Abstract
Selenium nanoparticles (SeNPs) are proposed as a safer and more effective selenium delivery system than sodium selenite (Na2SeO3). Here, we investigated the effects of replacing dietary Na2SeO3 with SeNPs synthesized by Lactobacillus casei ATCC 393 on the growth performance and gut health of early-weaned piglets. Seventy-two piglets (Duroc × Landrace × Large Yorkshire) weaned at 21 d of age were divided into the control group (basal diet containing 0.3 mg Se/kg from Na2SeO3) and SeNPs group (basal diet containing 0.3 mg Se/kg from SeNPs) during a 14-d feeding period. The results revealed that SeNPs supplementation increased the average daily gain (P = 0.022) and average daily feed intake (P = 0.033), reduced (P = 0.056) the diarrhea incidence, and improved (P = 0.013) the feed conversion ratio compared with Na2SeO3. Additionally, SeNPs increased jejunal microvilli height (P = 0.006) and alleviated the intestinal barrier dysfunction by upregulating (P < 0.05) the expression levels of mucin 2 and tight junction proteins, increasing (P < 0.05) Se availability, and maintaining mitochondrial structure and function, thereby improving antioxidant capacity and immunity. Furthermore, metabolomics showed that SeNPs can regulate lipid metabolism and participate in the synthesis, secretion and action of parathyroid hormone, proximal tubule bicarbonate reclamation and tricarboxylic acid cycle. Moreover, SeNPs increased (P < 0.05) the abundance of Holdemanella and the levels of acetate and propionate. Correlation analysis suggested that Holdemanella was closely associated with the regulatory effects of SeNPs on early-weaned piglets through participating in lipid metabolism. Overall, replacing dietary Na2SeO3 with biogenic SeNPs could be a potential nutritional intervention strategy to prevent early-weaning syndrome in piglets.
Keywords: Selenium nanoparticle, Early-weaned piglet, Gut homeostasis, Gut microbiota, Metabolites
1. Introduction
Early-weaning syndrome seriously affects the health and welfare of piglets and has become a bottleneck problem restricting the development of the large-scale swine industry (Saladrigas-García et al., 2021). Early weaning leads to decreased intestinal antioxidant capacity, increased free radicals, and impairment of the redox balance, which will seriously disrupt the intestinal barrier function, increase disease susceptibility, affect growth and development, and cause disease and even death in piglets (Moeser et al., 2017; Zhang and Piao, 2022). The practice of utilizing antibiotics for the prevention and control of agricultural bacterial diseases is a customary approach in the swine industry (Durso and Cook, 2014). However, bacterial resistance, drug residues, allergic reactions, and other hazards caused by the extensive use or abuse of antibiotics are becoming more and more serious (Gresse et al., 2017). Many countries and international organizations, including China, have already banned the use of antibiotic feed additives. Finding safe and effective alternatives to in-feed antibiotics is crucial in maintaining the profitability of the swine industry, especially in reducing the prevalence and severity of digestive disorders in the post-weaning period (Heo et al., 2013). Feeding functional feed additives may be a feasible strategy for promoting the growth and maintaining gut homeostasis in early-weaning piglets by enhancing nutrient digestion, small intestinal morphology, antioxidant capacity, and immunity, as well as by restoring gut microbiota (Lange et al., 2010; Zheng et al., 2021).
The trace element selenium (Se) plays a pivotal role in modulating diverse physiological and pathological processes in pigs (Surai, 2021; Xing et al., 2019). Se is an essential component of selenoproteins which is involved in diverse biological processes. Se is incorporated into selenocysteine during the biosynthesis of this amino acid, which is inserted into nascent protein chains of selenoproteins during their synthesis (Turanov et al., 2011). Up to now, glutathione peroxidase (GPx), thioredoxin reductase (TrxR), deiodinase (DIO), and 25 other types of selenoproteins have been discovered (Carlisle et al., 2020; Chen et al., 2018). Multiple studies demonstrate that dietary Se level is closely associated with the expression of selenoproteins and the maintenance of redox homeostasis (Yim et al., 2019). Furthermore, increased intestinal oxidative stress accompanies the weaning process of piglets (Tang et al., 2022). Due to the incomplete maturation of the intestinal tract in piglets and the further inhibition of intestinal development caused by premature weaning, intestinal anti-stress capacity is weakened. Consequently, the intestinal tract is susceptible to damage when exposed to oxidative stress. At this stage, exogenous substances, pathogens, or symbiotic bacteria that are ingested can easily penetrate the multiple barriers of the gut, trigger inflammatory responses, and promote the occurrence of inflammation, which in turn further exacerbates intestinal oxidative stress, leading to a vicious cycle (Wen et al., 2023). From a nutritional standpoint, adequate supplementation with Se to mitigate oxidative stress is an alternative way to reduce post-weaning gut health problems (Oliveira et al., 2017; Zoidis et al., 2018).
Sodium selenite (Na2SeO3), which has been used as a feed supplement for the past 45 years, is not the optimal form of supplying Se to animals, mainly due to its low bioavailability and environmental pollution potential when excreted into the environment and other disadvantages (Ortman and Pehrson, 1999; Surai, 2021). In addition, the therapeutic range of Se is quite narrow, meaning that it can be toxic at high doses and inadequate at low doses. Excessive intake of inorganic and organic Se species often results in toxic effects (Ferro et al., 2021; Khurana et al., 2019). Compared to Na2SeO3, Se nanoparticles (SeNPs) are considered a potential and optimal Se supplement for animals due to their diverse biological function such as antioxidant, immune regulation, and reproductive health enhancement (Khurana et al., 2019; Malyugina et al., 2021; Yang and Yang, 2023). The smaller size (less than 100 nm) and larger surface area of SeNPs can improve reactivity, solubility, and bioavailability, and it is easier to pass through biological barriers and be absorbed and utilized. Moreover, cell and mouse experiments indicate that the toxicity of SeNPs is much lower than that of selenate, selenite, and selenomethionine (Benko et al., 2012; Xu et al., 2018). More importantly, SeNPs are more efficient for the synthesis of selenoproteins, such as GPx, than traditional forms of Se and can improve the digestive system and promote nutrient digestion and absorption (Chen et al., 2022). Our previous research proved that Lactobacillus casei ATCC 393 has the capacity to biosynthesize SeNPs with low toxicity as well as antioxidant and anti-inflammatory properties (Xu et al., 2018). Further studies revealed that a SeNPs-enriched diet effectively mitigated intestinal barrier dysfunction through regulating selenoproteins expression, enhancing antioxidant capacity, improving mitochondrial stress responses, and maintaining gut microecological homeostasis in mice (Qiao et al., 2022a, 2022b).
Thus, to further verify the application effects of SeNPs in piglet feeding, this study compared the effects of diet supplemented with SeNPs synthesized by L. casei ATCC 393 or Na2SeO3 on growth performance, antioxidant properties, immune function, mitochondrial homeostasis, metabolic profile and gut microbiome of early-weaned piglets. To the best of our knowledge, this study is the first to investigate the effect of SeNPs synthesized by probiotics in pig feeding.
2. Materials and methods
2.1. Animal ethics statement
The animal experiments were approved by the Institutional Animal Care and Use Committee of the Northwestern Polytechnical University (No. 202001002) and conducted following the Chinese guidelines for animal welfare. The animal experiment complied with the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines.
2.2. Reagents
ELISA kits for transforming growth factor-β (TGF-β; Cat# F4722), D-lactic acid (D-LA; Cat# F3682), interleukin-10 (IL-10; Cat# F4577), tumor necrosis factor-α (TNF-α; Cat# F4535) and lipopolysaccharide (LPS; Cat# F8233) were purchased from Shanghai Fanke Industrial Co., Ltd (Shanghai, China). Interleukin-1β (IL-1β; Cat# JL18442), interleukin-18 (IL-18; Cat# JL20253), 8-hydroxy-2′-deoxyguanosine (8-OHdG; Cat# JL12294) and secretory immunoglobulin A (sIgA; Cat# JL11763) ELISA kits were obtained from Jianglai Biotechnology (Jianglaibio) Co., Ltd (Shanghai, China). Total superoxide dismutase (T-SOD) assay kit (Cat# S0101S) was obtained from Shanghai Beyotime Biotechnology Co., Ltd (Shanghai, China). Diamine oxidase (DAO) assay kit (Cat# BC1285), malondialdehyde (MDA) assay kit (Cat# BC0025), catalase (CAT) assay kit (Cat# BC0205), GPx activity assay kit (Cat# BC1195), and TrxR activity assay kit (Cat# BC1155) were obtained from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Primary antibodies for mucin 2 (MUC2; 1:500; Cat# A4767), claudin-1 (1:1000; Cat# A2196), zonula occludens-1 (ZO-1; 1:500; Cat# A0659), occludin (1:1000; Cat# A2601), NOD-like receptor thermal protein domain associated protein 3 (NLRP3; 1:1000; Cat# A5652), Nrf2 (1:1000; Cat# A0674), NADPH dehydrogenase-1 (NQO-1; 1:1000; Cat# A19586), heme oxygenase-1 (HO-1; 1:1000; Cat# A1346), AMP-activated kinase (AMPK; 1:1000; Cat# A1229), phospho-AMPK (pAMPK; 1:500; Cat# AP1345), IL-1β (1:1000; Cat# A1112), IL-18 (1:1000; Cat# A1115), caspase-1 (1:1000; Cat# A0964), ASC (1:1000; Cat# A16672), β-actin (1:5000; Cat# AC026) and Lamin B1 (1:1500; Cat# A1910) were procured from Wuhan ABclonal Biotechnology Co., Ltd. (Wuhan, China). SYBR green qPCR kit (Cat# AG11701), RNA extraction reagent (Cat# AG21102) and reverse transcription kit (Cat# AG11728) were obtained from Hunan Accurate Bio-Medical Technology Co., Ltd (Hunan, China).
2.3. Preparation of SeNPs
SeNPs were prepared according to our previously optimized procedure with appropriate modifications (Xu et al., 2018). Briefly, L. casei ATCC 393 in the exponential growth phase was incubated with 4 mM Na2SeO3 under anaerobic conditions for 48 h to synthesize SeNPs, followed by ultrasonic fragmentation, protein elution, liquid–liquid extraction, and freeze-drying to extract and purify SeNPs.
2.4. Animals and experimental design
A total of 72 piglets (Duroc × Landrace × Large Yorkshire) weaned at 21 d of age were divided into the control group (basal diet containing 0.3 mg Se/kg from Na2SeO3) and the SeNPs group (basal diet containing 0.3 mg Se/kg from SeNPs) according to the principle of similar average body weight and sex, and each group had 6 pens with 6 pigs per pen (3 males and 3 females). The basal diets used in the experiment were the same except for the different forms of added Se in the vitamin-mineral premix, in which the control group was given a basal diet that contained 0.3 mg/kg of Se in the form of Na2SeO3, while the SeNPs group was given a basal diet that contained 0.3 mg/kg of Se in the form of SeNPs. The actual Se concentrations of the basal diet, control group diet, and SeNPs group were 0.124, 0.502, and 0.487 mg/kg, respectively. The nutritional level of the basic diet meets the NRC (2012) nutritional needs for weaned piglets. The ingredients and nutrient composition of the experimental diet are shown in Table 1.
