Abstract
Patients with neurological diseases, such as schizophrenia, tend to show low K+-Cl- co-transporter 2 (KCC2) levels in the brain. The cause of these diseases has been associated with stress and neuroinflammation. However, since the pathogenesis of these diseases is not yet fully investigated, drug therapy is still limited to symptomatic therapy. Targeting KCC2, which is mainly expressed in the brain, seems to be an appropriate approach in the treatment of these diseases. In this review, we aimed to discuss about stress and inflammation, KCC2 and Gamma-aminobutyric acid (GABA) function, diseases which decrease the KCC2 levels in the brain, factors that regulate KCC2 activity, and the possibility to overcome neuronal dysfunction targeting KCC2. We also aimed to discuss the relationships between neurological diseases and LPS caused by Porphyromonas gingivalis (P. g), which is a type of oral bacterium. Clinical trials on oxytocin, sirtuin 1 (SIRT1) activator, and transient receptor potential cation channel subfamily V Member 1 activator have been conducted to develop effective treatment methods. We believe that KCC2 modulators that regulate mitochondria, such as oxytocin, glycogen synthase kinase 3β (GSK3β), and SIRT1, can be potential targets for neurological diseases.
Keywords: KCC2, GABA, Neurological diseases, Inflammation, Therapeutic target
1. Introduction
Stress and neuroinflammation have been considered as one of the most important risk factors of brain dysfunction. Moreover, brain dysfunction caused by stress has also been associated with memory impairment, attention impairment, executive dysfunction, social behavior impairment, communication and emotional impairment, agnosia, apraxia, and self-consciousness impairment. Brain dysfunction is known to be caused by not only stress but also trauma, cerebrovascular disease, autoimmune disease, and infection [1], [2]. Patients with mild brain dysfunction tend to lack a clinical insight into the disease and do not have physical or speech disabilities. Moreover, even in their daily activities, they often have problems in social situations, which induces stress not only on them but also on their families, leading to a decline in social productivity. However, the onset mechanism is still unclear.
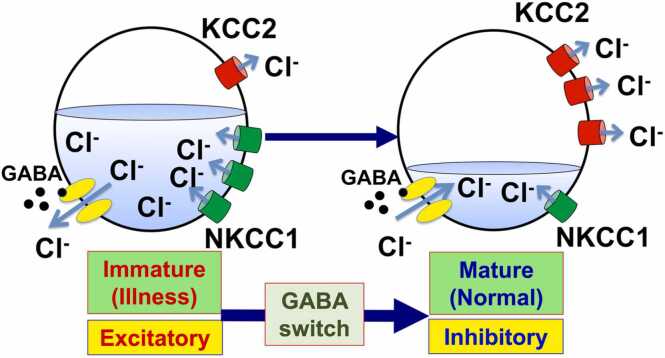
In recent years, the delay of the excitatory-to-inhibitory functional switch of Gamma-aminobutyric acid (GABA; GABA switch) has been reported as one of the causes of brain dysfunction. GABA is a dominant inhibitory neurotransmitter in the adult central nervous system (CNS), but before the GABA switch, GABA functions as an excitatory. In mice, the GABA switch occurs during the first to the second postnatal week. The correct timing of the GABA switch is determined by the functional balance of chloride co-transporters; one is the increase of K+-Cl- co-transporter 2 (KCC2), while the other is the decrease of Na+-K+-2Cl- co-transporter 1 (NKCC1). The expression change of these co-transporters decreases the intracellular chloride levels in the brain, which results in the GABA function from being excitatory to inhibitory [3] (Fig. 1). It is noteworthy that NKCC1 is expressed ubiquitously in vivo, whereas KCC2 protein expression is restricted to the brain [4], [5].
Fig. 1.
The GABA switch. When the Gamma-aminobutyric acid (GABA) binds to the GABAA receptor, which is a chloride channel, it opens the GABAA receptor. When the intracellular chloride ion concentration is high because of the low expression of K+-Cl- co-transporter 2 (KCC2), i.e., when the brain is immature, GABA makes chloride ions flow out from the cells, resulting in cell excitability (the membrane potential depolarizes). On the other hand, during normal brain development, KCC2 is up-regulated and Na+-K+-2Cl- co-transporter 1 (NKCC1) is down-regulated. Therefore, in mature brains with lowered intracellular chloride ions, chloride ions flow into the cells when GABA functions. As a result, cells respond in an inhibitory manner (the membrane potential hyperpolarizes). The excitatory-to-inhibitory functional switch of GABA is referred to as the GABA switch.
In some studies, the decreased expression of KCC2 in the nervous system was observed in Huntington’s disease [6], Rett syndrome [7], Angelman syndrome [8], stroke [9], epilepsy [10], and schizophrenia [11], [12]. Therefore, controlling the KCC2 expression that is, restoring the KCC2 expression and function, is critical to overcome these diseases. In recent years, oxytocin, which regulates KCC2 expression, has attracted attention to overcome neuropathy. It has been reported that the intranasal administration of oxytocin to patients with autism relieves their symptoms [13], [14], indicating that it has the potential to be applied to the treatment of many neurological disorders. Furthermore, oxytocin, inhibitors of the fms-like tyrosine kinase 3 (FLT3), glycogen synthase kinase 3β (GSK3β), activators of sirtuin 1 (SIRT1), and transient receptor potential cation channel subfamily V Member 1 (TRPV1) pathways have been reported to enhance KCC2 expression [7]. Moreover, a relationship between prohibitin 2 (PHB2), a multifunctional protein mainly localized in mitochondria, and KCC2 expression has also been reported [15].
In this review, we focus on the regulation of KCC2 in neurological diseases and discuss the possibility of targeting KCC2 to overcome neurological diseases.
2. Stress and inflammation
Inflammation, which is caused by stress, leads to brain dysfunction. The cause of stress is called a stressor. Several types of stressors affect the mind and body. It has been reported that restraint stress, oxidative stress, and maternal separation trigger dysfunctions in the brain [16], [17], [18]. Neuroinflammation, which is an inflammation of the nervous tissue, has been shown to cause psychiatric disorders, such as cognitive impairment [19].
One of the stressors that cause neuroinflammation is lipopolysaccharides (LPSs) [20]. LPSs derived from Escherichia coli (E. coli) up-regulate nterleukin-1 beta (IL-1β) and cause depression-like behaviors [21]. LPS derived from Porphyromonas gingivalis (P. g), which is a type of oral bacteria, causes not only periodontal disease but also is involved in Alzheimer’s disease [22].
Oxidative stress is also one of the important stressors that trigger inflammation. It is defined as an imbalance between the production of reactive oxygen species (ROS) and antioxidants. This imbalance leads to the damage of important biomolecules and impacts the whole body [23]. ROS are products of normal cellular metabolism and play vital roles in cell signaling pathways [24]. It has been indicated that most ROS are generated in cells by the mitochondrial respiratory chain [25], [26]. Additionally, LPS also stimulates ROS [27].
The oral and maxillofacial region is the most nerve-concentrated area in the body. According to the Penfield map [28], the maxillofacial region, along with the limbs, occupies a large portion of the somatosensory and motor cortices, indicating a strong relationship between the maxillofacial region and brain. Moreover, since P. g LPS in the oral cavity is commonly detected in the brains of Alzheimer’s disease patients [29], inflammation and dysfunction in the oral area might cause functional impairment in the brain. In fact, P. g LPS treatment has been reported to increase IL-1β expression in the cells of the nervous system, leading to inflammation and neural disfunction via KCC2 decrease [30].
