Information
Song Wang, Email: drwangsong@163.com.
Kaiguang Zhang, Email: zhangkaiguang@ustc.edu.cn.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
References
- 1.Smyth E.C., Nilsson M., Grabsch H.I., van Grieken N.C., Lordick F. Gastric cancer. Lancet. 2020;396:635–648. doi: 10.1016/S0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 2.Ajani J.A., Lee J., Sano T., Janjigian Y.Y., Fan D., Song S. Gastric adenocarcinoma. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.36. [DOI] [PubMed] [Google Scholar]
- 3.Mankaney G., Macaron C., Burke C.A. Refining risk factors for gastric cancer in patients with lynch syndrome to optimize surveillance esophagogastroduodenoscopy. Clin. Gastroenterol. Hepatol. 2020;18:780–782. doi: 10.1016/j.cgh.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Kunz P.L., Gubens M., Fisher G.A., Ford J.M., Lichtensztajn D.Y., Clarke C.A. Long-term survivors of gastric cancer: a California population-based study. J. Clin. Oncol. 2012;30:3507–3515. doi: 10.1200/JCO.2011.35.8028. [DOI] [PubMed] [Google Scholar]
- 5.Suzuki H., Oda I., Abe S., Sekiguchi M., Mori G., Nonaka S., Yoshinaga S., et al. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198–205. doi: 10.1007/s10120-015-0469-0. [DOI] [PubMed] [Google Scholar]
- 6.Banks M., Graham D., Jansen M., Gotoda T., Coda S., di Pietro M., Uedo N., et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68:1545–1575. doi: 10.1136/gutjnl-2018-318126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Li M., Chen S., Hu J., Guo Q., Liu R., Zheng H., et al. Endoscopic screening in asian countries is associated with reduced gastric cancer mortality: a meta-analysis and systematic review. Gastroenterology. 2018;155:347–354 e349. doi: 10.1053/j.gastro.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Gupta S., Li D., El Serag H.B., Davitkov P., Altayar O., Sultan S., Falck-Ytter Y., et al. AGA clinical practice guidelines on management of gastric intestinal metaplasia. Gastroenterology. 2020;158:693–702. doi: 10.1053/j.gastro.2019.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capelle L.G., de Vries A.C., Haringsma J., Ter Borg F., de Vries R.A., Bruno M.J., van Dekken H., et al. The staging of gastritis with the OLGA system by using intestinal metaplasia as an accurate alternative for atrophic gastritis. Gastrointest. Endosc. 2010;71:1150–1158. doi: 10.1016/j.gie.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Yue H., Shan L., Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2018;21:579–587. doi: 10.1007/s10120-018-0812-3. [DOI] [PubMed] [Google Scholar]
- 11.Pimentel-Nunes P., Libanio D., Marcos-Pinto R., Areia M., Leja M., Esposito G., Garrido M., et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European society of gastrointestinal endoscopy (ESGE), European Helicobacter and microbiota study group (EHMSG), European society of pathology (ESP), and sociedade portuguesa de Endoscopia digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365–388. doi: 10.1055/a-0859-1883. [DOI] [PubMed] [Google Scholar]
- 12.den Hollander W.J., Holster I.L., den Hoed C.M., Capelle L.G., Tang T.J., Anten M.P., Prytz-Berset I., et al. Surveillance of premalignant gastric lesions: a multicentre prospective cohort study from low incidence regions. Gut. 2019;68:585–593. doi: 10.1136/gutjnl-2017-314498. [DOI] [PubMed] [Google Scholar]
- 13.Jun J.K., Choi K.S., Lee H.Y., Suh M., Park B., Song S.H., Jung K.W., et al. Effectiveness of the Korean national cancer screening program in reducing gastric cancer mortality. Gastroenterology. 2017;152:1319–1328 e1317. doi: 10.1053/j.gastro.2017.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Lee J.W.J., Zhu F., Srivastava S., Tsao S.K., Khor C., Ho K.Y., Fock K.M., et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: a prospective multicentre cohort study (GCEP) Gut. 2022;71:854–863. doi: 10.1136/gutjnl-2021-324057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh J.M., Hur C., Ward Z., Schrag D., Goldie S.J. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: a cost-effectiveness analysis. Gut. 2016;65:563–574. doi: 10.1136/gutjnl-2014-308588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Re V., Orzes E., Canzonieri V., Maiero S., Fornasarig M., Alessandrini L., Cervo S., et al. Pepsinogens to distinguish patients with gastric intestinal metaplasia and Helicobacter pylori infection among populations at risk for gastric cancer. Clin. Transl. Gastroenterol. 2016;7:e183. doi: 10.1038/ctg.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung W.K., Wu M.S., Kakugawa Y., Kim J.J., Yeoh K.G., Goh K.L., Wu K.C., et al. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9:279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 18.Cai Q., Zhu C., Yuan Y., Feng Q., Feng Y., Hao Y., Li J., et al. Development and validation of a prediction rule for estimating gastric cancer risk in the Chinese high-risk population: a nationwide multicentre study. Gut. 2019;68:1576–1587. doi: 10.1136/gutjnl-2018-317556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hooi J.K.Y., Lai W.Y., Ng W.K., Suen M.M.Y., Underwood F.E., Tanyingoh D., Malfertheiner P., et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Lee Y.C., Chiang T.H., Chou C.K., Tu Y.K., Liao W.C., Wu M.S., Graham D.Y. Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 2016;150:1113–1124 e1115. doi: 10.1053/j.gastro.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Samloff I.M. Cellular localization of group I pepsinogens in human gastric mucosa by immunofluorescence. Gastroenterology. 1971;61:185–188. [PubMed] [Google Scholar]
- 22.Samloff I.M., Liebman W.M. Cellular localization of the group II pepsinogens in human stomach and duodenum by immunofluorescence. Gastroenterology. 1973;65:36–42. [PubMed] [Google Scholar]
- 23.Germana B., Di Mario F., Cavallaro L.G., Moussa A.M., Lecis P., Liatoupolou S., Comparato G., et al. Clinical usefulness of serum pepsinogens I and II, gastrin-17 and anti-Helicobacterpylori antibodies in the management of dyspeptic patients in primary care. Dig. Liver Dis. 2005;37:501–508. doi: 10.1016/j.dld.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Miki K., Ichinose M., Shimizu A., Huang S.C., Oka H., Furihata C., Matsushima T., et al. Serum pepsinogens as a screening test of extensive chronic gastritis. Gastroenterol. Jpn. 1987;22:133–141. doi: 10.1007/BF02774209. [DOI] [PubMed] [Google Scholar]
- 25.Sipponen P., Ranta P., Helske T., Kaariainen I., Maki T., Linnala A., Suovaniemi O., et al. Serum levels of amidated gastrin-17 and pepsinogen I in atrophic gastritis: an observational case-control study. Scand. J. Gastroenterol. 2002;37:785–791. [PubMed] [Google Scholar]
- 26.Dixon M.F., Genta R.M., Yardley J.H., Correa P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am. J. Surg. Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Arnold M., Park J.Y., Camargo M.C., Lunet N., Forman D., Soerjomataram I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut. 2020;69:823–829. doi: 10.1136/gutjnl-2019-320234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song H., Ekheden I.G., Zheng Z., Ericsson J., Nyren O., Ye W. Incidence of gastric cancer among patients with gastric precancerous lesions: observational cohort study in a low risk Western population. BMJ. 2015;351:h3867. doi: 10.1136/bmj.h3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamashima C., Ogoshi K., Okamoto M., Shabana M., Kishimoto T., Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitelli-Storelli F., Rubin-Garcia M., Pelucchi C., Benavente Y., Bonzi R., Rota M., Palli D., et al. Family history and gastric cancer risk: a pooled investigation in the stomach cancer pooling (stop) project consortium. Cancers. 2021;13 doi: 10.3390/cancers13153844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song H., Ekheden I.G., Ploner A., Ericsson J., Nyren O., Ye W. Family history of gastric mucosal abnormality and the risk of gastric cancer: a population-based observational study. Int. J. Epidemiol. 2018;47:440–449. doi: 10.1093/ije/dyx238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam J.H., Choi I.J., Kook M.C., Lee J.Y., Cho S.J., Nam S.Y., Kim C.G. OLGA and OLGIM stage distribution according to age and Helicobacter pylori status in the Korean population. Helicobacter. 2014;19:81–89. doi: 10.1111/hel.12112. [DOI] [PubMed] [Google Scholar]