Table 1.
Ingredients and nutrient composition of basal diets (%, dry matter basis).1
| Ingredients | Content | Nutrient composition2 | Content |
|---|---|---|---|
| Corn | 54.42 | Net energy, MJ/kg | 2535 |
| Soybean meal | 17.00 | Crude protein | 20.820 |
| Low-protein whey powder | 10.00 | SID Lysine | 1.477 |
| Whey protein concentrate | 10.00 | SID Threonine | 0.865 |
| Soybean oil | 2.00 | SID Methionine | 0.479 |
| 50% Choline chloride | 0.20 | SID (Methionine + Cystine) | 0.814 |
| Sodium chloride | 0.40 | SID Tryptophan | 0.279 |
| L-Lysine | 0.37 | SID Isoleucine | 0.899 |
| DL-Methionine | 0.14 | SID Valine | 0.930 |
| L-Threonine | 0.07 | Total Ca | 0.790 |
| Titanium dioxide | 0.40 | STTD P | 0.370 |
| Zinc oxide | 0.20 | ||
| Vitamin-mineral premix3 | 2.00 | ||
| Stone powder | 1.50 | ||
| Calcium dihydrogen phosphate | 1.30 | ||
| Total | 100.00 |
SID = standardized ileal digestibility; STTD P = standard total tract digestible phosphorus.
Control diet: the basal diet containing 0.3 mg Se/kg from sodium selenite (Na2SeO3); SeNPs diet: the basal diet containing 0.3 mg Se/kg from selenium nanoparticles (SeNPs).
Calculated values.
Vitamin-mineral premix provided the following per 1 kg of completed diet: vitamin A, 9920 IU; vitamin D3, 2240 IU; vitamin E, 24 mg; vitamin K3, 4 mg; vitamin B1, 2.4 mg; vitamin B2, 8 mg; vitamin B6, 6.4 mg; vitamin B12, 32 mg; Biotin, 64 mg; Fe (FeSO4), 90 mg; Cu (CuSO4), 12 mg; Mn (MnO), 52.5 mg; I (KI), 0.525 mg; Zn (ZnO), 60 mg; Se (Na2SeO3 or SeNPs), 0.3 mg.
All piglets were allowed ad libitum access to both feed and water. The feed intake and diarrhea condition of piglets were recorded daily during the experimental period (14 d). Also, on d 15, all piglets underwent a 12-h feed deprivation period and were subsequently weighed to measure their average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR = ADFI/ADG). For sample collection, one piglet close to the average body weight of each pen was sacrificed at the end of the study. Precaval vein blood was withdrawn, and used to prepare serum. Subsequently, the piglets were then anesthetized via electrical stunning and euthanized through exsanguination. The cecum contents and jejunum of piglets were collected and stored at −80 °C. In addition, fresh jejunal tissue was collected and fixed in 4% paraformaldehyde (PFA) or 2.5% glutaraldehyde for morphological analysis.
2.5. Diarrhea incidence
To determine the incidence and severity of diarrhea, the feces of the pigs were observed twice daily throughout the study and scored: 0, normal = hard pellet; 1 = soft, dry pellet; 2, mild diarrhea = soft, shaped wet pellet; 3, severe diarrhea = unshaped soft pellet or watery diarrhea, with a score ≥2 considered as diarrhea (Tang et al., 2021). Diarrhea incidence (%) = Σ (the number of piglets with diarrhea per pen × days of diarrhea)/(total number of piglets × number of experimental days) × 100.
2.6. Serum biochemical indicators
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin (ALB), alkaline phosphatase (ALP), blood urea nitrogen (BUN), creatinine (CREA) and lactate dehydrogenase (LDH) in serum of piglets were measured using an automatic biochemical analyzer (OLYMPUS AU400, Japan).
2.7. Assessment of intestinal barrier function
Serological detection is an important basis for the diagnosis of intestinal barrier dysfunction. Measurement of DAO enzyme activity and the content of D-LA and LPS in the serum of piglets were performed using corresponding kits, as these markers indicate damage to the intestinal barrier function.
2.8. Evaluation of histomorphology and mucus secretion capacity in the jejunum
The histomorphological changes and mucus secretion capacity (goblet cell number) in the jejunum were evaluated by H&E staining and AB-PAS staining, respectively. Briefly, the jejunum was fixed in 4% PFA and embedded in paraffin. Subsequently, tissue sections were stained according to standard protocols. Images of the stained tissue sections were acquired using a Tissue Slide Scanning System (Winmedic, Shandong, China).
2.9. Ultrastructural observation of microvilli and mitochondria in the jejunum
Approximately 0.5 cm of fresh jejunum tissue was taken from the piglet, gently rinsed to remove the mucous chyme adhering to the tissue, and then fixed in 2.5% glutaraldehyde. The samples were prepared following the standard procedures for sample processing (Graham and Orenstein, 2007). Then, the ultrastructure of microvilli and mitochondria were observed on a JEM-1400 FLASH electron microscope (JEOL, Tokyo, Japan).
2.10. Immunofluorescence staining of the jejunum
Jejunal tissue sections were subjected to immunofluorescence staining of MUC2 and tight junction proteins using previously described protocols (Yang et al., 2021a). Briefly, paraffin-embedded jejunal tissue was sectioned, dewaxed to water, and antigenically repaired using ethylenediaminetetraacetic acid. The tissue section was then incubated for 30 min in 1% BSA. The primary antibodies including rabbit polyclonal anti-MUC2 (1:500), anti-claudin-1 (1:200), anti-occludin (1:300), and anti-ZO-1 (1:200) were incubated with the sections for 24 h at 4 °C in the dark. Afterward, Cy3-conjugated secondary antibodies (1:300) were incubated with the sections for 1 h. Counterstaining with DAPI was used to visualize the nuclei. Images of the immunostained tissue sections were captured using a fluorescence microscope (Leica DMIL, Germany). After acquiring the image, we recolored the image using the pseudo-color function of ZEN Microscopy Software (ZEISS, Germany).
2.11. mRNA expression analysis
The mRNA levels of MUC2, selenoproteins, and mitochondrial biosynthesis-related genes in jejunum were measured as described previously (Qiao et al., 2022b). In brief, the TRIzol method is utilized to extract mRNA from the jejunum. Next, a 1-μg mRNA of satisfactory quality is selected for reverse transcription to obtain cDNA, which is subsequently subjected to qPCR. The oligonucleotide primer sequences are provided in Supplementary Table S1. The relative mRNA abundance of the target genes was normalized to β-actin and was then calculated using the 2−ΔΔCt method.
2.12. Se content measurement
Inductively coupled plasma-mass spectrometry (ICP-MS) was used to measure Se levels in serum and jejunum. Approximately 100 μL serum or 100 mg jejunum were dissolved in 5 mL of HNO3 and digested samples by microwave digestion. To volatilize the acid, the digestion solution was heated at 100 °C for at least 2 h. The measurement was performed after constant volume was reached.
2.13. Antioxidant capacity and immune response evaluation
The level of reactive oxygen species (ROS) in jejunum was detected by dihydroethidium (DHE) staining (Qiao et al., 2020). Total protein was isolated from jejunum using RIPA buffer containing protease inhibitor cocktail. Specifically, 50 mg of jejunal tissue was added to 1 mL of RIPA lysate and 1% (v/v) protease inhibitor and ground with a tissue homogenizer to obtain a tissue homogenate. After lysis on ice for 30 min, the supernatant was centrifuged at 10,000 × g for 10 min at 4 °C, and the protein concentration was determined by bicinchoninic acid (BCA). The MDA, total antioxidant capacity (T-AOC) and 8-OHdG levels, and the GPx, CAT, T-SOD and TrxR activities in jejunum were measured using the corresponding kits. The levels of sIgA, IL-1β, IL-10, IL-18, TGF-β and TNF-α in serum and jejunum were measured using the corresponding ELISA kits.
2.14. Mitochondrial DNA (mtDNA) in jejunum
The method for determining the mtDNA copy number was based on a previous study (Qiao et al., 2020). The relative copy number of mtDNA was calculated by comparing the expression of the mitochondrial cytochrome c oxidase I (COX1) gene to that of the nuclear hexokinase 2 (HK2) gene. The sequences of the oligonucleotide primers used for this analysis are provided in Supplementary Table S1.
2.15. Western blot analysis
The Western blot analysis was conducted according to the previously reported method (Dou et al., 2021). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) were loaded with equal volumes and amounts of protein from the samples (4 replicates per group) and subjected to electrophoresis to separate proteins by size. After separation, proteins were transferred to polyvinylidene fluoride (PVDF) membrane. The membranes were incubated overnight at 4 °C with the appropriate primary antibodies for AMPK, pAMPK, Nrf2, HO-1, NQO-1, NLRP3, IL-1β, IL-18, caspase-1, ASC, β-actin and Lamin-B1 followed by incubation for 1 h at room temperature with the appropriate secondary antibodies. After incubation with primary and secondary antibodies, the immunoreactive protein bands were visualized using an ECL Substrate kit and a T5200 Multi Detection System (Tanon, Shanghai, China).
2.16. Metabolomics analysis
Metabolomics analysis for serum in piglets was performed using UHPLC system coupled to a Q-Exactive HF-X mass spectrometer (Thermo Fisher Scientific Inc., USA) equipped with an ACQUITY HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm; Waters, USA) and electrospray ionization (ESI) source operating in positive mode and negative mode. Corresponding details are shown in Supplementary materials.
2.17. Gut microbiota and short-chain fatty acid (SCFA) analysis
The gut microbiota and SCFA analysis were conducted according to previously reported protocols (Hou et al., 2021). Total microbial genomic DNA was extracted from the cecum contents of piglets using the E.Z.N.A. DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer's instructions. The V3–V4 variable regions of the bacterial 16S rRNA gene were amplified utilizing degenerate PCR primers 338F/806R by an ABI GeneAmp 9700 PCR thermocycler (ABI, CA, USA). Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE300 platform (Illumina, San Diego, USA). Corresponding details can be found in Supplementary materials.
SCFA analysis of cecum contents in piglets was performed by gas chromatography-mass spectrometry (GC–MS) and ion source (AEI) operating in positive mode. Specifically, a saturated NaCl solution was used for resuspension of the cecal contents. Diethyl ether was used to extract the fatty acids after the samples were acidified with sulfuric acid (10%). The mixture was centrifuged at 10,000 × g for 10 min and Na2SO4 was then added to the supernatant to remove the water content. SCFA analysis for piglet cecum contents was performed using GC–MS on a TRACE1300-TSQ9000 Triple Quadrupole GC–MS/MS System.
2.18. Statistical analysis
The individual pen was used as a statistical unit for the growth performance and diarrhea incidence, and the individual pig was used as a statistical unit for other data. Before analysis, all data were tested for normality and homogeneity of variance using the Kolmogorov–Smirnov and Levene tests (with the significance level set at 5%) in SPSS 21.0 (SPSS, Inc, Chicago, USA). The difference in the diarrhea incidence was analyzed by the Mann–Whitney test. The other data were presented as the mean ± standard error of the mean (SEM) and statistical analysis was conducted using GraphPad Prism 7.0 software. The statistical significance of the comparisons between the two groups was evaluated using Student's unpaired t-test, and the difference was considered significant when P < 0.05, and a statistical tendency when 0.05 < P < 0.10. Spearman's correlational analysis was used to examine the correlation between differential metabolites and microbial taxa.