In recent years, it has been reported that endogenous damage-related molecules (damage-associated molecular patterns: DAMPs) induce inflammation by binding to Toll-like receptors (TRL) and receptors for advanced glycation end products (RAGE) [31]. DAMPs are thought to be released in association with cell stress, such as cell death and cell damage, and function as alarms that signal cell crisis. It has also been indicated that neuroinflammation is mediated by GSK3β-dependent TLR4 signaling in mice [32]. Moreover, it has also been shown that the up-regulation of IL-1β by binding LPS to TLR4 leads to the localization of RE1-silencing transcription factor (REST) and methyl CpG binding protein 2 (MECP2) to the nucleus and suppression of KCC2 gene expression [33]. MECP2 is a responsible gene in Rett syndrome, and the knockout of the gene has shown to enhance LPS-induced inflammatory response [34], [35]. Chronic stress has been reported to reduce hippocampal KCC2 expression and shifts GABA function from inhibitory to excitatory [36]. Furthermore, it has been reported that LPS decreases KCC2 expression and TLR4 and RAGE are involved in this LPS-induced stress response as receptors [15].
3. KCC2 expression and GABA function
GABA opens the GABAA receptor, which penetrates Cl-. GABA is the main inhibitory neurotransmitter in the mature CNS. However, GABA acts as excitatory in the immature brain. This is because NKCC1 expression tends to decrease, while KCC2 expression tends to increase during brain maturation. NKCC1 is a transporter that takes Cl- into cells and KCC2 is a transporter that releases Cl- outside the cells. When GABAA receptors are opened under a high intracellular Cl- concentration, i.e., the NKCC1 expression is high and KCC2 expression is low, Cl- are released outside the cell. This causes the depolarization of the plasma membrane potential and excites the cell. As the brain matures, the NKCC1 expression decreases, KCC2 expression increases, and intracellular Cl- concentration decreases. In this state, GABA makes the Cl- flow into the cells. As a result, GABA hyperpolarizes the membrane potential, and the cells respond in an inhibitory manner (Fig. 1). This functional change from an excitatory to inhibitory GABA function is called the GABA switch. Moreover, the GABA switch has been observed in a range of species, such as zebrafish [37], Xenopus [38], and chicks [39]. At least in vertebrates, it seems to be a general rule that has been conserved throughout evolution.
In the hippocampus, the amount of KCC2 mRNA is correlated with GABA function, overexpression of KCC2 changes the action of GABA from excitatory to inhibitory, and activation or inhibition of GABAA receptors alters the KCC2 expression and reversal potential of the plasma membrane [40]. These results suggested that KCC2 plays an important role in brain function better than NKCC1. It has been found that the correct timing of the GABA switch is important for normal brain maturation. It is known that exposure to various stresses at an early age delays the timing of this switch, causing behavioral abnormalities [18]. It has been indicated that oxytocin is involved in anti-inflammation [41] and in the GABA switch by up-regulating the KCC2 expression and phosphorylation via the oxytocin receptors [42].
4. KCC2 and neurological diseases
KCC2 is known to be involved in the pathogenesis of many neurological diseases, such as autism spectrum disorder (ASD) [43]. Table 1 shows the various diseases associated with decreased KCC2 expression [9], [10], [12], [34], [43], [44], [45], [46], [47], [48], [49], [50]. ASD is one of the developmental disorders that currently has no cure, which is characterized by poor communication with people and strong obsession with things. Moreover, ASD can also be accompanied by other neurodevelopmental disorders, such as intellectual disability, depression, bipolar disorder, obsessive-compulsive disorder, anxiety, and schizophrenia, and include Rett syndrome [51] and Angelman syndrome [8]. Rett syndrome is a genetic disorder characterized by autism, epilepsy, ataxic gait, and characteristic fisting movements. De novo mutations in the MECP2 gene in humans are responsible for more than 80% of all Rett syndrome cases [34], [52]. It has been reported that KCC2 expression is down-regulated in the brains of patients with Rett syndrome [53]. MECP2 deficiency in Rett syndrome also results in the downregulation of brain-derived neurotrophic factor (BDNF) because mutated MECP2 is unable to bind to the regulatory region of BDNF [54], while the overexpression of KCC2 has been reported to rescue the Rett syndrome phenotype [51]. Angelman syndrome is caused by the loss of function of the UBE3A, which is located in chromosome 15q11–q13 [44]. It is characterized by severe developmental delay, dyslexia, epilepsy, frequent laughter, and clumsy movements [55], [56]. In a mouse model of Angelman syndrome, decreased KCC2 expression and increased NKCC1 expression have been observed in the hippocampus [8]. This result indicates that not only KCC2 but also NKCC1 may be a therapeutic target for brain dysfunction.
Table 1.
Major diseases associated with decreased KCC2 expression.
| Disease | Symptoms | Cause | Ref |
|---|---|---|---|
| Autism spectrum disorder | Impaired social communication, restricted interests, stereotypies | Unknown (congenital abnormalities in brain function) | 43 |
| (Rett Syndrome) | Autism, epilepsy, ataxic gait, peculiar stereotypic movements (firming movements) | MECP2 gene mutation | 34 |
| (Angelman syndrome) | Epilepsy, shows clumsiness and laughs a lot at trifles | Loss of UBE3A gene | 44 |
| Mood disorders (depression, bipolar disorder) |
Intense sadness, lack of motivation, difficulty sleeping, getting tired easily, feeling sluggish |
Unknown cause (stress) | 45 |
| Epilepsy | Seizures, such as convulsions or loss of consciousness | Brain trauma, encephalitis, encephalopathy, meningitis, cerebral infarction, stress | 10 |
| Schizophrenia | Delusions, hallucinations, disorganized thinking, extremely disorganized or abnormal motor behavior, lose interest in everyday activities, socially withdraw | Unknown cause (stress) | 12 |
| Stroke | Paralysis of arms and face, inability to speak properly, dizziness, headache, unconsciousness | Arteriosclerosis caused by hypertension, hyperlipidemia, diabetes, smoking |
9 |
| Down syndrome | Flattened face, short neck, mental impairment, distraction, obsessive behaviors. |
21 trisomy | 46 |
| Huntington's disease | Involuntary movements such as chorea, psychiatric symptoms, behavioral disorders, cognitive impairment |
Huntingtin gene mutation | 47 |
| Diabetes | Increased blood sugar level, thirst, polydipsia, polyuria, malaise, weight loss, urinary sugar | Lifestyle habits such as overeating (especially high-fat diet), lack of exercise, obesity, stress, and aging | 48 |
| Spinal cord injury | Paralysis due to damage to the spinal cord | Traffic accident, fall | 49 |
| Neuropathic pain | Electric pain, stabbing pain, constricting pain, numbness pain, allodynia |
Nerve compression, injury, phantom limb pain, post-herpetic neuralgia, diabetes | 50 |
Typical mental disorders include affective disorders (depression and bipolar disorder), epilepsy, and schizophrenia. The WHO reports that the prevalence of bipolar disorder and epilepsy is about 1% of the population, respectively. The prevalence of schizophrenia is estimated at about 0.3%, while depression is estimated at about 4%. The genes responsible for these disorders have not been determined. However, these diseases are known to be caused by stress and decrease the expression of KCC2 [10], [12], [45]. The three types of strokes are as follows: cerebral infarction caused by clogged blood vessels (ischemia), cerebral hemorrhage caused by ruptured blood vessels, and subarachnoid hemorrhage. Among them, cerebral infarction accounts for most strokes, and ischemia has been shown to down-regulate KCC2 [9]. Moreover, it has been indicated that KCC2 is decreased in genetic diseases, such as Down’s syndrome and Huntington’s chorea, as well as in diabetes, spinal cord injury, and neuropathic pain [46], [47], [48], [49], [50].
Tsukahara et al. focused on the effects of repeated stress of forced drinking on the GABA response of female mice and found that repeated stress reduced their novel object recognition ability and social behavior. They found that the KCC2 levels decreased in the hippocampus [57]. Moreover, Furukawa et al. analyzed the effects of the maternal separation on the behavioral characteristics of juvenile mice and found that the mice that were isolated from their mother mice for 3 h every day exhibited more anxiety-like behavior than mice that were not separated [18].
