Abstract
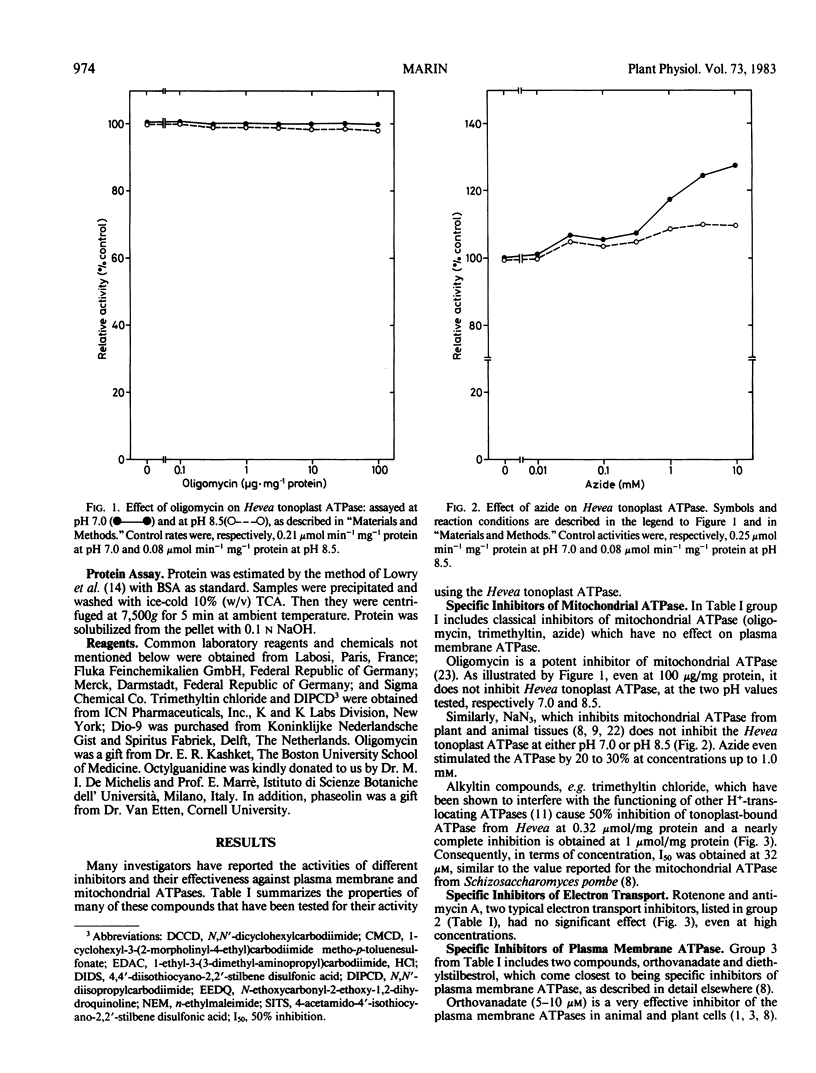
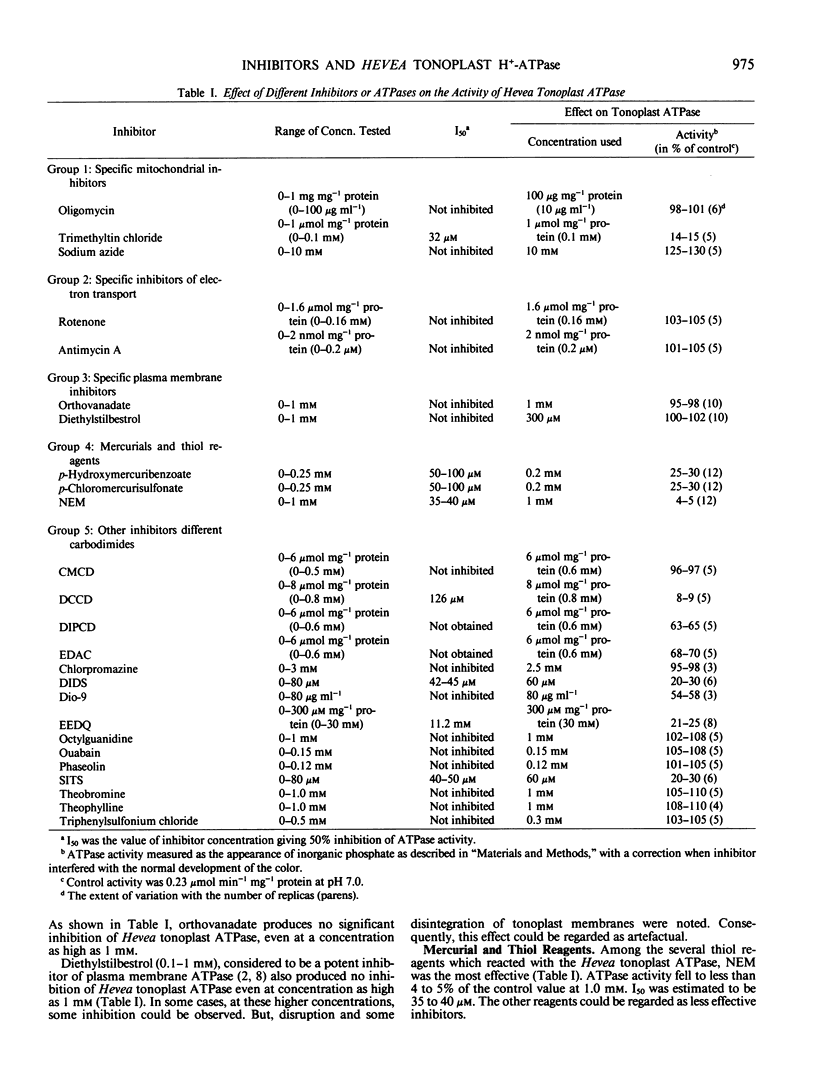
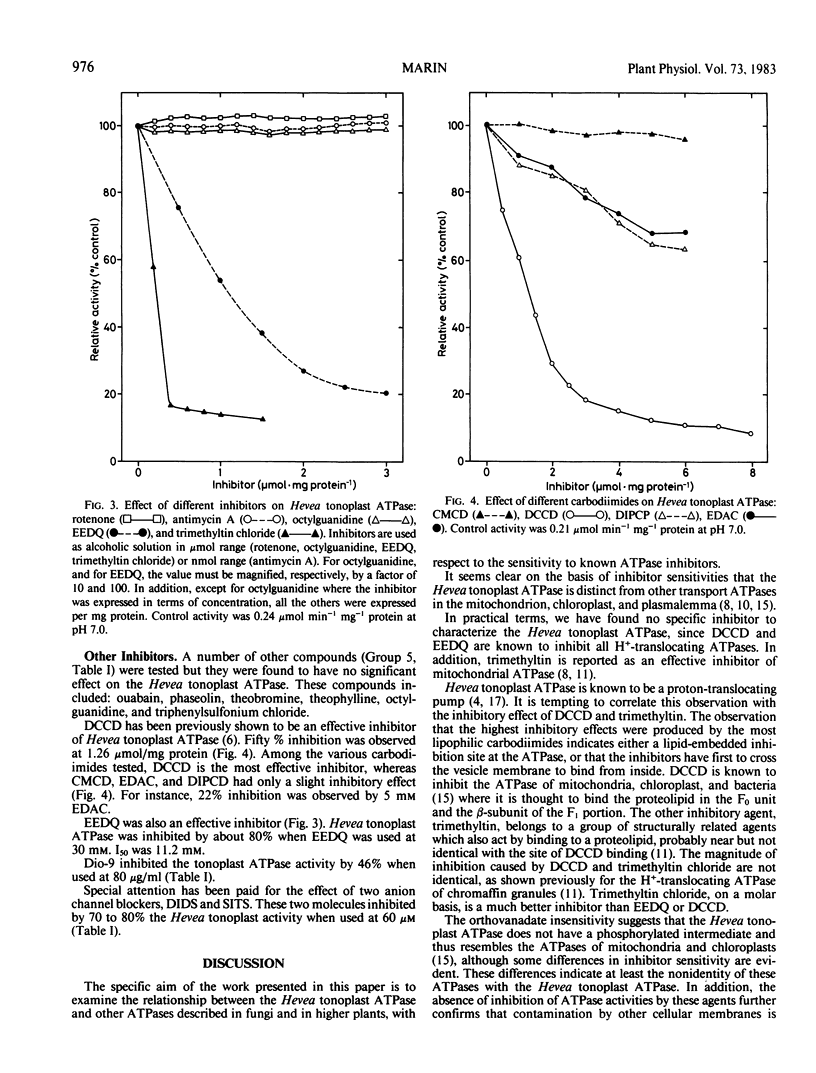
The tonoplast-bound H+-translocating ATPase from Hevea latex was found to be insensitive to vanadate, diethylstilbestrol, and octylguanidine, which are specific inhibitors of the plasma membrane ATPase. The inhibitors of the mitochondrial ATPase, oligomycin and azide, and also rotenone and antimycin A, were all without effect. In contrast, trimethyltin chloride strongly inhibited the activity of Hevea tonoplast ATPase.