3. Results
3.1. Effects of dietary SeNPs supplementation on growth performance and diarrhea incidence in early-weaned piglets
The effects of SeNPs, as a dietary Se supplement, on growth performance and diarrhea incidence in early-weaned piglets are shown in Table 2. Compared with the control group, SeNPs increased the ADG (P = 0.022) and ADFI (P = 0.033) in piglets. In addition, SeNPs treatment improved (P = 0.013) the FCR and reduced (P = 0.056) diarrhea incidence in piglets.
Table 2.
Effects of dietary SeNPs supplementation on growth performance and diarrhea incidence of early-weaned piglets.1
| Item | Control | SeNPs | SEM | P-value |
|---|---|---|---|---|
| Initial BW, kg | 5.58 | 5.53 | 0.132 | 0.854 |
| Final BW, kg | 9.01 | 10.34 | 0.373 | 0.066 |
| ADG, g | 245.50 | 343.90 | 22.856 | 0.022 |
| ADFI, g | 341.10 | 428.40 | 21.352 | 0.033 |
| FCR, % | 1.40 | 1.26 | 0.031 | 0.013 |
| Diarrhea incidence,2 % | 33.30 | 16.70 | 0.056 |
SeNPs = selenium nanoparticles; SEM = standard error of the mean; BW = body weight; ADG = average daily gain; ADFI = average daily feed intake; FCR = feed conversion ratio.
Data are expressed as means and SEM. Each group had 6 pens with 6 pigs per pen (3 males and 3 females).
The difference in the diarrhea incidence was analyzed by Mann–Whitney test (non-parametric statistical test).
3.2. Effects of dietary SeNPs supplementation on blood biochemical indicators in early-weaned piglets
As shown in Table 3, the ALB level in piglets in the SeNPs group was significantly higher (P = 0.002) than that in the control group. However, there was no difference in the levels of ALP, BUN, CREA, LDH, ALT, and AST, and the ratio of AST to ALT between the two groups (P > 0.05).
Table 3.
Effects of dietary SeNPs supplementation on blood biochemical indicators of early-weaned piglets.1
| Item | Control | SeNPs | SEM | P-value |
|---|---|---|---|---|
| ALT, U/L | 40.22 | 37.35 | 3.004 | 0.655 |
| AST, U/L | 52.63 | 49.72 | 3.732 | 0.715 |
| AST:ALT ratio | 1.36 | 1.80 | 0.327 | 0.530 |
| ALB, g/L | 19.38 | 24.58 | 1.023 | 0.002 |
| ALP, U/L | 296.40 | 324.50 | 38.215 | 0.619 |
| BUN, mM | 2.09 | 1.59 | 0.244 | 0.137 |
| CREA, μM | 56.38 | 60.66 | 2.432 | 0.281 |
| LDH, U/L | 658.80 | 642.00 | 28.263 | 0.773 |
SeNPs = selenium nanoparticles; SEM = standard error of the mean; AST = aspartate aminotransferase; ALT = alanine aminotransferase; ALB = albumin; ALP = alkaline phosphatase; BUN = blood urea nitrogen; CREA = creatinine; LDH = lactate dehydrogenase.
Data are expressed as means and SEM. n = 6.
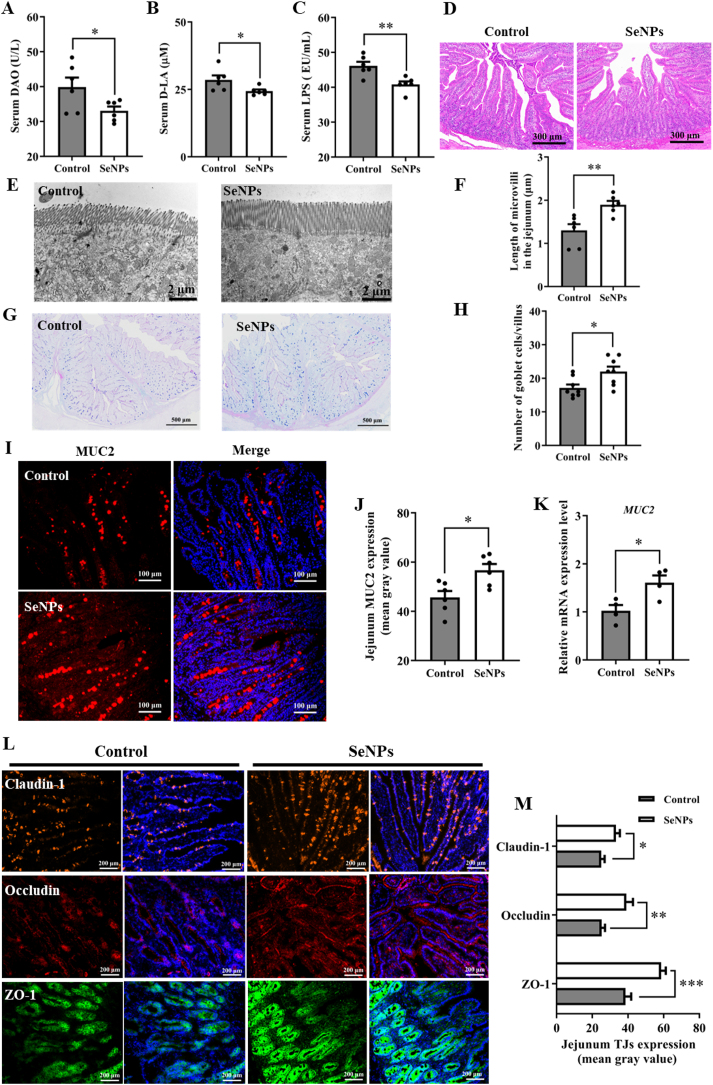
3.3. Effects of dietary SeNPs supplementation on intestinal barrier function in early-weaned piglets
The obtained data of intestinal barrier dysfunction biomarkers revealed that the activity of DAO (P = 0.046) and the levels of D-LA (P = 0.040) and LPS (P = 0.004) were higher in the control group than those in the SeNPs group (Fig. 1A–C). However, there were no obvious pathological changes and significant differences between the two groups in intestinal morphology (Fig. 1D). Notably, the TEM images showed that the height of jejunal microvilli of piglets in the SeNPs group was higher (P = 0.006) than that of piglets in the control group (Fig. 1E and F). Furthermore, dietary SeNPs supplementation increased the number of jejunal goblet cells in piglets (P = 0.017) (Fig. 1G and H). As shown in Fig. 1I–K, dietary supplementation with SeNPs increased the protein (P = 0.012) and mRNA (P = 0.023) expression levels of MUC2 in jejunum of piglets. Similarly, compared with the control group, SeNPs also increased the expression of claudin-1 (P = 0.017), occludin (P = 0.008), and ZO-1 (P < 0.001) in jejunum of piglets (Fig. 1L and M). These results indicated that replacing dietary Na2SeO3 with SeNPs effectively mitigated intestinal barrier damage in early-weaned piglets.
Fig. 1.
Dietary SeNPs supplementation improved intestinal barrier function of early-weaned piglets. (A) The activity of DAO in serum (n = 6). (B) The level of D-LA in serum (n = 6). (C) The level of LPS in serum (n = 6). (D) Jejunal histomorphology observed by H&E staining (n = 6). (E) TEM images of jejunal microvilli (n = 6). (F) Quantification of microvilli height in jejunum (n = 6). (G) Goblet cells in jejunum examined with AB-PAS staining (n = 6). (H) Quantification of jejunal goblet cell numbers (n = 8). (I) The expression level of MUC2 in jejunum detected by immunofluorescent staining (n = 6). (J) Quantification of MUC2 expression level in jejunum (n = 6). (K) The mRNA level of MUC2 in jejunum detected by qPCR (n = 4). (L) The expression levels of tight junction proteins (claudin-1, occludin, ZO-1) were detected by immunofluorescent staining (n = 6). (M) Quantification of the expression levels of claudin-1, occludin, and ZO-1 in jejunum (n = 6). Data are expressed as mean ± SEM. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001. SeNPs = selenium nanoparticles; DAO = diamine oxidase; D-LA = D-lactic acid; LPS = lipopolysaccharide; MUC2 = mucin 2; ZO-1 = zonula occludens-1; TJs = tight junctions.
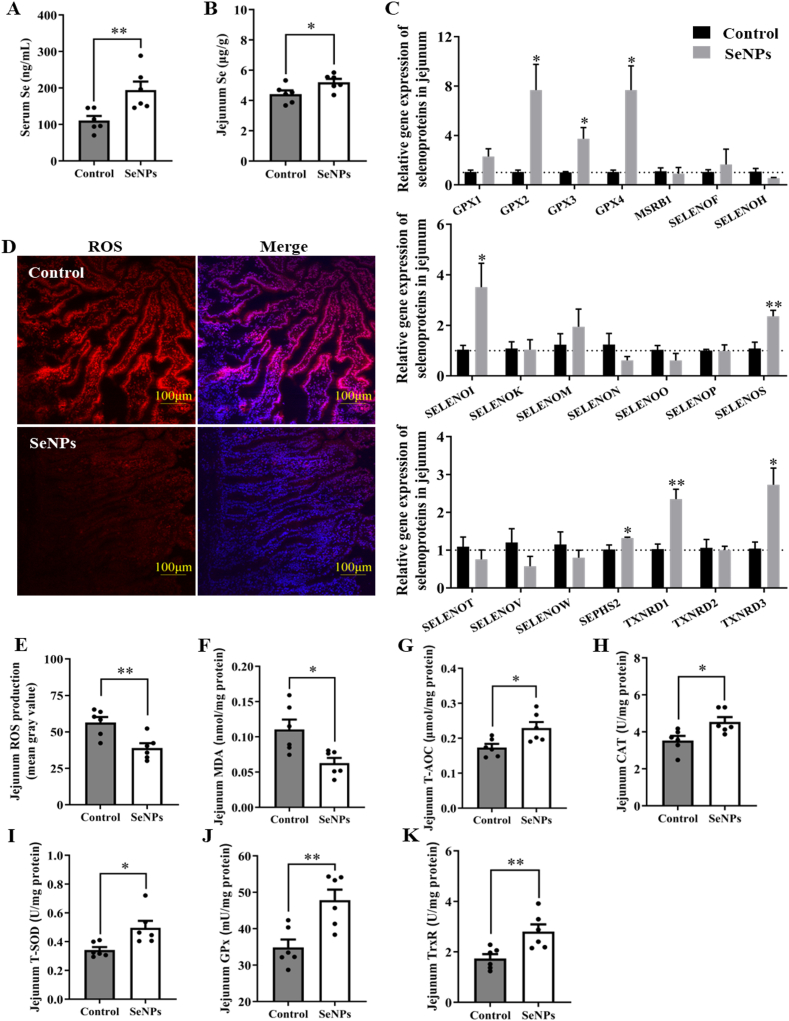
3.4. Effects of dietary SeNPs supplementation on the levels of selenoproteins and antioxidant capacity of jejunum in early-weaned piglets
Dietary supplementation with SeNPs increased the Se content in serum (P = 0.009) and jejunum (P = 0.037) of piglets, indicating an increased availability of Se in piglets of the SeNPs group (Fig. 2A and B). Therefore, we further detected the mRNA expression levels of 21 selenoproteins (except the DIO family) in jejunum of piglets and found that SeNPs increased (P < 0.05) GPX2, GPX3, GPX4, SELENOI, SELENOS, SEPHS2, TXNRD1 and TXNRD3 mRNA levels (Fig. 2C). Moreover, as expected, increased Se availability and mRNA expression levels of selenoproteins enhanced the antioxidant capacity in the jejunum by reducing (P < 0.05) the levels of ROS and MDA, and increasing (P < 0.05) the activities of the T-AOC, CAT, T-SOD, GPx, and TrxR (Fig. 2D–K).