5. Factors that regulates KCC2 activity
It has been reported that many factors can influence the KCC2 activity (as summarized in Table 2) [7], [15], [33], [42], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70]. The WNK (with-no-lysine kinase) family of serine-threonine kinases, together with SPAK, a STE20/SPS1-related proline/alanine-rich kinase, and OSR1, an oxidative stress-responsive kinase − 1, form a powerful signaling cascade that is involved in the control of KCC2 [71], [72]. WNK-SPAK/OSR1 directly phosphorylates KCC2 and inhibits the KCC2 function. It has been reported that the WNK-SPAK system is activated by the phosphatidylinositol-3 kinase (PI3K)/AKT signaling pathway [71].
Table 2.
Factors that regulate KCC2.
| Factor | Function | Ref |
|---|---|---|
| WNK/SPAK | Phosphorylates KCC2 extracellular domain and inhibit KCC2 | 58 |
| Brain-type creatine kinase | Directly binds to KCC2 and Activates KCC2 | 59 |
| PACSIN1 | A neuronal endocytic regulatory protein that inhibits KCC2 expression | 60 |
| Gephyrin | Anchor protein to stabilize KCC2 expression | 61 |
| GABAB Receptor | Directly bind to KCC2 and inhibit its expression | 62 |
| BDNF | Down-regulates KCC2 expression by CREB phosphorylation | 63 |
| MECP2 | Inhibit KCC2 expression by binding KCC2 promotor | 33 |
| FLT3 | Receptor tyrosine kinase that Inhibits KCC2 expression via AKT signaling | 7 |
| GSK3β | Inhibits KCC2 expression by inactivating KCC2 promoter (activate histone deacetylase and/or IL1β-REST signaling) |
64 |
| SIRT1 | Activate KCC2 expression by inhibiting REST gene expression | 65 |
| TRPV1 | Activate KCC2 expression | 7 |
| Estradiol | Down-regulates KCC2 expression in male mouse | 66 |
| Oxytocin | Activates KCC2 by inhibiting GSK3β | 42 |
| miR-137 | Directly down-regulates KCC2 expression | 67 |
| CLP257 | KCC2 activator, increase its function by KCC2 phosphorylation | 68 |
| CLP290 | KCC2 activator, pro-drug of CLP257 | 68 |
| Bisphenol A | KCC2 inhibitor by inactivating KCC2 promoter (inhibit histone acetyltransferase) |
69 |
| Furosemide | KCC2 inhibitor. Also inhibit NKCC1 | 70 |
| bumetanide | KCC2 inhibitor. Also inhibit NKCC1 | 70 |
| Kamikihito | Activates KCC2 by upregulating oxytocin expression | 15 |
Based on the study of Rett syndrome, the inhibitors of FLT3 and GSK3β, the activators of the SIRT1 and TRPV1 pathways, have been identified as factors that increase KCC2 expression [7]. FLT3 is known as a receptor tyrosine kinase and activates downstream MAPK, PI3K/AKT/mTOR, and STAT signaling by the binding of the FLT3 ligand [72]. It has been indicated that KW-2449, which is an inhibitor of FLT3, up-regulates KCC2 [7]. GSK3 is a serine/threonine protein kinase [73] and catalyzes the phosphorylation of many cellular substrates [74]. GSK3β is highly expressed particularly in the CNS and acts as a substrate for AKT, but it has been reported to be regulated both positively and negatively by AKT [75]. GSK3β has been reported to function as a positive effector of the WNK-SPAK pathway [76]. Several GSK3β inhibitors have been used in clinical trials [77]. SIRT1, which is a homolog of yeast silent information regulator 2, is a histone deacetylase, and its activation is known to extend lifespan in yeast to human [78], [79]. SIRT1 is basally expressed in the adult mammalian brain, predominantly in the neurons [79]. It has been reported that SIRT1 activator resveratrol down-regulates REST, which inhibits the KCC2 expression by binding to the promoter region of KCC2 [65]. Furthermore, SIRT1 has been shown to down-regulate miR-134, a brain specific miRNA, while miR-134 modulates synaptic plasticity and memory formation by down-regulating the cAMP response element binding protein (CREB) and BDNF expression [80]. SIRT1 has been shown to improve mitochondrial function via the deacetylation of peroxisome proliferator-activated receptor-γ co-activator-1α, which is involved in mitochondrial biogenesis [81]. Moreover, resveratrol is used in clinical trials [82]. Recently, the intranasal administration of rifampicin (a well-known antibiotic that also improves cognitive function) and resveratrol in combination has been shown to be a safer and more effective treatment method in improving cognitive function than the administration of rifampicin alone in animal models [83]. TRPV1, a capsaicin receptor, is a Ca2+-permeable non-selective cation channel which is involved in neuroinflammation and neuropathic pain [84], [85]. TRPV1 expresses in neurons and glia and is involved in neuroplasticity. Piperine is the main bioactive component of black pepper and is considered a TRPV1 activator which can also up-regulate KCC2 expression [7]. It has been shown that TRPV1 could induce long-term potentiation via BDNF, while BDNF has been shown to induce the generation of action potentials in the postsynaptic element by inducing TRPV1 and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors [86].
BDNF is the most major neurotrophic factor and widely expressed in the brain. They act through its receptor tropomyosin receptor kinase B (TrkB) and down-regulate KCC2 expression [63], [87]. BDNF mediates KCC2 downregulation which has been shown to be involved in neuropathic pain and seizures [88]. BDNF has been reported to regulate synaptic plasticity and memory. Decreased BDNF expression has also been associated with Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, and normal aging conditions [89]. It has been indicated that the dysregulation of neurotrophic factors including BDNF could play an important role in the etiology of bipolar disorder and major depressive disorder [90], [91]. Moreover, BDNF is known to be induced by ROS [92]. Mitochondria are the main intracellular source of ROS [26], and BDNF has been shown to increase the mitochondrial function of the brain, such as ATP synthesis [93]. TrkB is a tyrosine kinase and activates various signaling pathways, including the PI3K, AKT, MAPK, and phospholipase Cγ pathways that activate CREB by phosphorylation [94].
CLP257 and its carbamate prodrug CLP290 are KCC2 activators [68]. CLP257 was obtained as a result of the high-throughput screening of a library to identify small molecules that can decrease intracellular Cl- concentration. Gagnon et al., found that CLP257 significantly ameliorated the depolarization caused by the decreased expression of KCC2 in dorsal horn neurons in spinal cord slices during neuropathic pain, which ultimately improved intracellular Cl- accumulation. It has been suggested that CLP257 and estradiol are working through the same pathway to regulate KCC2 [95]. Estradiol has been shown to regulate KCC2 expression [66], [96] and activate the oxytocin and oxytocin receptors [97].
Oxytocin is a neuropeptide with a total length of nine amino acids and used as a neurotransmitter in the CNS [98], [99]. Oxytocin treatment through a nasal mucosa has been shown to improve autistic symptoms in a clinical trial [13], [14]. It has also been reported to cause GABA to switch “ON” by up-regulating the KCC2 expression and phosphorylation [42] and inhibits GSK-3β signaling [100]. Stress has been shown to activate the hypothalamic-pituitary-adrenal (HPA) axis and increase the glucocorticoid concentration in the blood, which leads to cognitive impairments and behavioral disorders [101]. Oxytocin administration reduces the glucocorticoid levels in the rat plasma due to stress [102], inhibits the HPA axis, and decreases anxiety to stress [103]. LPS treatment reduces the KCC2 expression, which in turn reduces the oxytocin receptor expression [30]. Moreover, oxytocin improves the ability to perceive and recognize emotions based on the facial expressions of others [104], [105], [106]. Based on these reports, oxytocin is reported to help improve the symptoms of neurological diseases. In fact, oxytocin has been verified to be effective in treating autism [14]. Moreover, a study has reported a dementia patient administered with oxytocin who was found to become less likely to feel anger or fear from the facial expressions of others, and the efficacy of oxytocin in treating PTSD patients has also been investigated [107], [108]. It has been reported that brain injuries caused by cranial X-ray irradiation decrease the KCC2 expression in the hippocampus, but nasal oxytocin administration after cranial X-ray irradiation improves the decrease in KCC2 as well as cognitive dysfunction [109].
