Abstract
The concept of sepsis has recently evolved from one of a ‘systemic inflammatory response syndrome caused by infection’ to a ‘severe, potentially fatal organic dysfunction caused by an inadequate or imbalanced host response to infection’. Organ dysfunction is closely related to sepsis. Multiple organ dysfunction syndrome (MODS) is the most serious outcome of sepsis, often leading to a poor prognosis. However, specific drugs for sepsis and MODS caused by sepsis remain undetermined, and the fatality rate is relatively high. Under the guidance of modern medicine, traditional Chinese medicine (TCM) has gained a wealth of experience in the prevention and treatment of sepsis and plays a key role via the effects of its numerous components, pathways and targets. This study used ‘Sepsis’, ‘Organ dysfunction’ and ‘Traditional Chinese medicine’ as strategies for searching the databases of Chinese National Knowledge Infrastructure, Wanfang, PubMed and The Web of Science. This paper presents an overview of the current status of TCM component formulations for preventing and treating sepsis with MODS to provide a theoretical basis for clinical treatment and drug development.
Keywords: Sepsis, Traditional Chinese medicine, Multiple organ dysfunction syndrome, Current status
1. Introduction
Sepsis leads to life-threatening organ dysfunction caused by a dysregulated bodily response to infection, which can be triggered in any part of the body [1]. An epidemiological study in the United States revealed that sepsis affects approximately 1.7 million adults every year, resulting in approximately 250,000 deaths [2]. However, epidemiological studies on sepsis in mainland China are limited. A study in Beijing in 2023 reported that the incidence of sepsis among hospitalised patients was 13.1 %, with an in-hospital mortality rate of 28.4 %. After adjusting for age and sex, the standardised incidence of sepsis was 421.85/100,000, with a mortality rate of 19.16/100,000 [3].
The optimal treatment for patients with sepsis requires the early recognition of symptoms and infectious lesions and rapid intervention to mitigate the extent of the immune response. Without prompt treatment, sepsis may lead to septic shock and multiple organ dysfunction syndrome (MODS), which is considered to be the terminal stage of systemic inflammatory response dysregulation, resulting in more than 5.3 million deaths worldwide each year [4]. A study by Xue et al. [5] demonstrated that the incidence of secondary MODS within 28 days in hospitalised patients with sepsis is 13.33 %. Moreover, a related study revealed that among patients with sepsis with secondary MODS, the fatality rate can be as high as 60%–80 % [6]. Thus, the prevention and treatment of MODS are crucial for the management of sepsis.
Sepsis with MODS is a research focus because of its extremely high morbidity and mortality rates and the lack of ideal clinical treatments. The current Western medical treatments for sepsis are mainly antibiotics and supportive care [7]. However, the use of antibiotics leads to a systemic microbiota imbalance and can easily trigger antibiotic resistance [8].
With advancements in modern medical technologies, the application of molecular biology principles and techniques in traditional Chinese medicine (TCM) has expa nded to yield improved results. According to the clinical manifestations of sepsis, the disease belongs to the category of febrile diseases in TCM [9]. Based on its aetiology, pathogenesis and pathological characteristics, sepsis is mostly classified as sepsis, severe sepsis and septic shock. As a powerful supplement to antibiotic drugs, TCM has been included in the conventional treatment of sepsis [10]. A meta-analysis of several clinical studies demonstrated that herbal medicine has a non-negligible role in the treatment of sepsis, with the mechanism of action effectively reducing the formation of inflammatory cells and cytokines [11]. The treatment of sepsis through TCM is effective and is advantageous for improving organ dysfunction and maintaining body homeostasis [12].
This review focuses on the current understanding of the role of TCM commonly used in the treatment of patients with sepsis and MODS and its effective components, thereby providing a theoretical basis for its clinical application.
2. Pathological mechanisms of sepsis with multiple organ dysfunction syndrome
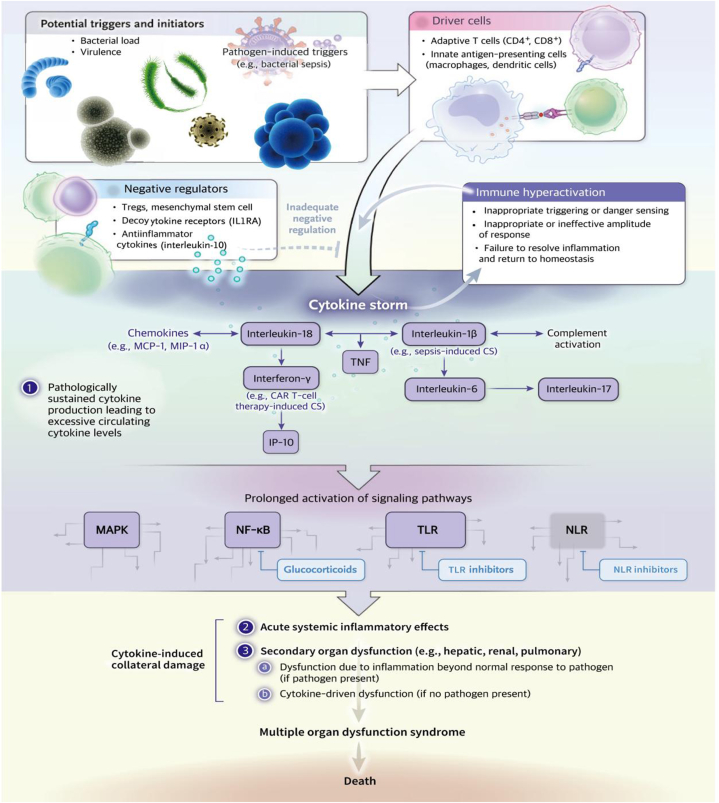
The pathological mechanisms underlying sepsis and MODS are complex and involve inflammatory reactions, oxidative stress and autophagy [13]. Inflammatory response and coagulation activation are two key host defence responses against infection [13]. These mechanisms do not work independently but rather cooperate and interact with each other in a complex and synchronous way [13].