Fig. 2.
Dietary SeNPs supplementation increased Se levels and enhanced the antioxidant capacity of early-weaned piglets. (A) The Se content in serum (n = 6). (B) The Se content in jejunum (n = 6). (C) The mRNA levels of selenoproteins in jejunum (n = 4). (D) ROS production assessed by DHE fluorescence (n = 6). (E) Quantification of ROS production in jejunum (n = 6). (F) The level of MDA in jejunum (n = 6). (G) The level of T-AOC in jejunum (n = 6). (H) The activity of CAT in jejunum (n = 6). (I) The activity of T-SOD in jejunum (n = 6). (J) The activity of GPx in jejunum (n = 6). (K) The activity of TrxR in jejunum (n = 6). Data are expressed as mean ± SEM. ∗P < 0.05; ∗∗P < 0.01. SeNPs = selenium nanoparticles; ROS = reactive oxygen species; MDA = malondialdehyde; T-AOC = total antioxidant capacity; CAT = catalase; T-SOD = total superoxide dismutase; GPx = glutathione peroxidase; TrxR = thioredoxin reductase.
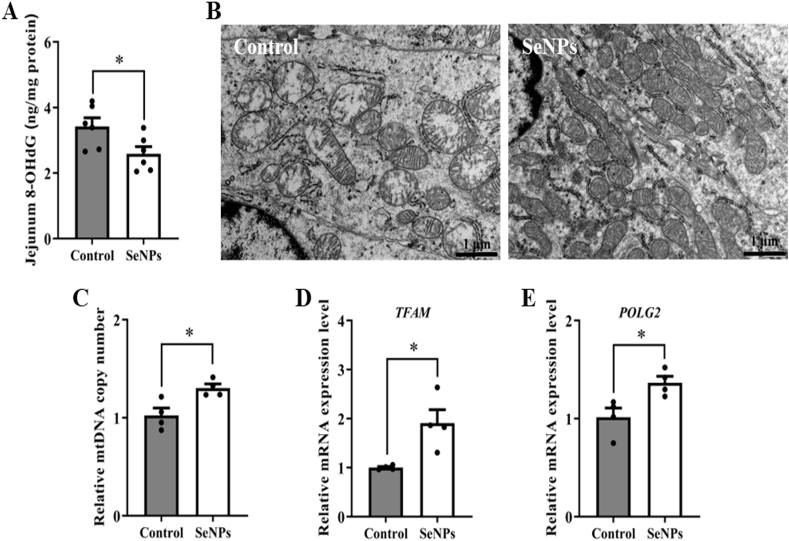
3.5. Effects of dietary SeNPs supplementation on mitochondria structure and function of jejunum in early-weaned piglets
The pro-oxidant 8-OHdG was used as a biomarker of mitochondrial DNA oxidative damage. The level of 8-OHdG was higher (P = 0.035) in the control group compared to the SeNPs group, which indicated that SeNPs effectively protected mitochondria against oxidative damage (Fig. 3A). The mitochondrial ultrastructure was impaired in piglets of the control group. Vacuoles were observed in mitochondria, and most of the mitochondrial membranes were disrupted. However, mitochondrial cristae and matrix density were normal and membranes were intact in piglets of the SeNPs group (Fig. 3B). In addition, compared with the control group, SeNPs increased (P < 0.05) the mtDNA copy number (Fig. 3C) and the mRNA levels of mitochondrial transcription factor A (TFAM) and DNA polymerase gamma 2 (POLG2) in jejunum (Fig. 3D and E).
Fig. 3.
Dietary SeNPs supplementation alleviated intestinal mitochondrial dysfunction of early-weaned piglets. (A) The level of 8-OHdG in jejunum (n = 6). (B) Mitochondrial ultrastructure observed by TEM (n = 6). (C) The mtDNA copy number was determined by qPCR analysis (n = 4). (D) The mRNA level of TFAM in jejunum (n = 4). (E) The mRNA level of POLG2 in jejunum (n = 4). Data are expressed as mean ± SEM. ∗P < 0.05. SeNPs = selenium nanoparticles; 8-OHdG = 8-hydroxy-2′-deoxyguanosine; mtDNA = mitochondrial DNA; TFAM = mitochondrial transcription factor A; POLG2 = DNA polymerase gamma 2.
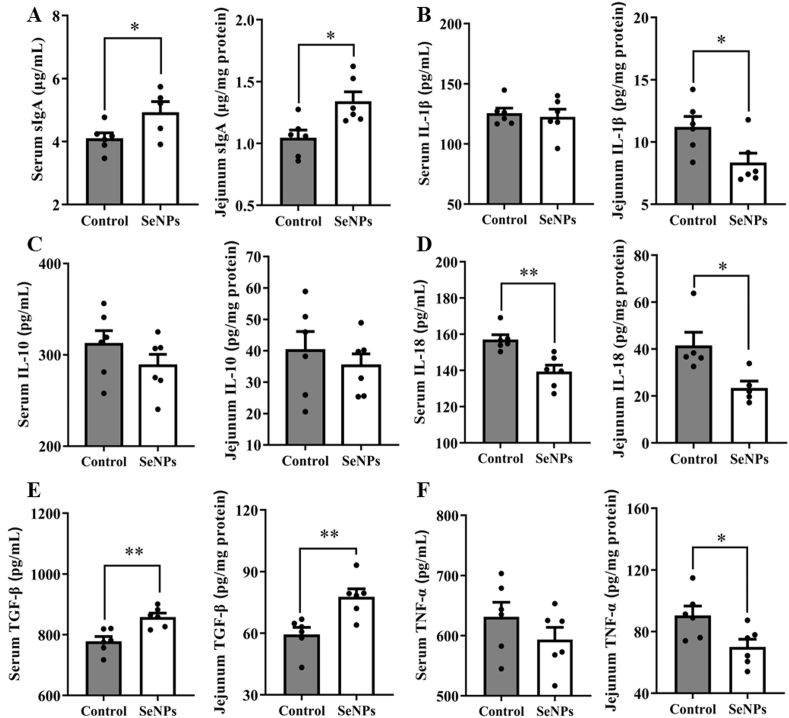
3.6. Effects of dietary SeNPs supplementation on immune responses
Dietary supplementation with SeNPs significantly altered the levels of immune response biomarkers (Fig. 4A–F). Compared with the control group, treatment with SeNPs increased (P < 0.05) the levels of sIgA and TGF-β, and decreased (P = 0.003) the level of IL-18 in serum. Moreover, the levels of IL-1β, IL-18, and TNF-α in jejunum of piglets from the SeNPs group were lower (P < 0.05) than those in the control group. However, the sIgA and TGF-β levels were higher (P < 0.05) than those in the control group.
Fig. 4.
Effects of dietary SeNPs supplementation on the immune responses of early-weaned piglets. (A) The level of sIgA in serum and jejunum (n = 6). (B) The level of IL-1β in serum and jejunum (n = 6). (C) The level of IL-10 in serum and jejunum (n = 6). (D) The level of IL-18 in serum and jejunum (n = 6). (E) The level of TGF-β in serum and jejunum (n = 6). (F) The level of TNF-α in serum and jejunum (n = 6). Data are expressed as mean ± SEM. ∗P < 0.05; ∗∗P < 0.01. SeNPs = selenium nanoparticles; sIgA = secretory immunoglobulin A; IL-1β = interleukin-1β; IL-10 = interleukin-10; IL-18 = interleukin-18; TGF-β = transforming growth factor-β; TNF-α = tumor necrosis factor-α.
3.7. Effects of dietary SeNPs supplementation on AMPK/Nrf2/NLRP3 signaling pathway in early-weaned piglets
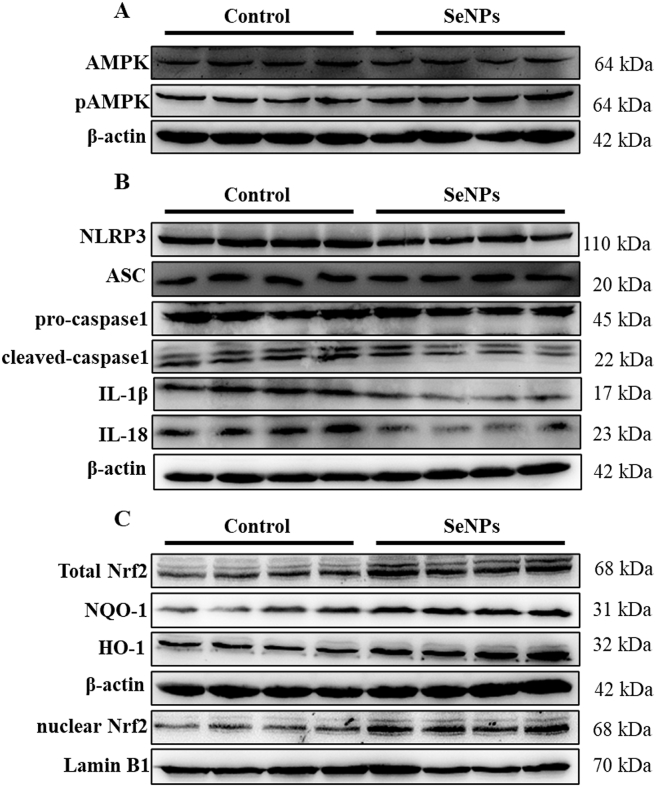
Results indicated that SeNPs up-regulated the expression level of pAMPK (P < 0.001) (Fig. 5A, Fig. S1A). In addition, treatment with SeNPs was found to decrease (P < 0.01) the NLRP3 expression and subsequent cleaved-caspase, IL-1β, and IL-18 expression levels (Fig. 5B, Fig. S1B). Furthermore, compared with the control group, dietary supplementation with SeNPs upregulated (P < 0.05) the expression levels of Nrf2 (expressions of both total and nuclear Nrf2 were increased) and its downstream antioxidant proteins NQO-1 and HO-1 (Fig. 5C, Fig. S1C).
Fig. 5.
