Abstract
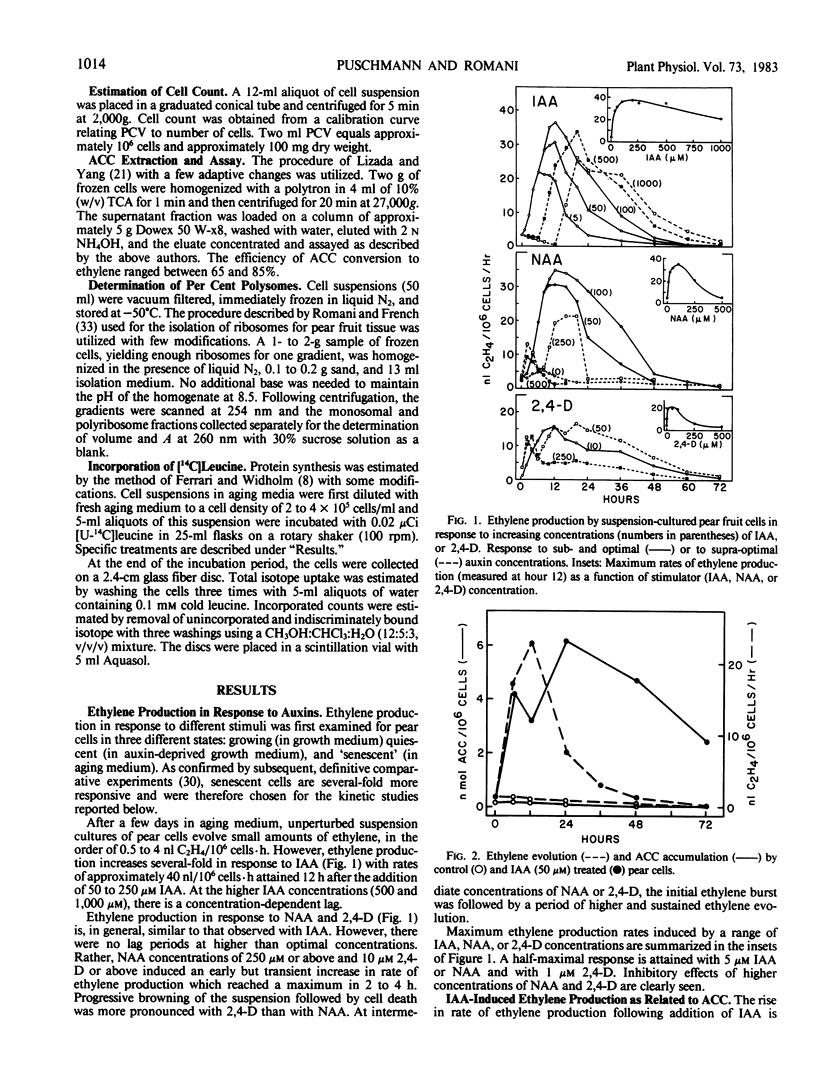