The induction of an infectious pathogen initiates an acute response involving macrophage activation and pro-inflammatory cytokine release, leading to a cytokine storm. This process is mediated by different pattern recognition receptors, including the most frequently studied Toll-like receptors (TLRs) [14]. Through damage or pathogen-related molecular patterns, TLRs interact with ligands and activate several signal pathways, including the mitogen-activated protein kinase (MAPK) and nuclear factor-kappa B (NF-κB) pathways, to produce inflammatory cytokines [14]. The NF-κB pathway is the central mediator of inflammation and is fundamentally involved in the molecular link between inflammation and thrombosis [15]. It is expressed in the vascular system and circulatory cells involved in thrombotic inflammation and mediates the expression of cytokines, chemokines and coagulation factors [15]. Through the formation of unrestricted extracellular traps of neutrophils or the endothelial damage caused by mechanical rupture, the complex cellular relationship between inflammation and thrombosis can lead to the rapid activation and aggregation of platelets and manifestations of thrombotic inflammatory diseases, which is one of the pathological mechanisms of sepsis [15].
Superoxide and hydrogen peroxide produce oxidative stress, disrupt mitochondrial dynamics and adenosine triphosphate synthesis and increase the cycle of neuronal damage [16]. In particular, they increase caspase-3 and caspase-9 cleavage and the Bax/Bcl2 ratio of hippocampal and cortical neurons, and they stimulate neurocognitive impairment [16]. This is an important pathological mechanism of cognitive impairment in sepsis-related encephalopathy [16].
Autophagy is another key defence mechanism employed by hosts to resist external pathogens and danger signals [17]. Autophagy plays a crucial role in inducing and regulating the inflammatory response of natural immune cells and is a key factor affecting the development of sepsis. It also plays a protective role in sepsis through the following mechanisms: the clearance of pathogens, the neutralisation of microbial toxins, the regulation of cytokine release, the reduction of apoptotic cancer targets and the promotion of antigen expression [17].
The overexpression of Beclin-1 reduces cardiac inflammation and fibre damage, maintains mitochondrial mass and ultimately improves cardiac performance against lipopolysaccharide damage [18]. Enhanced Beclin-1 signalling can inhibit the activation of mitochondrial active oxygen, thereby maintaining autophagy, even in cases of severe septicaemia (Fig. 1) [18].
Fig. 1.
Pathological mechanisms of sepsis and MODS.
Coagulation activation is part of the defence against infection, and coagulation in patients with sepsis is often abnormal [19]. The coagulation disorder associated with sepsis can increase the risk of multifunctional organ dysfunction caused by microvascular thrombosis [19]. Therefore, the regulation of the coagulation function in patients with sepsis and a reduction in the influence of abnormal coagulation changes on organ function are key mechanisms for the prevention and treatment of MODS in sepsis.
The aetiology and pathogenesis of sepsis are complex, and TCM emphasises syndrome differentiation and treatment. Wang summarised the pathogenesis of sepsis as ‘three syndromes and three methods’, namely, toxin-heat syndrome and methods for heat clearing and detoxification, blood stasis syndrome and methods for activating blood circulation and blood stasis removal, and acute deficiency syndrome and Fu Zheng−Gu Ben prescriptions [20,21]. In a study on the treatment of sepsis using TCM, compound TCM formulations were generally used instead of single drugs, and the treatment was based mainly on the syndrome differentiation of patients with sepsis [22]. Traditional Chinese medicine and its components also have the potential to block antibiotic-resistant bacteria, providing new therapeutic ideas for reversing resistance caused by the antibiotic treatment of sepsis with MODS [22].
3. Traditional Chinese medicine for the diagnosis and treatment of sepsis with multiple organ dysfunction syndrome
3.1. Toxic fever symptoms and methods for heat clearing and detoxification
Toxic fever symptoms of sepsis with MODS are characterised by a persistent high fever, dizziness, nausea and vomiting, a red tongue, a yellow or dry tongue coating and a weak and rapid pulse [23]. The TCM treatment method mainly focuses on heat clearance and detoxification [23].