Dietary SeNPs supplementation activated the AMPK/Nrf2/NLRP3 signaling pathway of early-weaned piglets. (A) The expression levels of AMPK and pAMPK proteins were measured by Western blot analysis. (B) The expression levels of NLRP3 inflammatory proteins were detected by Western blot analysis. (C) Nrf2 activation and the expression levels of its downstream proteins were measured by Western blot analysis. n = 4. SeNPs = selenium nanoparticles; AMPK = AMP-activated kinase; pAMPK = phospho-AMPK; NLRP3 = NOD-like receptor thermal protein domain associated protein 3; ASC = apoptosis-associated speck-like protein containing a caspase recruitment domain; IL-1β = interleukin-1β; IL-18 = interleukin-18; Nrf2 = nuclear factor erythroid 2-related factor 2; NQO-1 = NADPH dehydrogenase-1; HO-1 = heme oxygenase-1.
3.8. Dietary SeNPs supplementation altered serum metabolomic profile involved in lipid metabolism in early-weaned piglets
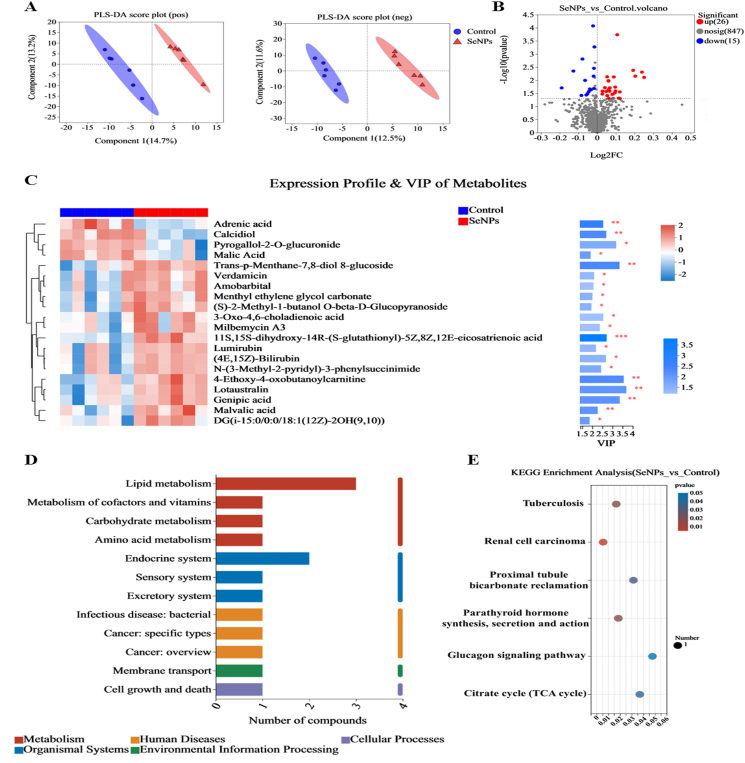
A total of 568 and 320 metabolites were identified in positive-ion (pos) and negative-ion (neg) modes, respectively. The supervised PLS-DA score plot revealed noticeable clustering and distinct separation of serum samples between the two experimental groups in both pos and neg modes (Fig. 6A). After merging the metabolites identified under pos and neg modes, the metabolites were annotated using the KEGG database. As shown in Fig. S2, the vast majority of annotated metabolites (65) were identified as phospholipids in lipids, accounting for about 50.39% of identified annotated metabolites associated with compounds with biological roles. KEGG functional pathway analysis of metabolites revealed that these metabolites were annotated in metabolism at Level 1, and at Level 2, the metabolites were primarily annotated as lipid metabolism, amino acid metabolism, and digestive system (Fig. S3).
Fig. 6.
Dietary SeNPs supplementation altered the serum metabolomic profiling involved in lipid metabolism of early-weaned piglets. (A) PLS-DA score plots in metabolomics analysis in pos and neg models. (B) Volcano plot of differential metabolites. (C) Clustering heat map and VIP bar chart of differential metabolites (Top 20). (D) KEGG function comment bar chart of differential metabolites. (E) The metabolic pathway impact prediction was based on the KEGG database. n = 6. ∗P < 0.05; ∗∗P < 0.01. SeNPs = selenium nanoparticles; pos = positive-ion; neg = negative-ion; PLS-DA = partial least squares-discriminant analysis; VIP = variable important in projection.
Metabolites with a VIP value greater than 1 and a q-value less than 0.05 were considered potential differential metabolites to distinguish between the control and SeNPs groups. As shown in Fig. 6B, the volcano plot shows the differential metabolites between the control group and the SeNPs group, and a total of 41 (26 upregulated and 15 downregulated) differential metabolites were identified (Supplementary Table S2). In addition, the clustering heat map and VIP bar chart of the top 20 differential metabolites reveal that compared with the control group, certain metabolites, such as adrenic acid, calcidiol, pyrogallol-2-O-glucuronide, and malic acid, were significantly downregulated, while the levels of other metabolites, such as verdamicin, amobarbital and menthyl ethylene glycol carbonate, were significantly increased (Fig. 6C). The KEGG database was used to perform functional pathway analysis of the differential metabolites, and differential metabolites were annotated in lipid metabolism (Fig. 6D). In addition, the major metabolic pathways that were affected by SeNPs treatment can be observed in the results, including parathyroid hormone synthesis, secretion and action, proximal tubule bicarbonate reclamation and tricarboxylic acid (TCA) cycle (Fig. 6E).
3.9. Effects of dietary SeNPs supplementation on gut microbiota and its metabolism in early-weaned piglets
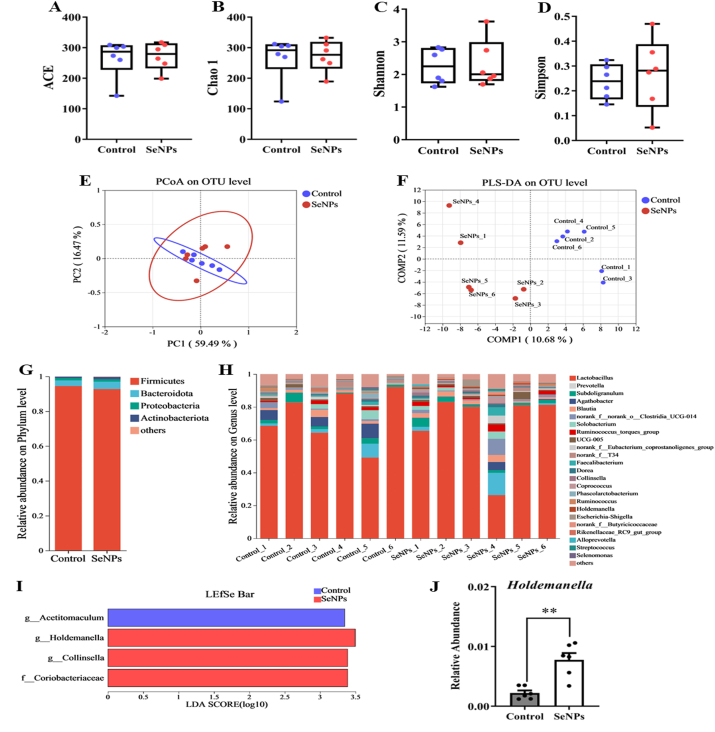
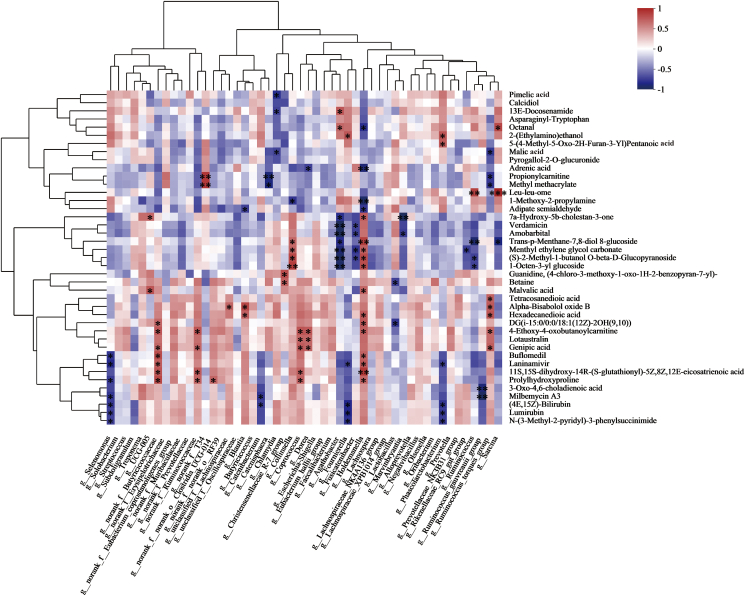
To investigate the effect of SeNPs on gut microbiota composition in early-weaned piglets, the α-diversity of the gut microbiota was first analyzed. As shown in Fig. 7A–D, treatment with SeNPs did not significantly (P > 0.05) alter the abundance and diversity of gut microbiota. However, principal coordinate analysis (PCoA) and partial least squares-discriminant analysis (PLS-DA) performed on the OTU level showed clear clustering of the gut microbiota in piglets receiving supplementation with SeNPs. The SeNPs group was found to be distinctly separated from the control group, indicating that the gut microbiota composition was impacted by SeNPs supplementation in early-weaned piglets (Fig. 7E and F). At the level of phylum, of the following phyla, Firmicutes, Bacteroidota, Proteobacteria, and Actinobacteriota, were found in relatively high abundance in cecal contents of piglets, and treatment with SeNPs had no significant effect on their composition (Fig. 7G). In addition, the relative abundance of different bacterial genera in the cecal microbiota was visualized using a bar graph shown in Fig. 7H. The LEfSe revealed the genera of bacterial that were differentially abundant between the control group and SeNPs group (Fig. 7I). Dietary supplementation with SeNPs resulted in an increase (P = 0.001) in the relative abundance of Holdemanella (Fig. 7J). Also, SeNPs treatment led to an increase (P < 0.05) in the concentration of total SCFA, as well as acetate and propionate specifically, in the cecal contents of the piglets compared to the control group (Table 4). Fig. 8 displayed the overall alterations in the distribution of distinctive metabolites and the proportional occurrence of microbial taxa at the genus rank. The obtained metabolic association heatmap indicated the abundance of Holdemanella was positively correlated with the levels of trans-p-menthane-7, 8-diol 8-glucoside, menthyl ethylene glycol carbonate, and 1-octen-3-yl glucoside, and negatively correlated with the level of 1-methoxy-2-propylamine.
Fig. 7.
Dietary SeNPs supplementation altered gut microbiota composition of early-weaned piglets. (A) ACE index. (B) Chao 1 index. (C) Shannon index. (D) Simpson index. (E) PCoA plots based on unweighted-UniFrac metrics. (F) PLS-DA score plots at the OTU level. (G) The relative abundance composition of fecal microbiota at the phylum level. (H) The relative abundance composition of fecal microbiota at the genus level. (I) Taxonomic abundance analysis (LEfSe analysis) revealed multiple differentially enriched taxa. (J) Relative abundance of Holdemanella. Data are expressed as mean ± SEM, n = 6. ∗P < 0.05; ∗∗P < 0.01. SeNPs = selenium nanoparticles; PLS-DA = partial least squares-discriminant analysis; PCoA = principal coordinate analysis; LEfSe = linear discriminant analysis effect size.
Table 4.