Kampo medicines are traditional herbal formulas prescribed based on the theory of Kampo developed independently in Japan. Recently, Kampo medicines, such as Kamishoyosan (KSS) and Kamikihito (KKT), were found to improve the stress-induced reduction of KCC2 via GSK3β signaling [30]. KSS has been shown to reduce aggressive biting behavior through serotonin production in the dorsal raphe nucleus of mice after stress [110]. KSS improves the KCC2 decrease through P. g LPS treatment by reducing the expression of RAGE and Tlr4 [15]. RAGE and TRL4 are known to be the receptor of P. g LPS. KKT is prescribed to patients with anxiety, depression, and insomnia [111]. Furthermore, KKT has been shown to increase the secretion of oxytocin in rats with acute stress [112].
One of the direct targets of miR-137 is KCC2 [67]. MiR-137 has been identified as a risk gene for the etiology of schizophrenia [67], bipolar disorder [113], and ASD [114]. MiR-137 also regulates GSK3β and mTOR through neuregulin and BDNF [115], [116]. The relationships between miR-137 and mitochondria have also been investigated [117]. Stress-induced mitochondrial dysfunction tends to suppress KCC2 [118]. The dysfunction of mitochondrial uncoupling protein UCP2 was reported to result in a decreased expression of KCC2 [119]. UCP2 provides protection against oxidative stress and plays an important role in decreasing the ROS production by mitochondria. UCP has shown to regulate insulin secretion by pancreatic beta cells and regulate free fatty acid metabolism, which are associated with the pathogenesis of diabetes [120]. 5-Aminolevulinic acid, which is a source of heme and biosynthesized in mitochondria, has been found to inhibit oxidative stress and ameliorate autistic-like behaviors [121]. Many mitochondrial components and metabolites can also function as DAMPs and promote inflammation when they are released into the cytosol or extracellular environment [122]. In fact, our studies have shown that P. g LPS treatment reduces mitochondrial function and KCC2 expression via TLR4 and RAGE, which act as receptors for DAMPS. Furthermore, the knockdown of the PHB2, one of the mitochondrial functional proteins, reduces KCC2 expression [9].
6. Conclusion: the possibility to overcome neuronal dysfunction
Targeting KCC2 to overcome neuronal dysfunction is a promising approach. Including the number of potential patients, it is estimated that a considerable number of patients are suffering from neuronal dysfunction. For example, according to a survey by the Ministry of Education, Culture, Sports, Science, and Technology, the percentage of children with developmental disorders, which are related to brain dysfunction, exceeds 6% in Japan. These patients are likely to have trouble in human relationships. As a result, it is difficult to adapt to social life, and stress is thought to easily lead to mental disorders, such as depression and schizophrenia as secondary disorders. Since the pathogenesis of these diseases is unknown, drug therapy is still limited to symptomatic therapy, and effective drug therapy is difficult. Targeting KCC2, which is mainly expressed in the brain and is deeply involved in neuronal dysfunction, is thought to be a better method with fewer side effects.
It has been shown that oxytocin treatment through a nasal mucosa improved autistic symptoms in a clinical trial [13], [14]. Therefore, oxytocin is very promising as a therapeutic method targeting KCC2. Furthermore, it could decrease KCC2 by using Kampo, such as KSS and KKT, without the direct administration of oxytocin. Clinical trials on SIRT1 and TRPV1 activators have also been promising. Due to its molecular weight, LPS is difficult to pass the blood–brain barrier in a healthy brain. However, P. g LPS in the oral cavity has been reported to be detected in the brains of Alzheimer’s disease patients [22]. With regard to the brain–gut axis [123], oral care is considered necessary. The method targeting mitochondria is also a promising approach. It has been reported that autologously derived mitochondrial transplantation successfully ameliorates heart injury [124]. Given the success of mitochondrial transplantation in patients with heart injury, it is highly likely that healthy mitochondrial transplantation in patients with psychiatric disorders to regulate KCC2 expression may also work. We have summarized the signaling cascades that regulate KCC2 reviewed in this paper (Fig. 2). Although further research is needed to confirm these findings, various approaches to psychiatric disorders targeting KCC2 will open a promising future for novel and effective treatments.
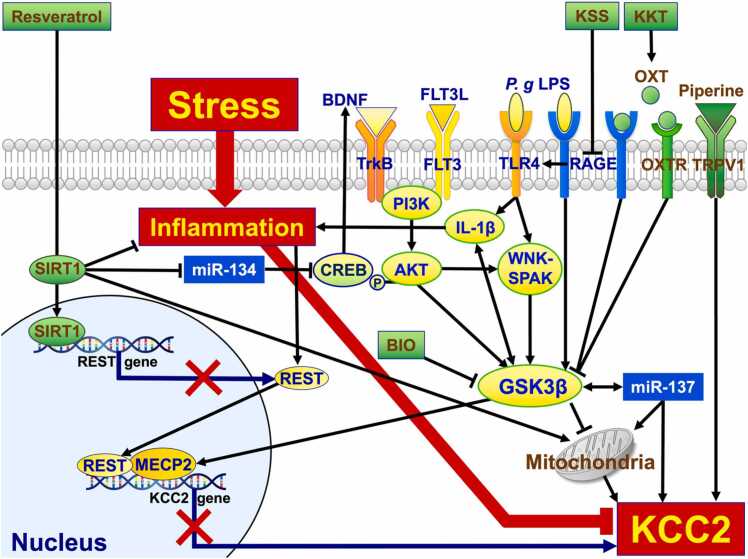
Fig. 2.