3.1.1. Rhubarb-derived anthraquinones
Using ‘sepsis’, ‘organ dysfunction’ and ‘heat clearing and detoxification’ as keywords, the author searched the PubMed and China National Knowledge Infrastructure databases to obtain studies on the treatment of sepsis using TCM compound preparations for heat clearance and detoxification. After sorting and analysing the agents evaluated in these studies, rhubarb was found to be used the most frequently in all heat-clearing and detoxifying prescriptions.
Rhubarb is commonly used in TCM because it contains anthracene derivatives, organic acids, volatile oils, glycosides, tannins and other active ingredients [24]. It can improve the digestive system, regulate the blood system, promote metabolism, influence the nervous system, improve kidney function, reduce and delay pain, prevent lung disease and inhibit antioxidant stress; additionally, it demonstrates anti-inflammatory, antitumor, antibacterial and antiviral activity [24]. Emodin, rhein, chrysophanol and other anthraquinones are the most-studied chemically-active ingredients of rhubarb [25]. They are also present in plants used in common TCM formulations, such as Polygonum cuspidatum and P. multiflorum, and they have high significance for research and practical application [25].
Xu et al. [26] revealed that emodin protects the myocardium of rats with sepsis by reducing the levels of myocardial oxidative stress, apoptosis and inflammatory factors. Dong et al. [27] reported that in mice with sepsis with lipopolysaccharide-induced acute brain injury, emodin pretreatment can significantly reduce the levels of inflammatory factors, such as interleukin-6 (IL-6), and nerve injury factors, such as plasma-specific enolase. Emodin has a protective effect on such mice, and it is speculated that the mechanism is related to the activation of the cholinergic pathway and inhibition of the inflammatory response. Another study demonstrated that chrysophanol promoted the polarisation of macrophages to the M2 type in model rats, thereby alleviating lung injury in the sepsis rat model, downregulating the expression of cleaved caspase-3 and cleaved caspase-9 in rat lung tissue and inhibiting NF-κB p65 and extracellular signal-regulated kinase 1/2 pathway activity [28]. An intraperitoneal injection of emodin in rats with sepsis can effectively reduce the levels of various oxidative stress indicators as well as the infiltration of inflammatory cells into heart, liver, lung and kidney tissues [29]. Emodin has a protective effect on the organs of rats with sepsis and can prevent sepsis oxidative stress and inflammatory reactions to a certain extent [29].
Lin et al. [30] revealed that the overall coagulation tendency in rats with sepsis is one of low coagulation, with fibrin and platelet activity first weakened and then enhanced. Emodin can improve the activity of endogenous coagulation factors and fibrinogen function in rats with sepsis and reduce their 48-h mortality [30]. Therefore, emodin is beneficial for regulating coagulation disorders in sepsis, especially in multiple organ failure caused by sepsis.
3.1.2. Berberine
Coptis chinensis is a commonly used drug for heat clearing and detoxification in TCM, and its usage frequency in heat-clearing and detoxifying prescriptions for sepsis is second only to that of rhubarb [31]. Berberine is an alkaloid obtained from C. chinensis and is one of its main effective components [31]. Berberine has an inhibitory effect on chronic and acute inflammation [31]. Berberine and C. chinensis can enhance the phagocytosis of leukocytes in vivo and in vitro and partially inhibit the inflammatory reaction process [31]. An administration of berberine to mice with sepsis induced by Escherichia coli injection can increase the survival rate of these mice and improve the antibacterial effect of antibiotics [32]. This is related mainly to its effects on T lymphocytes and reductions in the level of inflammatory factors, such as IL-6, indicating that berberine is beneficial in both the prevention and treatment of sepsis [32].
Moreover, berberine plays a positive role in the prevention and treatment of sepsis-induced MODS. Compared with untreated mice, berberine-pretreated mice with sepsis and acute respiratory distress syndrome had lower lung injury scores and serum inflammatory factor levels, with a significant reduction in the NF-κB p65 level in lung tissue [33]. Berberine may have a protective effect on the lung tissue of mice with sepsis, and its mechanism may involve the inhibition of an abnormally activated NF-κB channel in lung tissue [33]. Chen et al. [34] indicated that berberine could also protect rats with septic cardiomyopathy by inhibiting the activation of the TLR4/NF-κB signal pathway, increasing left ventricular end-diastolic pressure and reducing lipopolysaccharide-induced cardiomyocyte swelling.
Animal experiments and clinical studies have demonstrated that berberine can inhibit intestinal epithelial cell apoptosis in sepsis by inhibiting endogenous and exogenous apoptosis pathways and that it plays a protective role in the intestinal mucosa of patients and mice [35,36]. High-mobility group protein 1 (HMGB1) is a prototype damage-related molecular model protein, and together with its exogenous counterpart pathogen-related molecular model, it serves as an alarm system to prevent balance disorders in the body [37]. This protein acts as a chemotactic cytokine in infection, sepsis, hypoxia, ischaemia–reperfusion and other events [37], and it may be another important pathway for berberine in the prevention and treatment of sepsis. Shi et al. [38] reported that berberine targets the signal transduction of advanced glycation end products in HMGB1 to inhibit the decline of new neurons and restrain A1 astrocytes stressed by microglia, thereby reducing cognitive impairment caused by sepsis and preventing sepsis-related encephalopathy.