- 33.Esposito G., Dilaghi E., Cazzato M., Pilozzi E., Conti L., Carabotti M., Di Giulio E., et al. Endoscopic surveillance at 3 years after diagnosis, according to European guidelines, seems safe in patients with atrophic gastritis in a low-risk region. Dig. Liver Dis. 2021;53:467–473. doi: 10.1016/j.dld.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Grosso G., Bella F., Godos J., Sciacca S., Del Rio D., Ray S., Galvano F., et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017;75:405–419. doi: 10.1093/nutrit/nux012. [DOI] [PubMed] [Google Scholar]
- 35.Kodama M., Okimoto T., Mizukami K., Hirashita Y., Wada Y., Fukuda M., Matsunari O., et al. Gastric mucosal changes, and sex differences therein, after Helicobacter pylori eradication: a long-term prospective follow-up study. J. Gastroenterol. Hepatol. 2021;36:2210–2216. doi: 10.1111/jgh.15477. [DOI] [PubMed] [Google Scholar]
- 36.Wang S., Ye F., Sheng Y., Yu W., Liu Y., Liu D., Zhang K. Development and validation of nomograms to predict operative link for gastritis assessment any-stage and stages III-IV in the Chinese high-risk gastric cancer population. Front. Med. 2021;8 doi: 10.3389/fmed.2021.724566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah S.C., Gawron A.J., Li D. Surveillance of gastric intestinal metaplasia. Am. J. Gastroenterol. 2020;115:641–644. doi: 10.14309/ajg.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim N., Park Y.S., Cho S.I., Lee H.S., Choe G., Kim I.W., Won Y.D., et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter. 2008;13:245–255. doi: 10.1111/j.1523-5378.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 39.Hwang Y.J., Kim N., Lee H.S., Lee J.B., Choi Y.J., Yoon H., Shin C.M., et al. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication - a prospective study for up to 10 years. Aliment. Pharmacol. Ther. 2018;47:380–390. doi: 10.1111/apt.14424. [DOI] [PubMed] [Google Scholar]
- 40.Sung J.J., Lin S.R., Ching J.Y., Zhou L.Y., To K.F., Wang R.T., Leung W.K., et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterology. 2000;119:7–14. doi: 10.1053/gast.2000.8550. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y.C., Chen T.H., Chiu H.M., Shun C.T., Chiang H., Liu T.Y., Wu M.S., et al. The benefit of mass eradication of Helicobacter pylori infection: a community-based study of gastric cancer prevention. Gut. 2013;62:676–682. doi: 10.1136/gutjnl-2012-302240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitahara F., Kobayashi K., Sato T., Kojima Y., Araki T., Fujino M.A. Accuracy of screening for gastric cancer using serum pepsinogen concentrations. Gut. 1999;44:693–697. doi: 10.1136/gut.44.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zagari R.M., Rabitti S., Greenwood D.C., Eusebi L.H., Vestito A., Bazzoli F. Systematic review with meta-analysis: diagnostic performance of the combination of pepsinogen, gastrin-17 and anti-Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment. Pharmacol. Ther. 2017;46:657–667. doi: 10.1111/apt.14248. [DOI] [PubMed] [Google Scholar]
- 44.Huang R.J., Park S., Shen J., Longacre T., Ji H., Hwang J.H. Pepsinogens and gastrin demonstrate low discrimination for gastric precancerous lesions in a multi-ethnic United States cohort. Clin. Gastroenterol. Hepatol. 2022;20:950–952 e953. doi: 10.1016/j.cgh.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Wasielica-Berger J., Rogalski P., Pryczynicz A., Swidnicka-Siergiejko A., Dabrowski A. Methylene blue chromoendoscopy is more useful in detection of intestinal metaplasia in the stomach than mucosal pit pattern or vessel evaluation and predicts advanced Operative Link on Gastric Intestinal Metaplasia stages. Clin Endosc. 2023;56:203–213. doi: 10.5946/ce.2022.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esposito G., Pimentel-Nunes P., Angeletti S., Castro R., Libanio D., Galli G., Lahner E., et al. Endoscopic grading of gastric intestinal metaplasia (EGGIM): a multicenter validation study. Endoscopy. 2019;51:515–521. doi: 10.1055/a-0808-3186. [DOI] [PubMed] [Google Scholar]
- 47.Balachandran V.P., Gonen M., Smith J.J., DeMatteo R.P. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173–e180. doi: 10.1016/S1470-2045(14)71116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.