Summary of the KCC2 regulation. KCC2 is regulated by many factors. Factors that would up-regulate the KCC2 expression are presented in green, while factors that would down-regulate it are presented in yellow. Stress induces inflammation, while inflammation down-regulates KCC2. KCC2 is up-regulated when piperine binds to TRPV1, the capsaicin receptor. OXT, which is a neuropeptide, binds to both OXTR and RAGE. When OXT binds to these receptors, GSK3β is inactivated. Because GSK3β up-regulates IL-1β, OXT down-regulates inflammation and results in KCC2 downregulation. KKT is one of the Kampo medicines prescribed to patients presenting symptoms of anxiety, depression, and insomnia. KKT up-regulates OXT which results in the upregulation of KCC2. RAGE acts as a receptor not only for OXT but also for P. g LPS. When P. g LPS binds to RAGE, GSK3β is activated. RAGE activates TLR4, which also acts as the P. g LPS receptor. Moreover, TLR4 activates IL-1β and WNK-SPAK which leads to GSK3β activation. KSS inhibits RAGE, while BIO inhibits GSK3β. FLT3 and TrkB are receptor tyrosine kinases. As ligands, FLT3L binds to FLT3 and BDNF binds to TrkB. When these ligands bind to receptors, PI3K is activated. The PI3K activates AKT and up-regulates GSK3β. Akt has been reported to phosphorylate CREB and regulate BDNF. CREB is down-regulated by miR-134 post-translationally. MiR-134 is down-regulated by SIRT1, which is a histone deacetylase, and its activation is known to extend lifespan of yeast to human. SIRT1 is activated by resveratrol. Activated SIRT1 inhibits REST expression by binding to the transcriptional regulatory region of REST genes. REST translocates into the nucleus together with MECP2 via GSK3β and inhibits KCC2 expression. GSK3β inhibits mitochondrial activity, while mitochondrial dysfunction decreases KCC2 expression. Mitochondrial activity is regulated by not only GSK3β but also SIRT1 or miR-137. Please refer to the text for details. KCC2, K+-Cl- co-transporter 2; TRPV1, transient receptor potential cation channel subfamily V Member 1; OXT, oxytocin; OXTR, OXT receptor; RAGE, receptors for advanced glycation end products; GSK3β, glycogen synthase kinase 3β; IL-1β, nterleukin-1 beta; KKT, Kamikihito; P. g LPS, LPS derived from Porphyromonas gingivalis; TLR4, Toll-like receptor 4; WNK, with-no-lysine kinase; SPAK, STE20/SPS1-related proline/alanine-rich kinase; KSS, Kamishoyosan; BIO, 6-bromoindirubin-3'-oxime/ GSK-3 Inhibitor IX; FLT3, fms-like tyrosine kinase 3; FLT3L, FLT3 ligand; TrkB, tropomyosin receptor kinase B; BDNF, brain-derived neurotrophic factor; PI3K, phosphatidylinositol-3 kinase; CREB, cAMP response element binding protein; SIRT1, sirtuin 1; REST, RE1-silencing transcription factor; MECP2, methyl-CpG–binding protein 2.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Scientific field of dental Science: Dental pharmacology
Contributor Information
Kazuo Tomita, Email: ktomita@dent.kagoshima-u.ac.jp.
Tomoaki Sato, Email: tomsato@dent.kagoshima-u.ac.jp.
References
- 1.Mckee A.C., Daneshvar D.H. The neuropathology of traumatic brain injury. Handb Clin Neurol. 2015;127:45–66. doi: 10.1016/B978-0-444-52892-6.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parikh N.S., Merkler A.E., Iadecola C. Inflammation, autoimmunity, infection, and stroke: epidemiology and lessons from therapeutic intervention. Stroke. 2020;51(3):711–718. doi: 10.1161/STROKEAHA.119.024157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Ari Y., Khalilov I., Kahle K.T., Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- 4.Hübner C.A., Lorke D.E., Hermans-Borgmeyer I. Expression of the Na-K-2Cl-cotransporter NKCC1 during mouse development. Mech Dev. 2001;102:267–269. doi: 10.1016/s0925-4773(01)00309-4. [DOI] [PubMed] [Google Scholar]
- 5.Williams J.R., Sharp J.W., Kumari V.G., Wilson M., Payne J.A. The neuron-specific K-Cl cotransporter, KCC2. Antibody development and initial characterization of the protein. J Biol Chem. 1999;274(18):12656–12664. doi: 10.1074/jbc.274.18.12656. [DOI] [PubMed] [Google Scholar]
- 6.Andrews K., Josiah S.S., Zhang J. The therapeutic potential of neuronal K-Cl Co-transporter KCC2 in Huntington’s disease and its comorbidities. Int J Mol Sci. 2020;21(23):9142. doi: 10.3390/ijms21239142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang X., Drotar J., Li K., Clairmont C.D., Brumm A.S., Sullins A.J., et al. Pharmacological enhancement of KCC2 gene expression exerts therapeutic effects on human Rett syndrome neurons and Mecp2 mutant mice. Sci Transl Med. 2019;11(503) doi: 10.1126/scitranslmed.aau0164. eaau0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egawa K., Watanabe M., Shiraishi H., Sato D., Takahashi Y., Nishio S., et al. Imbalanced expression of cation-chloride cotransporters as a potential therapeutic target in an Angelman syndrome mouse model. Sci Rep. 2023;13(1):5685. doi: 10.1038/s41598-023-32376-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaenisch N., Witte O.W., Frahm C. Downregulation of potassium chloride co-transporter KCC2 after transient focal cerebral ischemia. Stroke. 2010;41(3):e151–e159. doi: 10.1161/STROKEAHA.109.570424. [DOI] [PubMed] [Google Scholar]
- 10.Cohen I., Navarro V., Clemenceau S., Baulac M., Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298(5597):1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- 11.Hyde T.M., Lipska B.K., Ali T., Mathew S.V., Law A.J., Metitiri O.E., et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31(30):11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arion D., Lewis D.A. Altered expression of regulators of the cortical chloride transporters NKCC1 and KCC2 in schizophrenia. Arch Gen Psychiatry. 2011;68(1):21–31. doi: 10.1001/archgenpsychiatry.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watanabe T., Kuroda M., Kuwabara H., Aoki Y., Iwashiro N., Tatsunobu N., et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138(pt11):3400–3412. doi: 10.1093/brain/awv249. [DOI] [PubMed] [Google Scholar]
- 14.Yamasue H., Okada T., Munesue T., Kuroda M., Fujioka T., Uno Y., et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Mol Psychiatry. 2020;25(8):1849–1858. doi: 10.1038/s41380-018-0097-2. [DOI] [PubMed] [Google Scholar]
- 15.Tomita K., Oohara Y., Igarashi K., Kitanaka J., Kitanaka N., Tanaka K., et al. Kamishoyosan and Kamikihito Protect Against Decreased KCC2 Expression Via Neuroinflammation Induced by the P. Gingivalis Lipopolysaccharide Treatment in PC-12 Cells and Improve Behavioral Abnormalities in Male Mice. Available at SSRN: https://ssrn.com/abstract=4464749 or doi: 10.2139/ssrn.4464749. [DOI] [PMC free article] [PubMed]
- 16.Chiba S., Numakawa T., Ninomiya M., Richards M.C., Wakabayashi C., Kunugi H. Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:112–119. doi: 10.1016/j.pnpbp.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Ng F., Berk M., Dean O., Bush A.I. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11:851–876. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa M., Tsukahara T., Tomita K., Iwai H., Sonomura T., Miyawaki S., et al. Neonatal maternal separation delays the GABA excitatory-to-inhibitory functional switch by inhibiting KCC2 expression. Biochem Biophys Res Commun. 2017;493:1243–1249. doi: 10.1016/j.bbrc.2017.09.143. [DOI] [PubMed] [Google Scholar]
- 19.Fourrier C., Singhal G., Baune B.T. Neuroinflammation and cognition across psychiatric conditions. CNS Spectr. 2019;24(1):4–15. doi: 10.1017/S1092852918001499. [DOI] [PubMed] [Google Scholar]