Among the different carbodiimides tested, which strongly inhibit the Hevea tonoplast ATPase, N,N′-dicyclohexylcarbodiimide was the most inhibitory. N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline was also an efficient inhibitor.
This unique inhibitor sensitivity of the Hevea tonoplast H+-translocating ATPase suggests that this enzyme differs in its mode of operation from all other known H+-translocating ATPases.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balke N. E., Hodges T. K. Inhibition of adenosine triphosphatase activity of the plasma membrane fraction of oat roots by diethylstilbestrol. Plant Physiol. 1979 Jan;63(1):48–52. doi: 10.1104/pp.63.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Josephson L., Warner R., Yanagisawa M., Lechene C., Guidotti G. Vanadate is a potent (Na,K)-ATPase inhibitor found in ATP derived from muscle. J Biol Chem. 1977 Nov 10;252(21):7421–7423. [PubMed] [Google Scholar]
- Cretin H. The proton gradient across the vacuo-lysosomal membrane of lutoids from the latex of Hevea brasiliensis. I. Further evidence for a proton-translocating ATPase on the vacuo-lysosomal membrane of intact lutoids. J Membr Biol. 1982;65(3):175–184. doi: 10.1007/BF01869961. [DOI] [PubMed] [Google Scholar]
- Goffeau A., Slayman C. W. The proton-translocating ATPase of the fungal plasma membrane. Biochim Biophys Acta. 1981 Dec 30;639(3-4):197–223. doi: 10.1016/0304-4173(81)90010-0. [DOI] [PubMed] [Google Scholar]
- Grubmeyer C., Spencer M. ATPase Activity of Pea Cotyledon Submitochondrial Particles: ACTIVATION, SUBSTRATE SPECIFICITY, AND ANION EFFECTS. Plant Physiol. 1980 Feb;65(2):281–285. doi: 10.1104/pp.65.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. G., Beers M. F., Scarpa A. H+ ATPase of chromaffin granules. Kinetics, regulation, and stoichiometry. J Biol Chem. 1982 Sep 25;257(18):10701–10707. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin W., Wagner G. J., Siegelman H. W., Hind G. Membrane-bound ATPase of intact vacuoles and tonoplasts isolated from mature plant tissue. Biochim Biophys Acta. 1977 Feb 14;465(1):110–117. doi: 10.1016/0005-2736(77)90359-5. [DOI] [PubMed] [Google Scholar]
- Maloney P. C. Energy coupling to ATP synthesis by the proton-translocating ATPase. J Membr Biol. 1982;67(1):1–12. doi: 10.1007/BF01868643. [DOI] [PubMed] [Google Scholar]
- Marin B., Marin-Lanza M., Komor E. The protonmotive potential difference across the vacuo-lysosomal membrane of Hevea brasiliensis (rubber tree) and its modification by a membrane-bound adenosine triphosphatase. Biochem J. 1981 Aug 15;198(2):365–372. doi: 10.1042/bj1980365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okorokov L. A., Kulakovskaya T. V., Kulaev I. S. Solubilization and partial purification of vacuolar ATPase of yeast Saccharomyces carlsbergensis. FEBS Lett. 1982 Aug 16;145(1):160–162. doi: 10.1016/0014-5793(82)81227-1. [DOI] [PubMed] [Google Scholar]
- Senior A. E., Fayle D. R., Downie J. A., Gibson F., Cox G. B. Properties of membranes from mutant strains of Escherichia coli in which the beta-subunit of the adenosine triphosphatase is abnormal. Biochem J. 1979 Apr 15;180(1):111–118. doi: 10.1042/bj1800111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]