Effects of dietary SeNPs supplementation on SCFA concentrations in cecal contents of early-weaned piglets (ng/mg).1
| Item | Control | SeNPs | SEM | P-value |
|---|---|---|---|---|
| Acetate | 4833.0 | 7009.0 | 520.15 | 0.028 |
| Butyrate | 284.3 | 259.9 | 32.48 | 0.726 |
| Caproate | 5.3 | 5.1 | 0.38 | 0.811 |
| Isobutyrate | 120.6 | 113.6 | 12.90 | 0.802 |
| Isovalerate | 44.3 | 50.8 | 9.34 | 0.746 |
| Propionate | 1036.0 | 1369.0 | 84.32 | 0.041 |
| Valerate | 8.3 | 12.2 | 1.63 | 0.256 |
| Total SCFA | 6331.0 | 8820.0 | 628.07 | 0.040 |
SeNPs = selenium nanoparticles; SEM = standard error of the mean; SCFA = short-chain fatty acids.
Data are expressed as means and SEM. n = 6.
Fig. 8.
Heatmap of correlations between microbial genus abundances and serum metabolites. Clustering heatmap of the correlation between differential metabolites and microbial taxa (the abscissa is the microbial taxa, and the ordinate is the differential metabolite). n = 6. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001.
4. Discussion
The weaning stage is regarded as one of the most crucial periods in the management of commercial pig farming, although pigs are naturally weaned at 12 to 17 weeks of age, large commercial farms typically wean piglets at 3 to 4 weeks of age (Gao et al., 2019; Zheng et al., 2021). After weaning, the sudden dietary change from breast milk (fats) to solid feed (carbohydrates) can result in underfed piglets, which in turn can lead to underutilization of dietary nutrients, local inflammation and oxidative stress, growth and expansion of pathogenic microorganisms, the occurrence of diarrhea, suboptimal growth performance, and even mortality (Campbell et al., 2013; Yang et al., 2021b). The digestive and absorptive processes of nutrients primarily take place in the gastrointestinal tract (Greenwood-Van Meerveld et al., 2017). Therefore, a healthy gut is a requirement for animal growth and a stronger immune system (Pluske et al., 2018). Functional alterations in the intestine are linked to the process of weaning, including morphological changes, barrier function, mucosal immunity, and gut microbiota (Zheng et al., 2021). Maintaining the function and balance of the cell population in gut relies on the organism's redox homeostasis. Oxidation-reduction signals can comprehensively affect intestinal cell life processes, including proliferation, death or apoptosis, and differentiation, and are closely related to barrier function and mucosal immune defense (Circu and Aw, 2011). Multiple lines of evidence suggest that redox imbalance occurs in weaned piglets regardless of weaning age (Xu et al., 2014). Oxidative stress has the potential to negatively impact disease resistance and gut homeostasis (Novais et al., 2021). The excessive generation of ROS is the primary manifestation of oxidative stress, which can cause oxidative damage to cellular components (Rubattu et al., 2014). To tightly regulate cellular ROS levels, cells elicit adaptive responses to regulate ROS production or use antioxidant systems to eliminate potentially harmful levels of ROS (Rabinovitch et al., 2017).
Se plays a crucial role in pig nutrition by contributing to the synthesis of selenoproteins, which are the key components of the body's antioxidant system (Surai and Fisinin, 2015). Various Se species, such as inorganic compounds like selenate and selenite, organic compounds like selenocysteine, and SeNPs, are available as nutritional supplements (Skalickova et al., 2017). SeNPs are of particular interest due to their excellent biological activities, minimal toxicity, stability and high bioavailability (Liu et al., 2020). The present study revealed that the Se content in serum and jejunum of SeNPs group was higher than those of Na2SeO3 group by 1.76-fold and 1.18-fold, respectively. Elevated Se content increased Se availability and the mRNA levels of some selenoproteins (GPX2, GPX3, GPX4, SELENOI, SELENOS, SEPHS2, TXNRD1 and TXNRD3), mainly including antioxidant enzymes, thereby enhancing the antioxidant capacity of piglets. Moreover, replacing dietary Na2SeO3 with biogenic SeNPs improved intestinal barrier function. The beneficial effect of Se on the gut barrier is mainly attributed to the role of selenoproteins in redox reaction homeostasis, which improves the level and activity of antioxidant enzymes (GPx and TrxR) and scavenge excess ROS (Qiao et al., 2022b). However, SeNPs only increased the expression levels of specific selenoproteins in jejunum, which may be related to their role in intestinal health and growth performance. These selenoproteins are important factors involved in antioxidant defense, apoptosis, signal transduction and metabolic regulation (Labunskyy et al., 2014). In contrast, other selenoproteins may not have an important role in these aspects. In addition, the expression of selenoproteins may also be tissue specific. Lu et al. (2019) evaluated the effect of dietary Se on selenoprotein mRNA levels in porcine spleen tissue and found that high nutritional levels of Se addition only increased the mRNA expression levels of GPX1, GPX2, TXNRD3, SELENOH, SELENON, SELENOP, and SELENOV in the porcine spleen.
Decreased albumin levels can reflect the poor nutritional status of the organism (Ekanah, 2015). It was due to the improved effect of SeNPs on the intestinal barrier function of piglets that SeNPs improved the nutrient absorption capacity of piglets and consequently significantly increased the albumin levels. In addition, Se and iodine have a synergistic effect, which is particularly important for a healthy thyroid. Iodine is a component of thyroid hormones, while Se, as the active component of selenoprotein (DIO), helps to convert thyroid hormones into their active form (Schomburg and Köhrle, 2008). It has been shown that thyroid hormones can promote albumin metabolism (Kim et al., 2010). In the gut, goblet cells secrete MUC2 as the predominant mucin protein. The expression of MUC2 is crucial for preventing diseases, as research has demonstrated that knockout mice lacking MUC2 spontaneously develop colitis (Vancamelbeke and Vermeire, 2017). In this study, our results showed that SeNPs significantly increased the protein and mRNA expression levels of MUC2 in jejunum of piglets.
In recent years, new prevention and treatment strategies targeting the organelle function of the intestinal cell population, particularly the mitochondria, have emerged as potential targets for repairing intestinal barrier damage and preventing and treating intestinal diseases. Mitochondria are a crucial hub controlling innate immune responses, programmed cell death, inflammation processes, and bacterial pathogenesis mechanisms. Therefore, mitochondrial function is crucial for maintaining gut homeostasis (Rath et al., 2018). Mitochondria have a key role in generating cellular energy, but they are also a significant source of ROS, which is generated as a byproduct of cellular respiration (Zorov et al., 2014). The correlation between mitochondrial homeostasis, oxidative stress, and intestinal barrier dysfunction may be an important issue in weaned piglets facing high energy demands (Bhatti et al., 2017). Studies have reported that during the initial week after weaning in piglets, there is a decrease in both the activities of mitochondrial respiratory complexes and the content of mtDNA in the intestine (Cao et al., 2018). Therefore, mitochondria may be a potential target for preventing intestinal barrier dysfunction (Mottawea et al., 2016). Here, SeNPs protected intestinal mitochondria homeostasis in early-weaning piglets, mainly manifested by more intact mitochondrial ultrastructure and mtDNA abundance. Furthermore, oxidative stress can regulate intestinal stem cell differentiation and crypt–villus axis epithelial cell renewal and apoptosis (Rath et al., 2018), which in this study manifested as shortened jejunal microvilli. Moreover, it is noteworthy that weaning stress in piglets triggers mitochondrial autophagy, which constitutes the primary mechanism responsible for the maintenance of mitochondrial quality and serves as a pivotal factor in the development of oxidative stress-induced intestinal damage (Novais et al., 2021).
In addition, metabolomics showed that SeNPs regulated lipid metabolism and participated in parathyroid hormone synthesis, secretion and action, proximal tubule bicarbonate reclamation, and the TCA cycle, which may be linked to the impact of ROS (Castelli et al., 2021; Vujic et al., 2021). ROS can affect the activity of metabolic enzymes, such as TCA cycle enzymes, through reversible oxidation of cysteine residues or irreversible carbonylation of lysine residues (van der Reest et al., 2018). Additionally, lipid metabolism is intricately linked with the generation of ROS, which can be fine-tuned by feedback mechanisms (Bartolacci et al., 2021). The hydrolysis of triglycerides into fatty acids, which are subsequently oxidized in the mitochondria, is a significant contributor to the production of ROS (Currie et al., 2013). Overall, efficient mitochondrial oxidative phosphorylation and redox homeostasis are needed to meet the post-weaning energy requirements of piglets, providing a rationale for dietary Se supplementation. In addition to the above evidence, adding SeNPs to the diet can increase the expression of mitochondrial biosynthesis-related genes, which also supports that supplementation with SeNPs is efficient in protecting mitochondria.
AMPK is well established as a key metabolic regulator, which can quickly regulate the energy changes of cells, participate in pathophysiological processes, and at the same time activate pathways that regulate the maintenance of cellular energy homeostasis (Ma et al., 2017). Furthermore, ROS can act as molecular regulators of cellular signaling and function for AMPK, triggering downstream signaling pathways and AMPK-dependent metabolic adaptations that specifically maintain oxidative metabolism stability and mitochondrial homeostasis (Rabinovitch et al., 2017). He et al. (2022) discovered that incorporating glutamine to the diet of piglets can inhibit intestinal inflammation caused by LPS from Escherichia coli by modulating the interaction between AMPK signaling pathway activation and mitochondria. Based on the excellent antioxidant and ROS scavenging abilities of SeNPs, it is reasonable to speculate that SeNPs can maintain mitochondrial homeostasis by regulating the AMPK-mediated signaling pathway and protect cells against oxidative damage, which has been confirmed by our previous research (Qiao et al., 2022a, 2022b). In addition, Nrf2, as the primary transcription factor involved in antioxidant response element-associated antioxidative signaling, plays a significant role in the elimination of oxidative stress-induced ROS (Xu et al., 2020). AMPK is capable of phosphorylating Nrf2 directly, leading to its nuclear accumulation and activation of downstream antioxidant and inflammatory pathways, effectively alleviating inflammation and oxidative stress (Joo et al., 2016; Xu et al., 2020). The current study demonstrated that replacing dietary Na2SeO3 with biogenic SeNPs not only enhanced the antioxidant, but also improved the immune function as evidenced by the increased expression of sIgA and TGF-β and the decreased pro-inflammatory cytokines expression (IL-1β and IL-18), which may involve in the NLRP3 signaling pathway. Mitochondria serve as ubiquitous facilitators of NLRP3 inflammasome activation prompted by various inflammatory stimuli (Sadatomi et al., 2017; Zhou et al., 2011). These findings indicate that the NLRP3 inflammasome senses mitochondrial dysfunction, which may explain the frequent co-occurrence of mitochondrial damage and inflammatory disorders (Zhou et al., 2011). Here, dietary supplementation with SeNPs promoted the nuclear accumulation of Nrf2 by activating AMPK, leading to an increase in the expression of downstream antioxidant enzymes and inhibition of NLRP3 inflammasome expression.