- 20.Catorce M.N., Gevorkian G. LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr Neuropharmacol. 2016;14:155–164. doi: 10.2174/1570159x14666151204122017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor J.C., Lawson M.A., André C., Moreau M., Lestage J., Castanon N., et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominy S.S., Lynch C., Ermini F., Benedyk M., Marczyk A., Konradi A., et al. Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv. 2019;5:eaau3333. doi: 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durackova Z. Some current insights into oxidative stress. Physiol Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 24.Jabs T. Reactive oxygen intermediates as mediators of programmed cell death in plants and animals. Biochem Pharm. 1999;57:231–245. doi: 10.1016/s0006-2952(98)00227-5. [DOI] [PubMed] [Google Scholar]
- 25.Poyton R.O., Ball K.A., Castello P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 26.Indo H.P., Davidson M., Yen H.-C., Suenaga S., Tomita K., Nishii T., et al. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106–118. doi: 10.1016/j.mito.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Hsu H.Y., Wen M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem. 2002;277(25):22131–22139. doi: 10.1074/jbc.M111883200. [DOI] [PubMed] [Google Scholar]
- 28.Penfield W., Rasmussen T. Macmillan; New York: 1950. The cerebral cortex of man; a clinical study of localization of function. [Google Scholar]
- 29.Poole S., Singhrao S.K., Kesavalu L., Curtis M.A., Crean S. Determining the presence of periodontopathic viru- lence factors in short-term postmortem Alzheimer’s disease brain tissue. J Alzheimers’ Dis. 2013;36:665–677. doi: 10.3233/JAD-121918. [DOI] [PubMed] [Google Scholar]
- 30.Tomita K., Yamanishi-Taira S., Igarashi K., Oogai U., Kuwahara Y., Roudkenar M.H., et al. Oxytocin ameliorates KCC2 decrease induced by oral bacteria-derived LPS that affect rat primary cultured cells and PC-12 cells. Peptides. 2022;150 doi: 10.1016/j.peptides.2021.170734. [DOI] [PubMed] [Google Scholar]
- 31.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y., Pardo M., Armini R.S., Martinez A., Mouhsine H., Zagury J.F., et al. Stress-induced neuroinflammation is mediated by GSK3-dependent TLR4 signaling that promotes susceptibility to depression-like behavior. Brain Behav Immun. 2016;53:207–222. doi: 10.1016/j.bbi.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzi D., Menna E., Canzi A., Desiato G., Mantovani C., Matteoli M. The communication between the immune and nervous systems: the role of il-1beta in synaptopathies. Front Mol Neurosci. 2018;11:111. doi: 10.3389/fnmol.2018.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amir R.E., Van den Veyver I.B., Wan M., Tran C.Q., Francke U., Zoghbi H.Y. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 35.Nance E., Kambhampati S.P., Smith E.S., Zhang Z., Zhang F., Singh S., et al. Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome. J Neuroinflamm. 2017;14:252. doi: 10.1186/s12974-017-1004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacKenzie G., Maguire J. Chronic stress shifts the GABA reversal potential in the hippocampus and increases seizure susceptibility. Epilepsy Res. 2015;109 doi: 10.1016/j.eplepsyres.2014.10.003. 13e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saint-Amant L., Drapeau P. Motoneuron activity patterns related to the earliest behavior of the zebrafish embryo. J Neurosci. 2000;20:3964–3972. doi: 10.1523/JNEUROSCI.20-11-03964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohrbough J., Spitzer N.C. Regulation of intracellular Cl– levels by Na+-dependent Cl– cotransport distinguishes depolarizing from hyperpolarizing GABAA receptor-mediated responses in spinal neurons. J Neurosci. 1996;16:82–91. doi: 10.1523/JNEUROSCI.16-01-00082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu T., Trussell L.O. Mixed excitatory and inhibitory GABA-mediated transmission in chick cochlear nucleus. J Physiol. 2001;535:125–131. doi: 10.1111/j.1469-7793.2001.t01-1-00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3(9):728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 41.Jankowski M., Bissonauth V., Gao L., Gangal M., Wang D., Danalache B., et al. Anti-inflammatory effect of oxytocin in rat myocardial infarction. Basic Res Cardiol. 2010;105:205–218. doi: 10.1007/s00395-009-0076-5. [DOI] [PubMed] [Google Scholar]
- 42.Leonzino M., Busnelli M., Antonucci F., Verderio C., Mazzanti M., Chini B. The timing of the excitatory-to-inhibitory GABA switch is regulated by the oxytocin receptor via KCC2. Cell Rep. 2016;15(1):96–103. doi: 10.1016/j.celrep.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Penzes P. Pumping up the synapse. Neuron. 2007;56(6):942–944. doi: 10.1016/j.neuron.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Kishino T., Lalande M., Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 45.Ueno T., Okabe A., Akaike N., Fukuda A., Nabekura J. Diversity of neuron-specific K+-Cl- cotransporter expression and inhibitory postsynaptic potential depression in rat motoneurons. J Biol Chem. 2002;277(7):4945–4950. doi: 10.1074/jbc.M109439200. [DOI] [PubMed] [Google Scholar]
- 46.Lysenko L.V., Kim J., Madamba F., Tyrtyshnaia A.A., Ruparelia A., Kleschevnikov A.M. Developmental excitatory-to-inhibitory GABA polarity switch is delayed in Ts65Dn mice, a genetic model of Down syndrome. Neurobiol Dis. 2018;115:1–8. doi: 10.1016/j.nbd.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Dargaei Z., Bang J.Y., Mahadevan V., Khademullah C.S., Bedard S., Parfitt G.M., et al. Restoring GABAergic inhibition rescues memory deficits in a Huntington’s disease mouse model. Proc Natl Acad Sci USA. 2018;115:E1618–E1626. doi: 10.1073/pnas.1716871115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgado C., Pinto-Ribeiro F., Tavares I. Diabetes affects the expression of GABA and potassium chloride cotransporter in the spinal cord: a study in streptozotocin diabetic rats. Neurosci Lett. 2008;438(1):102–106. doi: 10.1016/j.neulet.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 49.Boulenguez P., Liabeuf S., Bos R., Bras H., Jean-Xavier C., Brocard C., et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16(3):302–307. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 50.Coull J.A., Boudreau D., Bachand K., Prescott S.A., Nault F., Sík A., et al. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424(6951):938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 51.Tang X., Kim J., Zhou L., Wengert E., Zhang L., Wu Z., et al. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc Natl Acad Sci USA. 2016;113(3):751–756. doi: 10.1073/pnas.1524013113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levy S.E., Mandell D.S., Schultz R.T. Autism. Lancet. 2009;374(9701):1627–1638. doi: 10.1016/S0140-6736(09)61376-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hinz L., Torrella Barrufet J., Heine V.M. KCC2 expression levels are reduced in post mortem brain tissue of Rett syndrome patients. Acta Neuropathol Commun. 2019;7(1):196. doi: 10.1186/s40478-019-0852-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abuhatzira L., Makedonski K., Kaufman Y., Razin A., Shemer R. MeCP2 deficiency in the brain decreases BDNF levels by REST/CoREST-mediated repression and increases TRKB production. Epigenetics. 2007;2(4):214–222. doi: 10.4161/epi.2.4.5212. [DOI] [PubMed] [Google Scholar]
- 55.Saitoh S., Harada N., Jinno Y., Hashimoto K., Imaizumi K., Kuroki Y., et al. Molecular and clinical study of 61 Angelman syndrome patients. Am J Med Genet. 1994;52(2):158–163. doi: 10.1002/ajmg.1320520207. [DOI] [PubMed] [Google Scholar]
- 56.Williams C.A., Angelman H., Clayton-Smith J., Driscoll D.J., Hendrickson J.E., Knoll J.H., et al. Angelman syndrome: consensus for diagnostic criteria. Angel Syndr Found Am J Med Genet. 1995;56(2):237–238. doi: 10.1002/ajmg.1320560224. [DOI] [PubMed] [Google Scholar]
- 57.Tsukahara T., Masuhara M., Iwai H., Sonomura T., Sato T. Repeated stress-induced expression pattern alterations of the hippocampal chloride transporters KCC2 and NKCC1 associated with behavioral abnormalities in female mice. Biochem Biophys Res Commun. 2015;465(1):145–151. doi: 10.1016/j.bbrc.2015.07.153. [DOI] [PubMed] [Google Scholar]