3.2. Blood stasis syndrome and methods for activating blood circulation and removing blood stasis
Blood stasis syndrome is characterised by high fever, dizziness, piercing pain similar to that caused by a needle bayonet, fixed pain that is often aggravated at night, lumps, subcutaneous ecchymosis or bleeding [15]. Patients may experience symptoms such as oliguria, anuria, oedema, dark tongue or ecchymosis of the tongue, obvious blood stasis and ecchymosis at the bottom of the tongue, as well as a delayed pulse or astringent chord [15]. It is the main blood factor of MODS caused by sepsis [15]. The treatment for blood stasis syndrome should be based on the activation of blood circulation to remove blood stasis [15].
Blood stasis caused by heat and toxins plays a key role in the occurrence and development of sepsis [39]. Traditional Chinese medicine includes many prescriptions to promote blood circulation and remove blood stasis. This paper mainly describes the two effective components of TCM that appear most frequently in prescriptions to promote blood circulation and remove sepsis-related blood stasis: safflower yellow and tetramethylpyrazine.
3.2.1. Safflower yellow
Safflower can activate blood circulation, remove blood stasis, disperse dampness and cause detumescence [40]. Safflower yellow is the primary known effective component of safflower and has demonstrated anti-inflammatory, immune-function-enhancing and antioxidant properties [40]. Wang [41] reported that the three main components in Xuebijing injection – safflower yellow A, hydroxy-safflower yellow A and dehydrated safflower yellow B – have protective effects on lipopolysaccharide-induced lung injury in mice with sepsis and can inhibit the infiltration of inflammatory cells in the lungs of mice with acute lung injury as well as the formation of neutrophil extracellular trapping nets. Li et al. [42] revealed that increasing the dose of safflower yellow A significantly reduced the levels of inflammatory factor tumour necrosis factor α (TNF-α) and serum tissue factor at a lower dose, increasing levels of the serum tissue factor pathway inhibitor. In addition to its positive effects on lung tissue, safflower yellow can more effectively treat myocardial injury in patients with sepsis compared with conventional methods, and the incidence of major adverse cardiac events was lower in hospitalised patients treated with safflower yellow [43].
3.2.2. Tetramethylpyrazine
Ligusticum chuanxiong promotes blood circulation and Qi circulation, soothes the liver, eliminates wind and relieves pain [44]. As a non-volatile alkaloid of Rhizoma chuanxiong, ligustrazine is also an effective component of many TCM ingredients and has significant biological activity [44].
Clinical studies on ligustrazine and sepsis have focused mainly on the clinical application of Danshen ligustrazine injections, and animal experiments have evaluated the effect of ligustrazine on sepsis-related organ dysfunction [[45], [46], [47], [48], [49]]. For sepsis-related renal injury, Ying et al. [45] reported that ligustrazine significantly reduced the renal water content and level of urinary renal injury molecule-1 in mice with sepsis-related acute renal damage, thereby reducing the degree of renal injury. The mechanism underlying these effects is speculated to involve the inhibition of caspase-3, the downregulation of the anti-N-methyl-d-aspartate receptor and the antiapoptotic activity of ligustrazine.
In terms of sepsis-related brain and lung injury, Huang et al. [46] reported that ligustrazine reduced inflammatory cell infiltration and the sepsis-induced damage of brain and lung tissue in rats. Ligustrazine demonstrates a protective effect on the basal layer and cerebral cortex and can significantly increase the expression of the brain tight-junction-related proteins claudin-5 and occludin and reduce sepsis-induced damage to the blood–brain barrier. Wang et al. [47] reported that ligustrazine inhibited the p38 MAPK/cAMP response element binding protein, reducing the inflammatory reaction in the hippocampi of mice with sepsis-related encephalopathy.
In addition, Liu et al. [48] demonstrated that ligustrazine inhibited the apoptosis of pulmonary microvascular endothelial cells induced by endoplasmic reticulum stress protein kinase RNA-like endoplasmic reticulum kinase signal transduction, increasing the survival rate and oxygen partial pressure in rats with sepsis and alleviating sepsis-induced acute lung injury. In a study on the hepatoprotective activity of ligustrazine, the liver function of ligustrazine-pretreated rats with sepsis-induced acute liver injury was reported to be significantly better than that of ligustrazine-treated mice, and ligustrazine was shown to play a favourable protective role in the mitochondrial membrane transport function of rat hepatocytes [49].
Zhang et al. [50] revealed that ligustrazine reduced the expression of iron elements in mouse liver tissue by inhibiting Janus kinase/signal transduction and transcriptional activator phosphorylation, thereby reducing liver injury and systemic inflammatory injury. Moreover, Xiao et al. [51] reported that Salvia miltiorrhiza and ligustrazine injection exhibited a hepatoprotective effect on mice with septic hepatopathy. A clinical study demonstrated that ligustrazine antagonised TNF-α and improved microcirculation, and in combination with routine treatment, it effectively improved myocardial damage in patients with sepsis [52]. A prophylactic study reported that S. miltiorrhiza and ligustrazine injections could improve the inflammatory response and significantly reduce the incidence of systemic inflammatory response syndrome in patients with severe burns, although there was no significant difference in the incidence of MODS between groups [53]. Many animal experiments have demonstrated that ligustrazine is effective in the treatment of sepsis and MODS, but research on its clinical application remains limited, and most existing studies evaluated only TCM prescriptions [50,51]. Therefore, more ligustrazine drug research can be expected in the future.