Increasing evidence indicates that early weaning stress is a cause of gut microbiota dysbiosis, which is strongly linked to the development of diarrhea and enteric infections in piglets (Qin et al., 2022). According to the findings of Li et al. (2018), weaning stress altered both the microbial composition and metabolic profiles of the piglet intestine. Therefore, using non-antibiotic nutritional supplements that can potentially restore gut microbiota balance is an effective approach to assist in the weaning transition of piglets (Gresse et al., 2017). Zhai et al. (2018) found that dietary supplementation of Na2SeO3 at supranutritional Se levels could alter the gut microbiota, leading to potential impacts on the host's intestinal barrier and immune responses. The protective effect of SeNPs on mitochondria may also impact the variety of gut microbiota. Research has indicated that the dysregulation of intestinal cell mitochondrial function and genetic variations in the genome can impact both the composition and functionality of the gut microbiome (Yardeni et al., 2019). Our previous study showed that SeNPs improved microbial diversity and increased the abundance of SCFA-producing probiotics. Furthermore, excess nutrient intake of SeNPs can potentially optimize gut microbiota to prevent intestinal barrier dysfunction by fecal microbiota transplantation in mice (Qiao et al., 2022b). In this study, there was no significant disparity detected in the α-diversity among the experimental groups, and short-term (14-d) SeNPs administration may not be sufficient to effectively change the richness and variety of the intestinal microbiota of piglets. However, replacing dietary Na2SeO3 with SeNPs significantly increased the abundance of Holdemanella, upregulated by 3.51-fold. Some studies have suggested that Holdemanella may have a potential role in maintaining a state of optimal health and wellbeing, and may improve blood sugar metabolism (Romaní-Pérez et al., 2021; Zhang et al., 2022). The Holdemanella can have antitumorigenic and anti-inflammatory effects by releasing SCFA and long-chain fatty acid, such as (R)-3-hydroxy-octadecanoic acid (Romaní-Pérez et al., 2021). Comparable changes in the levels of SCFA have been noted in piglets consuming a diet supplemented with SeNPs, as SCFA are a gut microbiota-derived metabolite, and SeNPs significantly increased the concentrations of the total SCFA, acetate and propionate compared with Na2SeO3 group. Furthermore, the correlation analysis demonstrated that Holdemanella was the key response bacterial genus for treatment with SeNPs, and its relative abundance showed a significant correlation with those of 17 metabolites out of a total of 41 differential metabolites that might participate in lipid metabolism. Given the regulatory effects of Se supplementation on the gut microbiota and its metabolites, Callejón-Leblic et al. (2021) found that the role of Se supplementation in regulating GPx and selenoprotein levels was influenced by microbiota disruption, suggesting an intertwined mechanism, which provides evidence for a critical Se intake-microbiome-metabolism interaction, but further studies are needed to elucidate the specific mechanisms.
In general, SeNPs improved the growth performance and gut health of early-weaned piglets. In follow-up studies, we need to increase the sample size of piglets to further verify the protective effect of SeNPs. Additionally, although this study has found that SeNPs are involved in lipid metabolism, the regulatory mechanism behind this is still unclear. Therefore, targeted lipid metabolomics should be further utilized in future experiments to explore the relationship between SeNPs and lipid metabolism. Furthermore, this study only focused on the impact of SeNPs on early-weaned piglets, thus it is necessary to investigate the impact of SeNPs on other growth stages to determine their applicability as a nutritional intervention strategy.
5. Conclusion
In summary, the present study demonstrated that replacing dietary Na2SeO3 with SeNPs synthesized by L. casei ATCC 393 significantly improved the growth performance of early-weaned piglets, alleviated the early weaning-induced intestinal barrier dysfunction, contributed to maintaining intestinal homeostasis, and further reduced diarrhea incidence by enhancing the antioxidant capacity and immunity, thereby improving mitochondrial structure and function by regulating the AMPK/Nrf2/NLRP3 signaling pathway. This study provides a novel Se delivery platform for early-weaned piglets' diets and also provides a potential nutrition intervention strategy to regulate intestinal homeostasis of early-weaned piglets.
Author contributions
Lei Qiao: Conceptualization, Data curation, Funding acquisition, Methodology, Writing-original draft; Xina Dou: Data curation, Methodology, Supervision; Xiaofan Song: Data curation, Methodology, Supervision; Jiajing Chang: Methodology, Supervision; Xiaonan Zeng: Methodology, Supervision; Lixu Zhu: Methodology, Supervision; Hongbo Yi: Data curation, Methodology, Writing-review & editing; Chunlan Xu: Conceptualization, Funding acquisition, Supervision, Writing-review & editing.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 32072746) and the Innovation Foundation for Doctor Dissertation of Northwestern Polytechnical University (No. CX2021029, No. CX2022062).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aninu.2023.08.003.
Contributor Information
Hongbo Yi, Email: yihongbo@gdaas.cn.
Chunlan Xu, Email: clxu@nwpu.edu.cn.
Appendix supplementary data
The following is the Supplementary data to this article:
References
- Bartolacci C., Andreani C., El-Gammal Y., Scaglioni P.P. Lipid metabolism regulates oxidative stress and ferroptosis in RAS-driven cancers: a perspective on cancer progression and therapy. Front Mol Biosci. 2021;8 doi: 10.3389/fmolb.2021.706650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko I., Nagy G., Tanczos B., Ungvari E., Sztrik A., Eszenyi P., et al. Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ Toxicol Chem. 2012;31:2812–2820. doi: 10.1002/etc.1995. [DOI] [PubMed] [Google Scholar]
- Bhatti J.S., Bhatti G.K., Reddy P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders — a step towards mitochondria based therapeutic strategies. BBA-Mol Basis Dis. 2017;1863:1066–1077. doi: 10.1016/j.bbadis.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejón-Leblic B., Selma-Royo M., Collado M.C., Abril N., García-Barrera T. Impact of antibiotic-induced depletion of gut microbiota and selenium supplementation on plasma selenoproteome and metal homeostasis in a mice model. J Agric Food Chem. 2021;69:7652–7662. doi: 10.1021/acs.jafc.1c02622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.M., Crenshaw J.D., Polo J. The biological stress of early weaned piglets. J Anim Sci Biotechnol. 2013;4:19. doi: 10.1186/2049-1891-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S.T., Wang C.C., Wu H., Zhang Q.H., Jiao L.F., Hu C.H. Weaning disrupts intestinal antioxidant status, impairs intestinal barrier and mitochondrial function, and triggers mitophagy in piglets. J Anim Sci. 2018;96:1073–1083. doi: 10.1093/jas/skx062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle A.E., Lee N., Matthew-Onabanjo A.N., Spears M.E., Park S.J., Youkana D., et al. Selenium detoxification is required for cancer-cell survival. Nat Metab. 2020;2:603–611. doi: 10.1038/s42255-020-0224-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli S., De Falco P., Ciccarone F., Desideri E., Ciriolo M.R. Lipid catabolism and ROS in cancer: a bidirectional liaison. Cancers. 2021;13:5484. doi: 10.3390/cancers13215484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Yao P., Zhang W., Zhang Y., Xin N., Wei H., et al. Selenium nanoparticles: enhanced nutrition and beyond. Crit Rev Food Sci Nutr. 2022:1–12. doi: 10.1080/10408398.2022.2101093. [DOI] [PubMed] [Google Scholar]
- Chen X.D., Zhao Z.P., Zhou J.C., Lei X.G. Evolution, regulation, and function of porcine selenogenome. Free Radic Biol Med. 2018;127:116–123. doi: 10.1016/j.freeradbiomed.2018.04.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu M.L., Aw T.Y. Redox biology of the intestine. Free Radic Res. 2011;45:1245–1266. doi: 10.3109/10715762.2011.611509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie E., Schulze A., Zechner R., Walther T.C., Farese R.V. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18:153–161. doi: 10.1016/j.cmet.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou X., Qiao L., Chang J., Yan S., Song X., Chen Y., et al. Lactobacillus casei ATCC 393 and it's metabolites alleviate dextran sulphate sodium-induced ulcerative colitis in mice through the NLRP3-(Caspase-1)/IL-1β pathway. Food Funct. 2021;12:12022–12035. doi: 10.1039/d1fo02405a. [DOI] [PubMed] [Google Scholar]
- Durso L.M., Cook K.L. Impacts of antibiotic use in agriculture: what are the benefits and risks? Curr Opin Microbiol. 2014;19:37–44. doi: 10.1016/j.mib.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Ekanah K. Relationship between plasma levels of albumin, selenium, chromium and manganese of healthy subjects and patients with HIV/AIDS, diabetes mellitus and cardiovascular disease in akwa-ibom and cross river states of Nigeria. J Public Health Epidemiol. 2015;7:154–158. [Google Scholar]
- Ferro C., Florindo H.F., Santos H.A. Selenium nanoparticles for biomedical applications: from development and characterization to therapeutics. Adv Healthc Mater. 2021;10 doi: 10.1002/adhm.202100598. [DOI] [PubMed] [Google Scholar]
- Gao J., Yin J., Xu K., Li T., Yin Y. What is the impact of diet on nutritional diarrhea associated with gut microbiota in weaning piglets: a system review. Biomed Res Int. 2019 doi: 10.1155/2019/6916189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham L., Orenstein J.M. Processing tissue and cells for transmission electron microscopy in diagnostic pathology and research. Nat Protoc. 2007;2:2439–2450. doi: 10.1038/nprot.2007.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B., Johnson A.C., Grundy D. Gastrointestinal physiology and function. Handb Exp Pharmacol. 2017;239:1–16. doi: 10.1007/164_2016_118. [DOI] [PubMed] [Google Scholar]
- Gresse R., Chaucheyras-Durand F., Fleury M.A., Van de Wiele T., Forano E., Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. 2017;25:851–873. doi: 10.1016/j.tim.2017.05.004. [DOI] [PubMed] [Google Scholar]
- He L., Zhou X., Wu Z., Feng Y., Liu D., Li T., et al. Glutamine in suppression of lipopolysaccharide-induced piglet intestinal inflammation: the crosstalk between AMPK activation and mitochondrial function. Anim Nutr. 2022;10:137–147. doi: 10.1016/j.aninu.2022.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J.M., Opapeju F.O., Pluske J.R., Kim J.C., Hampson D.J., Nyachoti C.M. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr (Berl) 2013;97:207–237. doi: 10.1111/j.1439-0396.2012.01284.x. [DOI] [PubMed] [Google Scholar]
- Hou L., Wang L., Qiu Y., Xiong Y., Xiao H., Yi H., et al. Effects of protein restriction and subsequent realimentation on body composition, gut microbiota and metabolite profiles in weaned piglets. Animals. 2021;11:686. doi: 10.3390/ani11030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo M.S., Kim W.D., Lee K.Y., Kim J.H., Koo J.H., Kim S.G. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- Kim M.K., Kwon H.S., Baek K.H., Lee J.H., Park W.C., Sohn H.S., et al. Effects of thyroid hormone on A1C and glycated albumin levels in nondiabetic subjects with overt hypothyroidism. Diabetes Care. 2010;33:2546–2548. doi: 10.2337/dc10-0988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange Cd, Pluske J.R., Gong J., Gong J., Nyachoti C.M. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest Sci. 2010;134:124–134. [Google Scholar]
- Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94:739–777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Guo Y., Wen Z., Jiang X., Ma X., Han X. Weaning stress perturbs gut microbiome and its metabolic profile in piglets. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33649-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.J., Qin Y., Zhao Z.H., Zhang Y., Yang J.H., Zhai D.H., et al. Lentinan-functionalized selenium nanoparticles target tumor cell mitochondria via TLR4/TRAF3/MFN1 pathway. Theranostics. 2020;10:9083–9099. doi: 10.7150/thno.46467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Wang P., Teng T., Shi B., Shan A., Lei X.G. Effects of dietary selenium deficiency or excess on selenoprotein gene expression in the spleen tissue of pigs. Animals. 2019;9:1122. doi: 10.3390/ani9121122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E.H., Poffenberger M.C., Wong A.H., Jones R.G. The role of AMPK in T cell metabolism and function. Curr Opin Immunol. 2017;46:45–52. doi: 10.1016/j.coi.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Malyugina S., Skalickova S., Skladanka J., Slama P., Horky P. Biogenic selenium nanoparticles in animal nutrition: a review. Agriculture. 2021;11:1244. [Google Scholar]
- Moeser A.J., Pohl C.S., Rajput M. Weaning stress and gastrointestinal barrier development: implications for lifelong gut health in pigs. Anim Nutr. 2017;3:313–321. doi: 10.1016/j.aninu.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottawea W., Chiang C.K., Mühlbauer M., Starr A.E., Butcher J., Abujamel T., et al. Altered intestinal microbiota-host mitochondria crosstalk in new onset Crohn's disease. Nat Commun. 2016;7 doi: 10.1038/ncomms13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novais A.K., Deschêne K., Martel-Kennes Y., Roy C., Laforest J.P., Lessard M., et al. Weaning differentially affects mitochondrial function, oxidative stress, inflammation and apoptosis in normal and low birth weight piglets. PLoS One. 2021;16 doi: 10.1371/journal.pone.0247188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council) The National Academies Press; Washington, DC: 2012. Nutrient requirements of swine: Eleventh Revised Edition. [Google Scholar]
- Oliveira T.F.B., Bertechini A.G., Philomeno R., Silva V.A. Dietary levels and sources of selenium for post weaning piglets. Cienc Rural. 2017;47(12) [Google Scholar]
- Ortman K., Pehrson B. Effect of selenate as a feed supplement to dairy cows in comparison to selenite and selenium yeast. J Anim Sci. 1999;77:3365–3370. doi: 10.2527/1999.77123365x. [DOI] [PubMed] [Google Scholar]
- Pluske J.R., Turpin D.L., Kim J.C. Gastrointestinal tract (gut) health in the young pig. Anim Nutr. 2018;4:187–196. doi: 10.1016/j.aninu.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L., Dou X., Song X., Chang J., Pi S., Zhang X., et al. Protective effect of biogenic selenium nanoparticles against diquat-induced acute toxicity via regulation of gut microbiota and its metabolites. Food Chem Toxicol. 2022;170 doi: 10.1016/j.fct.2022.113480. [DOI] [PubMed] [Google Scholar]
- Qiao L., Dou X., Yan S., Zhang B., Xu C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL/6 mice through their antioxidant activity. Food Funct. 2020;11:3020–3031. doi: 10.1039/d0fo00132e. [DOI] [PubMed] [Google Scholar]
- Qiao L., Zhang X., Pi S., Chang J., Dou X., Yan S., et al. Dietary supplementation with biogenic selenium nanoparticles alleviate oxidative stress-induced intestinal barrier dysfunction. NPJ Sci Food. 2022;6:30. doi: 10.1038/s41538-022-00145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W., Xu B., Chen Y., Yang W., Xu Y., Huang J., et al. Dietary ellagic acid supplementation attenuates intestinal damage and oxidative stress by regulating gut microbiota in weanling piglets. Anim Nutr. 2022;11:322–333. doi: 10.1016/j.aninu.2022.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch R.C., Samborska B., Faubert B., Ma E.H., Gravel S.P., Andrzejewski S., et al. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017;21:1–9. doi: 10.1016/j.celrep.2017.09.026. [DOI] [PubMed] [Google Scholar]
- Rath E., Moschetta A., Haller D. Mitochondrial function - gatekeeper of intestinal epithelial cell homeostasis. Nat Rev Gastroenterol Hepatol. 2018;15:497–516. doi: 10.1038/s41575-018-0021-x. [DOI] [PubMed] [Google Scholar]
- Romaní-Pérez M., López-Almela I., Bullich-Vilarrubias C., Rueda-Ruzafa L., Gómez Del Pulgar E.M., Benítez-Páez A., et al. Holdemanella biformis improves glucose tolerance and regulates GLP-1 signaling in obese mice. FASEB J. 2021;35 doi: 10.1096/fj.202100126R. [DOI] [PubMed] [Google Scholar]
- Rubattu S., Pagliaro B., Pierelli G., Santolamazza C., Castro S.D., Mennuni S., et al. Pathogenesis of target organ damage in hypertension: role of mitochondrial oxidative stress. Int J Mol Sci. 2014;16:823–839. doi: 10.3390/ijms16010823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadatomi D., Nakashioya K., Mamiya S., Honda S., Kameyama Y., Yamamura Y., et al. Mitochondrial function is required for extracellular ATP-induced NLRP3 inflammasome activation. J Biochem. 2017;161:503–512. doi: 10.1093/jb/mvw098. [DOI] [PubMed] [Google Scholar]
- Saladrigas-García M., D'Angelo M., Ko H.L., Nolis P., Ramayo-Caldas Y., Folch J.M., et al. Understanding host-microbiota interactions in the commercial piglet around weaning. Sci Rep. 2021;11 doi: 10.1038/s41598-021-02754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg L., Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008;52:1235–1246. doi: 10.1002/mnfr.200700465. [DOI] [PubMed] [Google Scholar]
- Skalickova S., Milosavljevic V., Cihalova K., Horky P., Richtera L., Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90. doi: 10.1016/j.nut.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Surai P.F. In: Selenium in pig nutrition and health. Surai P.F., editor. Wageningen Academic Publishers; Netherlands: 2021. [Google Scholar]
- Surai P.F., Fisinin V.I. Selenium in pig nutrition and reproduction: boars and semen quality-a review. Asian-Australas J Anim Sci. 2015;28:730–746. doi: 10.5713/ajas.14.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q., Xu E., Wang Z., Xiao M., Cao S., Hu S., et al. Dietary Hermetia illucens larvae meal improves growth performance and intestinal barrier function of weaned pigs under the environment of enterotoxigenic Escherichia coli K88. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.812011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Xiong K., Fang R., Li M. Weaning stress and intestinal health of piglets: a review. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1042778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turanov A.A., Xu X.M., Carlson B.A., Yoo M.H., Gladyshev V.N., Hatfield D.L. Biosynthesis of selenocysteine, the 21st amino acid in the genetic code, and a novel pathway for cysteine biosynthesis. Adv Nutr. 2011;2:122–128. doi: 10.3945/an.110.000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancamelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Reest J., Lilla S., Zheng L., Zanivan S., Gottlieb E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat Commun. 2018;9:1581. doi: 10.1038/s41467-018-04003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujic A., Koo A.N.M., Prag H.A., Krieg T. Mitochondrial redox and TCA cycle metabolite signaling in the heart. Free Radic Biol Med. 2021;166:287–296. doi: 10.1016/j.freeradbiomed.2021.02.041. [DOI] [PubMed] [Google Scholar]
- Wen X., Tang L., Zhong R., Liu L., Chen L., Zhang H. Role of mitophagy in regulating intestinal oxidative damage. Antioxidants. 2023;12:480. doi: 10.3390/antiox12020480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing H., Zheng S., Zhang Z., Zhu F., Xue H., Xu S. Pharmacokinetics of selenium in healthy piglets after different routes of administration: application of pharmacokinetic data to the risk assessment of selenium. Biol Trace Elem Res. 2019;191:403–411. doi: 10.1007/s12011-019-1644-7. [DOI] [PubMed] [Google Scholar]
- Xu C., Qiao L., Guo Y., Ma L., Cheng Y. Preparation, characteristics and antioxidant activity of polysaccharides and proteins-capped selenium nanoparticles synthesized by Lactobacillus casei ATCC 393. Carbohydr Polym. 2018;195:576–585. doi: 10.1016/j.carbpol.2018.04.110. [DOI] [PubMed] [Google Scholar]
- Xu J., Xu C., Chen X., Cai X., Yang S., Sheng Y., et al. Regulation of an antioxidant blend on intestinal redox status and major microbiota in early weaned piglets. Nutrition. 2014;30:584–589. doi: 10.1016/j.nut.2013.10.018. [DOI] [PubMed] [Google Scholar]
- Xu W., Zhao T., Xiao H. The implication of oxidative stress and AMPK-Nrf2 antioxidative signaling in pneumonia pathogenesis. Front Endocrinol. 2020;11:400. doi: 10.3389/fendo.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Yang H. Recent development in Se-enriched yeast, lactic acid bacteria and bifidobacteria. Crit Rev Food Sci Nutr. 2023;63:411–425. doi: 10.1080/10408398.2021.1948818. [DOI] [PubMed] [Google Scholar]
- Yang X., Zheng M., Zhou M., Zhou L., Ge X., Pang N., et al. Lentinan supplementation protects the gut-liver axis and prevents steatohepatitis: the role of gut microbiota involved. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.803691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Wang Y., He T., Ziema Bumbie G., Wu L., Sun Z., et al. Effects of dietary yucca schidigera extract and oral candida utilis on growth performance and intestinal health of weaned piglets. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.685540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardeni T., Tanes C.E., Bittinger K., Mattei L.M., Schaefer P.M., Singh L.N., et al. Host mitochondria influence gut microbiome diversity: a role for ROS. Sci Signal. 2019;12 doi: 10.1126/scisignal.aaw3159. [DOI] [PubMed] [Google Scholar]
- Yim S.H., Clish C.B., Gladyshev V.N. Selenium deficiency is associated with pro-longevity mechanisms. Cell Rep. 2019;27:2785–2797. doi: 10.1016/j.celrep.2019.05.001. e2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai Q., Cen S., Li P., Tian F., Zhao J., Zhang H., et al. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ Sci Technol Lett. 2018;5:724–730. [Google Scholar]
- Zhang C., Liang D., Li X., Liu J., Fan M., Jing M., et al. Characteristics of gut microbial profiles of offshore workers and its associations with diet. Front Nutr. 2022;9 doi: 10.3389/fnut.2022.904927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Piao X. Different dietary protein sources influence growth performance, antioxidant capacity, immunity, fecal microbiota and metabolites in weaned piglets. Anim Nutr. 2022;8:71–81. doi: 10.1016/j.aninu.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L., Duarte M.E., Sevarolli Loftus A., Kim S.W. Intestinal health of pigs upon weaning: challenges and nutritional intervention. Front Vet Sci. 2021;8 doi: 10.3389/fvets.2021.628258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- Zoidis E., Seremelis I., Kontopoulos N., Danezis G.P. Selenium-dependent antioxidant enzymes: actions and properties of selenoproteins. Antioxidants. 2018;7:66. doi: 10.3390/antiox7050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.