- 58.de Los Heros P., Alessi D.R., Gourlay R., Campbell D.G., Deak M., Macartney T.J., et al. The WNK-regulated SPAK/OSR1 kinases directly phosphorylate and inhibit the K+ -Cl− co-transporters. Biochem J. 2014;458:559–573. doi: 10.1042/BJ20131478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Medina I., Friedel P., Rivera C., Kahle K.T., Kourdougli N., Uvarov P., et al. Current view on the functional regulation of the neuronal K(+)-Cl(-) cotransporter KCC2. Front Cell Neurosci. 2014;8:27. doi: 10.3389/fncel.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahadevan V., Khademullah C.S., Dargaei Z., Chevrier J., Uvarov P., Kwan J., et al. Native KCC2 interactome reveals PACSIN1 as a critical regulator of synaptic inhibition. eLife. 2017;6 doi: 10.7554/eLife.28270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Al Awabdh S., Donneger F., Goutierre M., Séveno M., Vigy O., Weinzettl P., et al. Gephyrin interacts with the K-Cl cotransporter KCC2 to regulate its surface expression and function in cortical neurons. J Neurosci. 2022;42(2):166–182. doi: 10.1523/JNEUROSCI.2926-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright R., Newey S.E., Ilie A., Wefelmeyer W., Raimondo J.V., Ginham R., et al. Neuronal chloride regulation via KCC2 is modulated through a GABAB receptor protein complex. J Neurosci. 2017;37(22):5447–5462. doi: 10.1523/JNEUROSCI.2164-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivera C., Li H., Thomas-Crusells J., Lahtinen H., Viitanen T., Nanobashvili A., et al. BDNF-induced TrkB activation down-regulates the K+-Cl- cotransporter KCC2 and impairs neuronal Cl- extrusion. J Cell Biol. 2002;159(5):747–752. doi: 10.1083/jcb.200209011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Delpire Advances in the development of novel compounds targeting cation-chloride cotransporter physiology. 2021;320(3):C324–C340. doi: 10.1152/ajpcell.00566.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guida N., Laudati G., Anzilotti S., Secondo A., Montuori P., Di Renzo G., et al. Resveratrol via sirtuin-1 downregulates RE1-silencing transcription factor (REST) expression preventing PCB-95-induced neuronal cell death. Toxicol Appl Pharm. 2015;288(3):387–398. doi: 10.1016/j.taap.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 66.Galanopoulou A.S., Moshé S.L. Role of sex hormones in the sexually dimorphic expression of KCC2 in rat substantia nigra. Exp Neurol. 2003;184(2):1003–1009. doi: 10.1016/S0014-4886(03)00387-X. [DOI] [PubMed] [Google Scholar]
- 67.Mi T.W., Sun X.W., Wang Z.M., Wang Y.Y., He X.C., Liu C., et al. Loss of microRNA-137 impairs the homeostasis of potassium in neurons via KCC2. Exp Neurobiol. 2020;29(2):138–149. doi: 10.5607/en19072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gagnon M., Bergeron M.J., Lavertu G., Castonguay A., Tripathy S., Bonin R.P., et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat Med. 2013;19:1524–1528. doi: 10.1038/nm.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yeo M., Berglund K., Hanna M., Guo J.U., Kittur J., Torres M.D., et al. Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci USA. 2013;110(11):4315–4320. doi: 10.1073/pnas.1300959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Payne J.A. Functional characterization of the neuronal-specific K-Cl cotransporter: implications for [K+]o regulation. Am J Physiol. 1997;273(5):C1516–C1525. doi: 10.1152/ajpcell.1997.273.5.C1516. [DOI] [PubMed] [Google Scholar]
- 71.Nishida H., Sohara E., Nomura N., Chiga M., Alessi D.R., Rai T., et al. Phosphatidylinositol 3-kinase/Akt signaling pathway activates the WNK-OSR1/SPAK-NCC phosphorylation cascade in hyperinsulinemic db/db mice. Hypertension. 2012;60(4):981–990. doi: 10.1161/HYPERTENSIONAHA.112.201509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi S. Downstream molecular pathways of FLT3 in the pathogenesis of acute myeloid leukemia: biology and therapeutic implications. J Hematol Oncol. 2011;4:13. doi: 10.1186/1756-8722-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li D.W., Liu Z.Q., Chen W., Yao M., Li G.R. Association of glycogen synthase kinase-3β with Parkinson’s disease. Mol Med Rep. 2014;9:2043–2050. doi: 10.3892/mmr.2014.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beurel E., Grieco S.F., Jope R.S. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharm Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X., Yao Z. Chronic over-nutrition and dysregulation of GSK3 in diseases. Nutr Metab. 2016:13–49. doi: 10.1186/s12986-016-0108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sato A., Shibuya H. Glycogen synthase kinase 3β functions as a positive effector in the WNK signaling pathway. PLoS One. 2018;13(3) doi: 10.1371/journal.pone.0193204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arciniegas Ruiz S.M., Eldar-Finkelman H. Glycogen synthase kinase-3 inhibitors: preclinical and clinical focus on CNS-a decade onward. Front Mol Neurosci. 2022;14 doi: 10.3389/fnmol.2021.792364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kaeberlein M., McVey M., Guarente L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 1999;13:2570–2580. doi: 10.1101/gad.13.19.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharm Sci. 2012;33(9):494–501. doi: 10.1016/j.tips.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Gao J., Wang W.Y., Mao Y.W., Gräff J., Guan J.S., Pan L., et al. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466(7310):1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 82.Turner R.S., Thomas R.G., Craft S., van Dyck C.H., Mintzer J., Reynolds B.A., et al. Alzheimer's disease cooperative study. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85(16):1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Umeda T., Sakai A., Shigemori K., Yokota A., Kumagai T., Tomiyama T. Oligomer-targeting prevention of neurodegenerative dementia by intranasal rifampicin and resveratrol combination - a preclinical study in model mice. Front Neurosci. 2021;15 doi: 10.3389/fnins.2021.763476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Serra M.P., Boi M., Carta A., Murru E., Carta G., Banni S., et al. Anti-inflammatory effect of beta-caryophyllene mediated by the involvement of TRPV1, BDNF and trkB in the rat cerebral cortex after hypoperfusion/reperfusion. Int J Mol Sci. 2022;23(7):3633. doi: 10.3390/ijms23073633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Merighi A., Salio C., Ghirri A., Lossi L., Ferrini F., Betelli C., et al. BDNF as a pain modulator. Prog Neurobiol. 2008;85(3):297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 86.Satheesh N.J., Uehara Y., Fedotova J., Pohanka M., Büsselberg D., Kruzliak P. TRPV currents and their role in the nociception and neuroplasticity. Neuropeptides. 2016;57:1–8. doi: 10.1016/j.npep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 87.Coull J.A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 88.Mapplebeck J.C.S., Lorenzo L.E., Lee K.Y., Gauthier C., Muley M.M., De Koninck Y., et al. Chloride dysregulation through downregulation of KCC2 mediates neuropathic pain in both sexes. Cell Rep. 2019;28(3):590–596. doi: 10.1016/j.celrep.2019.06.059. e4. [DOI] [PubMed] [Google Scholar]
- 89.Miranda M., Morici J.F., Zanoni M.B., Bekinschtein P. Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci. 2019;13:363. doi: 10.3389/fncel.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duman R.S., Monteggia L.M. A neurotrophic model for stress- related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Autry A.E., Monteggia L.M. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharm Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Siamilis S., Jakus J., Nyakas C., Costa A., Mihalik B., Falus A., et al. The effect of exercise and oxidant- antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47:453–457. doi: 10.1038/sc.2008.125. [DOI] [PubMed] [Google Scholar]