3.3. Acute deficiency syndrome and Fu Zheng–Gu Ben prescription
Acute deficiency syndrome manifests as either Yin withdrawal syndrome or Yang withdrawal syndrome [21]. The main symptoms of Yin withdrawal syndrome are a conscious trance or restlessness, reddening of the face, oliguria or anuria, a red and dry tongue and a bradycardia [21]. The main symptoms of Yang withdrawal syndrome are a cold sweat, inverse cold limbs, sudden dizziness, a light or purple tongue and a slightly weak or irregular pulse. Acute deficiency syndrome is mainly treated by Fu Zheng–Gu Ben prescriptions [21].
3.3.1. Ginsenoside
Ginseng is a key medicine for invigorating Qi in TCM and is frequently used in Fu Zheng–Gu Ben prescriptions. Ginseng contains chemical components such as ginsenosides, ginseng polysaccharides, volatile oils and amino acids, which have pharmacological effects such as anti-fatigue, anti-ageing, anti-oxidative and immunity-improving activities [54]. Many experiments have been conducted to study the effects of ginsenoside on the prevention and treatment of MODS in sepsis, including liver injury, lung injury, myocardial injury and sepsis-related encephalopathy [[55], [56], [57], [58], [59], [60]].
Wu et al. [55] reported that ginsenosides increased the expression of taurine-upregulated gene 1 and reduced the expression of miR-200a-3p to stimulate the silencing information regulator 1/AMP-activated protein kinase pathway, thereby enhancing autophagy to improve liver injury and mitochondrial dysfunction caused by sepsis. Another study revealed that ginsenoside protected mouse type 2 alveolar epithelial cells from lipopolysaccharide-induced apoptosis by enhancing autophagy and inducing the expression of NF E2 p45-related factor 2, which is conducive to the prevention and treatment of sepsis-induced lung injury [56]. Zhang et al. [57] also noted that ginsenosides significantly alleviated lung inflammation in mice with sepsis-induced acute lung injury, reducing the levels of inflammatory factors in alveolar lavage fluid and the NF-κB level in lung tissue, suggesting that ginsenoside can also control lung injury and inhibit channels related to NF-κB. Ji et al. [58] reported that lipopolysaccharides induced the apoptosis of pulmonary epithelial cells in a time-dependent and concentration-dependent manner. At a certain lipopolysaccharide concentration, pulmonary epithelial cell autophagy initially increases and then decreases after 12–16 h. The initial increase in autophagy reduces lipopolysaccharide-induced apoptosis, and ginsenosides play a protective role in sepsis-induced acute lung injury by appropriately increasing the rate of autophagy.
The aforementioned studies demonstrate that ginsenosides alleviate sepsis-induced lung injuries through various mechanisms.
Myocardial dysfunction caused by sepsis is a cause of death in many patients with severe disease, and the abnormal expression of TLR4, NF-κB and nucleotide oligomerisation domain-like receptor 3 are key pathways of cardiomyocyte apoptosis and inflammatory response in sepsis [59]. Ginsenosides can reduce lipopolysaccharide-induced cardiomyocyte apoptosis and the inflammatory response in mice by blocking these pathways and restoring damaged cardiac function to a certain extent [59]. In encephalopathy, ginsenosides can block the abnormal activation of NF-κB and MAPK signalling pathways to control the levels of inflammatory factors and chemokines, thereby reducing brain damage [60].
4. Summary
As a key form of complementary and integrative medicine, TCM plays a crucial role in rescuing acute and critical illnesses complicated by sepsis and MODS. The results of this study show that it has many effects in sepsis treatment, such as anti-inflammation, improvement of microcirculation, alleviation of gastrointestinal dysfunction, enhancement of the immune system and protection of affected organs from injury, exerting specific positive effects on MODS caused by sepsis. In future research, based on the conclusions of this study, further exploration will be conducted on the efficacy and mechanism of TCM in treating MODS caused by sepsis. We look forward to future drug development and more prospective randomised controlled clinical trials to verify the application value of the effective components of TCM.
Funding statement
This research did not receive any funding support.
Ethics statement
As all analyses were based on previously published studies, no ethical approval or patient consent was required.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Fan T.T., Cheng B.L., Fang X.M., et al. Application of Chinese medicine in the management of critical conditions: a review on sepsis. Am. J. Chin. Med. 2020;48(6):1315–1330. doi: 10.1142/S0192415X20500640. [DOI] [PubMed] [Google Scholar]
- 2.Rhee C., Jones T.M., Hamad Y., et al. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2) doi: 10.1001/jamanetworkopen.2018.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W., Yang X., Pin Y., et al. National incidence and mortality of hospitalized sepsis in China. Crit. Care. 2023;27(1):84. doi: 10.1186/s13054-023-04385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou R.J., Tao J., Qiu J.X., et al. DNA-PKcs promotes sepsis-induced multiple organ failure by triggering mitochondrial dysfunction. J. Adv. Res. 2022;41:39–48. doi: 10.1016/j.jare.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue H., Yan X.H., Liang L., et al. Logistic regression analysis of risk factors for multiple organ dysfunction syndrome secondary to sepsis. Pediatr. Crit. Care Med. 2017;18(4):319–329. [Google Scholar]
- 6.Wang B., Wang Z.Y., Xu R., et al. Correlation between lactate/albumin ratio level and incidence of multiple organ dysfunction syndrome as well as mortality in patients with sepsis Chin. J Infect Control. 2011;16(5):417–422. (in Chinese) [Google Scholar]
- 7.Varkouhi A.K., Monteiro A., Tsoporis J.N., Mei S., Stewart D.J., Dos S.C. Genetically modified mesenchymal stromal/stem cells: application in critical illness. Stem Cell Rev. Rep. 2020;16(5):812–827. doi: 10.1007/s12015-020-10000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faura J., Bustamante A., Miró-Mur F., Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J. Neuroinflammation. 2021;18(1):127. doi: 10.1186/s12974-021-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X.H., Xu D.Q., Chen Y.Y., Yue S.J., Fu R.J., Huang L., Tang Y.P. Traditional Chinese Medicine: a promising strategy to regulate inflammation, intestinal disorders and impaired immune function due to sepsis. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.952938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Shi Y., Li Z., Zuo L., Tang M., Jing Z., et al. Metabolomic insights into the synergistic effect of biapenem in combination with xuebijing injection against sepsis. Front. Pharmacol. 2020;11:502. doi: 10.3389/fphar.2020.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen Y., Feng C., Chen W., Chen C., Kuang S., Liu F., et al. Effect of traditional Chinese medicine on serum inflammation and efficacy in patients with sepsis: a systematic review and meta-analysis. Ann. Palliat. Med. 2021;10(12):12456–12466. doi: 10.21037/apm-21-3179. [DOI] [PubMed] [Google Scholar]
- 12.Yang Xingcai, Hong Wei, Zheng Tianhong, et al. Clinical study on the protective effect of traditional Chinese medicine anti-inflammatory mixture on multiple organ function in sepsis patients. Chinese Journal of Emergency Medicine. 2017;37(10):6. doi: 10.3969/j.issn.1002-1949.2017.10.004. [DOI] [Google Scholar]
- 13.Iba T., Levy J.H. Inflammation and thrombosis: roles of neutrophils, platelets and endothelial cells and their interactions in thrombus formation during sepsis. J. Thromb. Haemostasis. 2018;16(2):231–241. doi: 10.1111/jth.13911. [DOI] [PubMed] [Google Scholar]
- 14.Li Z., Qi X., Zhang X., et al. TRDMT1 exhibited protective effects against LPS-induced inflammation in rats through TLR4-NF-κB/MAPK-TNF-α pathway. Animal Model Exp Med. 2022;5(2):172–182. doi: 10.1002/ame2.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mussbacher M., Salzmann M., Brostjan C., et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis. Front. Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu M., Mei X.L., Zhao Y.N. Sepsis and cerebral dysfunction: BBB damage, neuroinflammation, oxidative stress, apoptosis and autophagy as key mediators and the potential therapeutic approaches. Neurotox Res. 2021;39(2):489–503. doi: 10.1007/s12640-020-00270-5. [DOI] [PubMed] [Google Scholar]
- 17.Huang M., Cai S., Su J. The pathogenesis of sepsis and potential therapeutic targets. Int. J. Mol. Sci. 2019;20(21):5376. doi: 10.3390/ijms20215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y., Cai Y., Zang Q.S. Cardiac autophagy in sepsis. Cells. 2019;8(2):141. doi: 10.3390/cells8020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan X.W., Jiang N., Chen B., et al. Research progress of single-flavor traditional Chinese medicine in the treatment of sepsis. Journal of Basic Chinese Medicine. 2020;23(5):736–739. (in Chinese) [Google Scholar]
- 20.Wang J.D., Li Z.J., Li Y.P. Dialectical treatment of sepsis from "three evidences and three methods. Chin. Crit. Care Med. 2016;18(11):643–644. (in Chinese) [Google Scholar]