- 93.Markham A., Cameron I., Franklin P., Spedding M. BDNF increases rat brain mitochondrial respiratory coupling at complex I, but not complex II. Eur J Neurosci. 2004;20(5):1189–1196. doi: 10.1111/j.1460-9568.2004.03578.x. [DOI] [PubMed] [Google Scholar]
- 94.Yoshii A., Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev Neurobiol. 2010;70(5):304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jin X., Kim W.B., Kim M.N., Jung W.W., Kang H.K., Hong E.H., et al. Oestrogen inhibits salt-dependent hypertension by suppressing GABAergic excitation in magnocellular AVP neurons. Cardiovasc Res. 2021;117(10):2263–2274. doi: 10.1093/cvr/cvaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pozzi D., Rasile M., Corradini I., Matteoli M. Environmental regulation of the chloride transporter KCC2: switching inflammation off to switch the GABA on. Transl Psychiatry. 2020;10(1):349. doi: 10.1038/s41398-020-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berio E., Divari S., Starvaggi Cucuzza L., Biolatti B., Cannizzo F.T. 17β-estradiol upregulates oxytocin and the oxytocin receptor in C2C12 myotubes. PeerJ. 2017;5 doi: 10.7717/peerj.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bakos J., Srancikova A., Havranek T., Bacova Z. Molecular mechanisms of oxytocin signaling at the synaptic connection. Neural Plast. 2018;2018 doi: 10.1155/2018/4864107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon S., Kim Y.K. The role of the oxytocin system in anxiety disorders. Adv Exp Med Biol. 2020;1191:103–120. doi: 10.1007/978-981-32-9705-0_7. [DOI] [PubMed] [Google Scholar]
- 100.Wang P., Wang S.C., Yang H., Lv C., Jia S., Liu X., et al. Therapeutic potential of oxytocin in atherosclerotic cardiovascular disease: mechanisms and signaling pathways. Front Neurosci. 2019;13:454. doi: 10.3389/fnins.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- 102.Windle R.J., Shanks N., Lightman S.L., Ingram C.D. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 103.Cochran D.M., Fallon D., Hill M., Frazier J.A. The role of oxytocin in psychiatric disorders: a review of biological and therapeutic research findings. Harv Rev Psychiatry. 2013;21(5):219–247. doi: 10.1097/HRP.0b013e3182a75b7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Domes G., Heinrichs M., Michel A., Berger C., Herpertz S.C. Oxytocin improves "mind-reading" in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 105.Lischke A., Berger C., Prehn K., Heinrichs M., Herpertz S.C., Domes G. Intranasal oxytocin enhances emotion recognition from dynamic facial expressions and leaves eye-gaze unaffected. Psychoneuroendocrinology. 2012;37(4):475–481. doi: 10.1016/j.psyneuen.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 106.Marsh A.A., Yu H.H., Pine D.S., Blair R.J. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacol (Berl) 2010;209(3):225–232. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- 107.Jesso S., Morlog D., Ross S., Pell M.D., Pasternak S.H., Mitchell D.G., et al. The effects of oxytocin on social cognition and behaviour in frontotemporal dementia. Brain. 2011;134(Pt 9):2493–2501. doi: 10.1093/brain/awr171. [DOI] [PubMed] [Google Scholar]
- 108.Donadon M.F., Martin-Santos R., Osório F.L. The associations between oxytocin and trauma in humans: a systematic review. Front Pharm. 2018;9:154. doi: 10.3389/fphar.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Igarashi K., Iwai H., Tanaka K.I., Kuwahara Y., Kitanaka J., Kitanaka N., et al. Neuroprotective effect of oxytocin on cognitive dysfunction, DNA damage, and intracellular chloride disturbance in young mice after cranial irradiation. Biochem Biophys Res Commun. 2022;612:1–7. doi: 10.1016/j.bbrc.2022.04.099. [DOI] [PubMed] [Google Scholar]
- 110.Igarashi K., Kuchiiwa T., Kuchiiwa S., Iwai H., Tomita K., Sato T. Kamishoyosan (a Japanese traditional herbal formula), which effectively reduces the aggressive biting behavior of male and female mice, and potential regulation through increase of Tph1, Tph2, and Esr2 mRNA levels. Brain Res. 2021;1768 doi: 10.1016/j.brainres.2021.147580. [DOI] [PubMed] [Google Scholar]
- 111.Lee J.Y., Oh H.K., Ryu H.S., Yoon S.S., Eo W., Yoon S.W. Efficacy and safety of the traditional herbal medicine, Gamiguibi-tang, in patients with cancer-related sleep disturbance: a prospective, randomized, wait-list-controlled, pilot study. Integr Cancer Ther. 2018;17(2):524–530. doi: 10.1177/1534735417734914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsukada M., Ikemoto H., Lee X.P., Takaki T., Tsuchiya N., Mizuno K., et al. Kamikihito, a traditional Japanese Kampo medicine, increases the secretion of oxytocin in rats with acute stress. J Ethnopharmacol. 2021;276 doi: 10.1016/j.jep.2021.114218. [DOI] [PubMed] [Google Scholar]
- 113.Ripke S., O'Dushlaine C., Chambert K., Moran J.L., Kähler A.K., Akterin S., et al. Genome-wide association analysis identi- fies 13 new risk loci for schizophrenia. Nat Genet. 2013;45(10):1150–1159. doi: 10.1038/ng.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duan J., Shi J., Fiorentino A., Leites C., Chen X., Moy W., et al. A rare functional noncoding variant at the GWAS-implicated MIR137/MIR2682 locus might confer risk to schizophrenia and bipolar disorder. Am J Hum Genet. 2014;95(6):744–753. doi: 10.1016/j.ajhg.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pinto D., Delaby E., Merico D., Barbosa M., Merikangas A., Klei L., et al. Convergence of genes and cellular pathways dysregulated in autism spectrum disorders. Am J Hum Genet. 2014;94(5):677–694. doi: 10.1016/j.ajhg.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thomas K.T., Anderson B.R., Shah N., Zimmer S.E., Hawkins D., Valdez A.N., et al. Inhibition of the schizophrenia-associated MicroRNA miR-137 disrupts Nrg1α neurodevelopmental signal transduction. Cell Rep. 2017;20(1):1–12. doi: 10.1016/j.celrep.2017.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li W., Zhang X., Zhuang H., Chen H.G., Chen Y., Tian W., et al. MicroRNA-137 is a novel hypoxia-responsive microRNA that inhibits mitophagy via regulation of two mitophagy receptors FUNDC1 and NIX. J Biol Chem. 2014;289(15):10691–10701. doi: 10.1074/jbc.M113.537050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Channakkar A.S., Singh T., Pattnaik B., Gupta K., Seth P., Adlakha Y.K. MiRNA-137-mediated modulation of mitochondrial dynamics regulates human neural stem cell fate. Stem Cells. 2020;38(5):683–697. doi: 10.1002/stem.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bai Y., Bai Y., Wang S., Wu F., Wang D.H., Chen J., et al. Targeted upregulation of uncoupling protein 2 within the basal ganglia output structure ameliorates dyskinesia after severe liver failure. Free Radic Biol Med. 2018;124:40–50. doi: 10.1016/j.freeradbiomed.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 120.Fisler J., Warden C.H. Uncoupling proteins, dietary fat and the metabolic syndrome. Nutr Metab. 2006;38:3. doi: 10.1186/1743-7075-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Matsuo K., Yabuki Y., Fukunaga K. 5-aminolevulinic acid inhibits oxidative stress and ameliorates autistic-like behaviors in prenatal valproic acid-exposed rats. Neuropharmacology. 2020;168 doi: 10.1016/j.neuropharm.2020.107975. [DOI] [PubMed] [Google Scholar]
- 122.Marchi S., Guilbaud E., Tait S.W.G., Yamazaki T., Galluzzi L. Mitochondrial control of inflammation. Nat Rev Immunol. 2023;23(3):159–173. doi: 10.1038/s41577-022-00760-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cryan J.F., O'Riordan K.J., Cowan C.S.M., Sandhu K.V., Bastiaanssen T.F.S., Boehme M., et al. The microbiota-gut-brain axis. Physiol Rev. 2019;99(4):1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 124.Masuzawa A., Black K.M., Pacak C.A., Ericsson M., Barnett R.J., Drumm C., et al. Transplantation of autologously derived mitochondria protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2013;304(7):H966–H982. doi: 10.1152/ajpheart.00883.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]