- 21.Cao S.H., Wang J., Li Y. From "simultaneous treatment of bacteriotoxins" to "four evidences and four methods"-Dialectical ideas on the combination of traditional Chinese medicine and western medicine in the treatment of multiple organ dysfunction syndrome deepening and perfection. Chin. Crit. Care Med. 2014;17(11):641–643. (in Chinese) [Google Scholar]
- 22.Su T., Qiu Y., Hua X., Ye B., Luo H., Liu D., et al. Novel opportunity to reverse antibiotic resistance: to explore traditional Chinese medicine with potential activity against antibiotics-resistance bacteria. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.610070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y.F., Zhang Q., Wang Y.M. Dialectical application of traditional Chinese medicine prescription in adjuvant treatment of sepsis. Shaanxi Traditional Chinese Medicine. 2017;40(8):1105–1108. (in Chinese) [Google Scholar]
- 24.Jin L.X., Jin L.J., Luan Z.Q., et al. Research progress on chemical constituents and PharmacoIogy of rhubarb. Information on Traditional Chinese Medicine. 2009;37(1):121–126. (in Chinese) [Google Scholar]
- 25.Wang Dan. 2023. Preparation of EMO/PSM Nanodrug Delivery System and Evaluation of its Therapeutic Effect on Experimental UC [J] [Google Scholar]
- 26.Xu R.M., Shao Z.Y., Wang D.W. Protective effect of emodin on myocardial injury in sepsis rats. Med J West China. 2019;32(1):1443–1446. (in Chinese) [Google Scholar]
- 27.Dong Y., Liu G., Zhang L., et al. Neuroprotective effect of emodin on acute brain injury in sepsis mice. Med J Chin PLA. 2019;44(1):13–19. (in Chinese) [Google Scholar]
- 28.Wei P., Guo J.X., Yu D.Y., et al. Chrysophanol alleviates lung injury of sepsis rats by promoting macrophage M2 polarization. Immunological Journal. 2020;36(9):748–755. (in Chinese) [Google Scholar]
- 29.Hu L.Y., Tan J.Y., Shen J., et al. Protective effect of emodin on oxidative stress and inflammation of various organs in rat model of cecum ligation perforation sepsis. J Clin Pathol Res. 2021;39(7):1388–1395. (in Chinese) [Google Scholar]
- 30.Lin Q.W., Song J.H., Zeng Q.B., et al. Effect ofemodin on coagulation disorders of septic rats. Military Medical Journal of Southeast China. 2018;20(5):464–470. (in Chinese) [Google Scholar]
- 31.Huang L. Research progress on chemical constituents and pharmacological activities of active ingredients of Coptis chinensis. Cardiovascular Disease Electronic Journal of integrated traditional Chinese and Western Medicine. 2020;8(17):136–137. (in Chinese) [Google Scholar]
- 32.Pierpaoli E., Cirioni O., Simonetti O., et al. Potential application of berberine in the treatment of Escherichia coli sepsis. Nat. Prod. Res. 2021 Nov;35(22):4779–4784. doi: 10.1080/14786419.2020.1721729. [DOI] [PubMed] [Google Scholar]
- 33.Wu X.L., Yu L., Long D., et al. Study on the protective effects and mechanism of berberine hydrochloride on sepsis induced acute respiratory distress syndrome in mice. The Journal of Practical Medicine. 2021;35(22):3452–3456. (in Chinese) [Google Scholar]
- 34.Chen H., Liu Q., Liu X., et al. Berberine attenuates septic cardiomyopathy by inhibiting TLR4/NF-κB signalling in rats. Pharmaceut. Biol. 2021 Dec;59(1):121–128. doi: 10.1080/13880209.2021.1877736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H.M., Xing Y., Tang X.Y., et al. Berberine inhibits enterocyte apoptosis in septic mice. Chin. J. Pathophysiol. 2016;32(9):1660–1665. (in Chinese) [Google Scholar]
- 36.Wang L., Xie W.J., Liu J., et al. Protective effect of berberine on intestinal mucosal barrier function in patients with sepsis. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2017;27(16):1733–1735. (in Chinese) [Google Scholar]
- 37.Xue J., Suarez J.S., Minaai M., et al. HMGB1 as a therapeutic target in disease. J. Cell. Physiol. 2021 May;236(5):3406–3419. doi: 10.1002/jcp.30125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi J., Xu H., Cavagnaro M.J., et al. Blocking HMGB1/RAGE signaling by berberine alleviates A1 astrocyte and attenuates sepsis-associated encephalopathy. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.760186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao Y., Qu Z., Yao W., et al. Research progress on the system of "coagulation disorder-blood stasis syndrome-activating blood circulation and removing blood stasis" in sepsis. J. Ethnopharmacol. 2020;261 [Google Scholar]
- 40.Wang Z.M., Xiao H.B., Li X.Y., et al. Research progress on pharmacological actions and clinical applications of Carthami Flos. China Journal of Traditional Chinese Medicine and Pharmacy. 2018;36(11):6608–6611. (in Chinese) [Google Scholar]
- 41.Wang Y.P., Guo Y., Wen P.S., et al. Three ingredients of safflower alleviate acute lung injury and inhibit NET release induced by lipopolysaccharide. Mediat. Inflamm. 2020;2020 doi: 10.1155/2020/2720369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q.Y., Jin X.F., Zhu H.R., et al. Effects of different doses of safflower yellow A on lung protection and inflammatory factors in sepsis. Jilin Medical Journal. 2016;42(11):2565–2569. (in Chinese) [Google Scholar]
- 43.Chu Y., Qi H., Liu X., et al. Safflower yellow treats the myocardial injury in patients with severe sepsis. Chinese Traditional Patent Medicine. 2012;39(4):706–710. (in Chinese) [Google Scholar]
- 44.Pu Z.H., Dai M., Peng C., et al. Research progress on the material basis and pharmacological effects of alkaloids from Chuanxiong. China Pharmacy. 2011;31(8):1020–1024. (in Chinese) [Google Scholar]
- 45.Ying J., Wu J., Zhang Y., et al. Ligustrazine suppresses renal NMDAR1 and caspase-3 expressions in a mouse model of sepsis-associated acute kidney injury. Mol. Cell. Biochem. 2020 Jan;464(1–2):73–81. doi: 10.1007/s11010-019-03650-4. [DOI] [PubMed] [Google Scholar]
- 46.Huang Z.S., Xie D.Q., Xu L.J., et al. Tetramethylpyrazine ameliorates lipopolysaccharide-induced sepsis in rats via protecting blood-brain barrier, impairing inflammation and nitrous oxide systems. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.562084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang J., Zhu H., Huang C.S., et al. Role of p38 MAPK/CREB signaling pathway in tetramethylpyrazine-induced reduction of hippocampal inflammatory responses in mice with sepsis-associated encephalopathy. Chinese Journal of Anesthesiology. 2019;41(7):870–873. (in Chinese) [Google Scholar]
- 48.Liu W., Liu K., Zhang S., et al. Tetramethylpyrazine showed therapeutic effects on sepsis-induced acute lung injury in rats by inhibiting endoplasmic reticulum stress protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling-induced apoptosis of pulmonary microvascular endothelial cells. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2018;24:1225–1231. doi: 10.12659/MSM.908616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheng Y., Wang J., Tao X., et al. Study on the protective effect of ligustrazine on the transporting function of hepatocellular mitochondria membrane in the septic rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;30(10):996–1000. doi: 10.3760/cma.j.issn.2095-4352.2018.010.019. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y.W., Wang J., Zhu H., et al. Ligustrazine regulates expression of hepcidin in septic mice and its mechanism. Zhejiang Medical Journal. 2018;43(21):2311–2315. (in Chinese) [Google Scholar]
- 51.Xiao L., Mei G.C., Wu L.H., et al. Protective effect of danshen chuangxiongqin injection on acute liver injury in septic young rats based on Nrf2/HO-1 pathway. J. Emerg. Tradit. Chin. Med. 2019;28(9):1585–1589. (in Chinese) [Google Scholar]
- 52.Liu B.W., Hu W., Hu W.H., et al. Study on the cardioprotective effect of ligustrazine on patients with septic myocardial damage. Zhejiang Journal of Integrated Traditional Chinese and Western Medicine. 2016;28(8):639–641. (in Chinese) [Google Scholar]
- 53.Lin Y., Ruan S.B., Chen X.D., et al. Effect of salviae miltiorrhizae and ligustrazine hydrochloride injection on the inflammatory factors of severe burn patients and the prevention of sepsis. Hebei Medicine. 2013;24(3):382–385. (in Chinese) [Google Scholar]
- 54.Yu X.n., Feng X.G., Zhang J.M., et al. Research progress on chemical constituents and pharmacological effects of Panax ginseng. Ginseng Research. 2016;31(1):47–51. (in Chinese) [Google Scholar]
- 55.Wu P., Yu X., Peng Y., et al. Ginsenoside Rg3 alleviates septic liver injury by regulating the lncRNA TUG1/miR-200c-3p/SIRT1 axis. J. Inflamm. 2021;18(1):31. doi: 10.1186/s12950-021-00296-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji Q., Sun Z., Yang Z., et al. Protective effect of ginsenoside Rg1 on LPS-induced apoptosis of lung epithelial cells. Mol. Immunol. 2021 Aug;136:168–174. doi: 10.1016/j.molimm.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Z.B., Xu Q.P. Experimental study of ginsenoside Rg1 combined with antibiotics in the treatment of acute lung injury in mice with sepsis. J. Sichuan Univ. (Eng. Sci. Ed.) 2013;51(3):371–375. doi: 10.12182/20200560204. (in Chinese) [DOI] [PubMed] [Google Scholar]
- 58.Ji Q.J., Sun Z.R., Yang Z.Z., et al. Effects of Rg_1 on LPS-induced apoptosis and autophagy of lung epithelial cells. Zhongguo Zhongyao Zazhi. 2020;44(8):1648–1653. doi: 10.19540/j.cnki.cjcmm.20190111.002. [DOI] [PubMed] [Google Scholar]
- 59.Luo M., Yan D., Sun Q., et al. Ginsenoside Rg1 attenuates cardiomyocyte apoptosis and inflammation via the TLR4/NF-kB/NLRP3 pathway. J. Cell. Biochem. 2020 Apr;121(4):2994–3004. doi: 10.1002/jcb.29556. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y., Chi M., Qiao X., et al. Anti-inflammatory effect of ginsenoside Rg1 on LPS-induced septic encephalopathy and associated mechanism. Curr. Neurovascular Res. 2022;19(1):38–46. doi: 10.2174/1567202619666220414093130. [DOI] [PubMed] [Google Scholar]