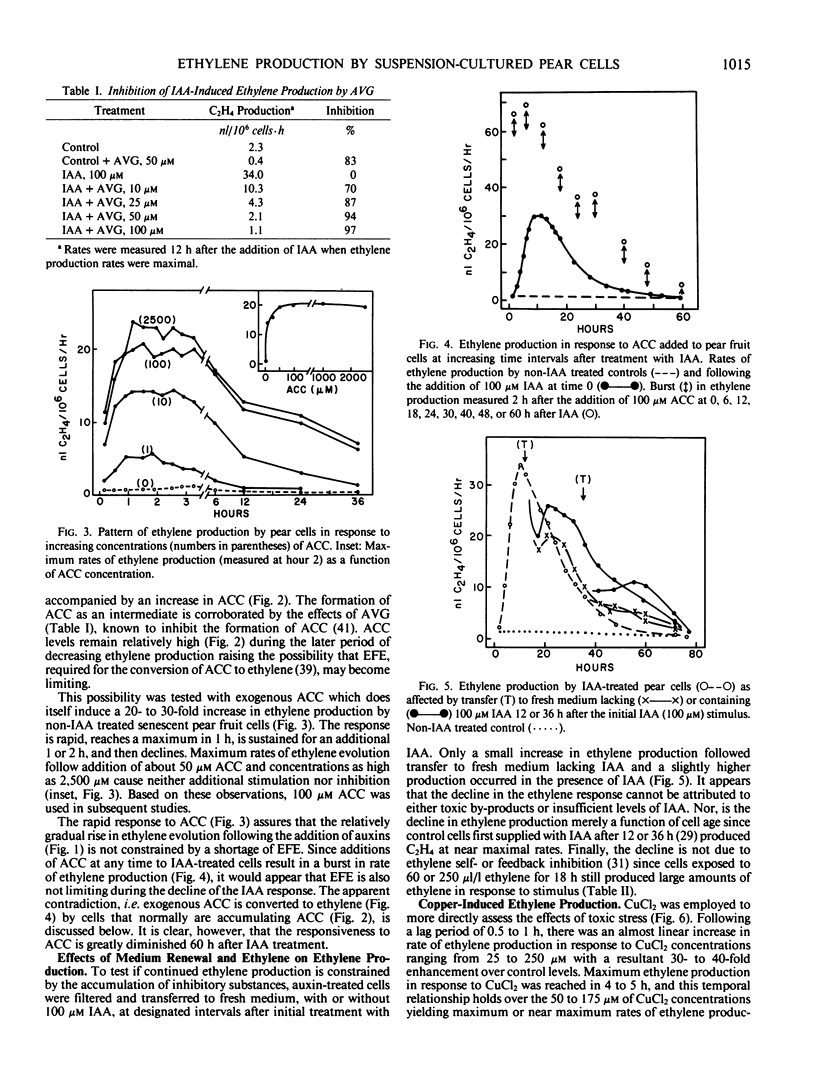
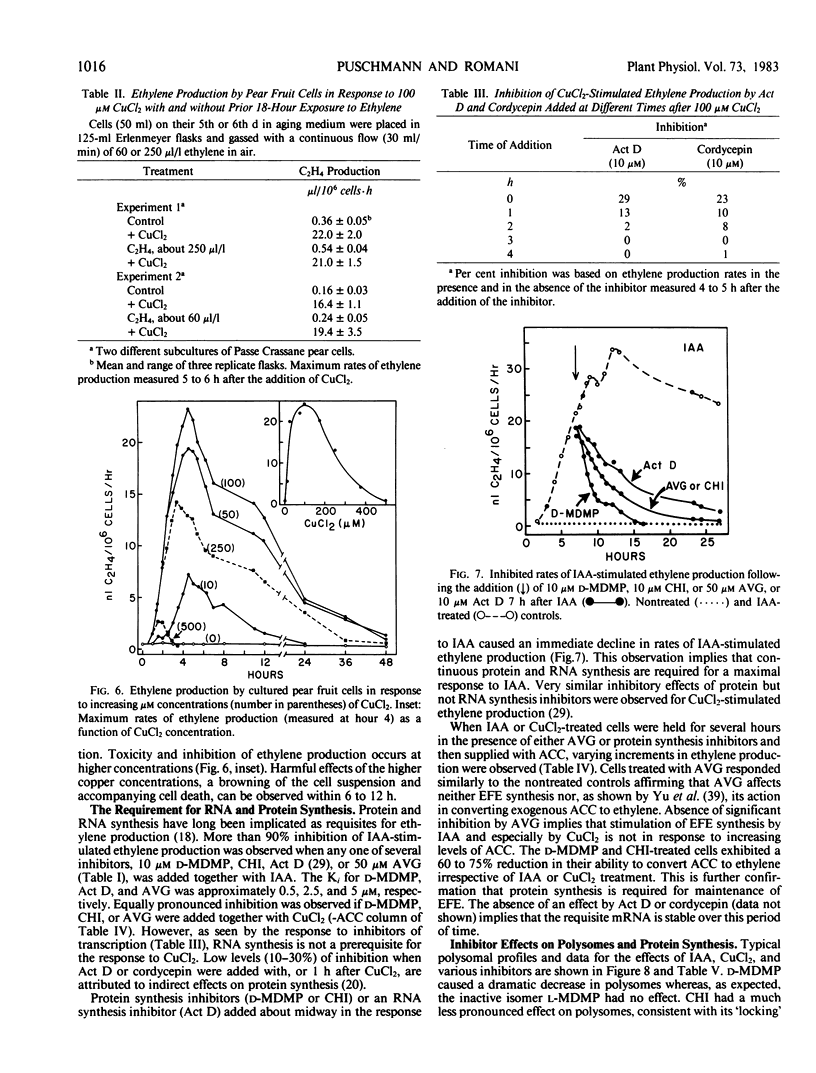
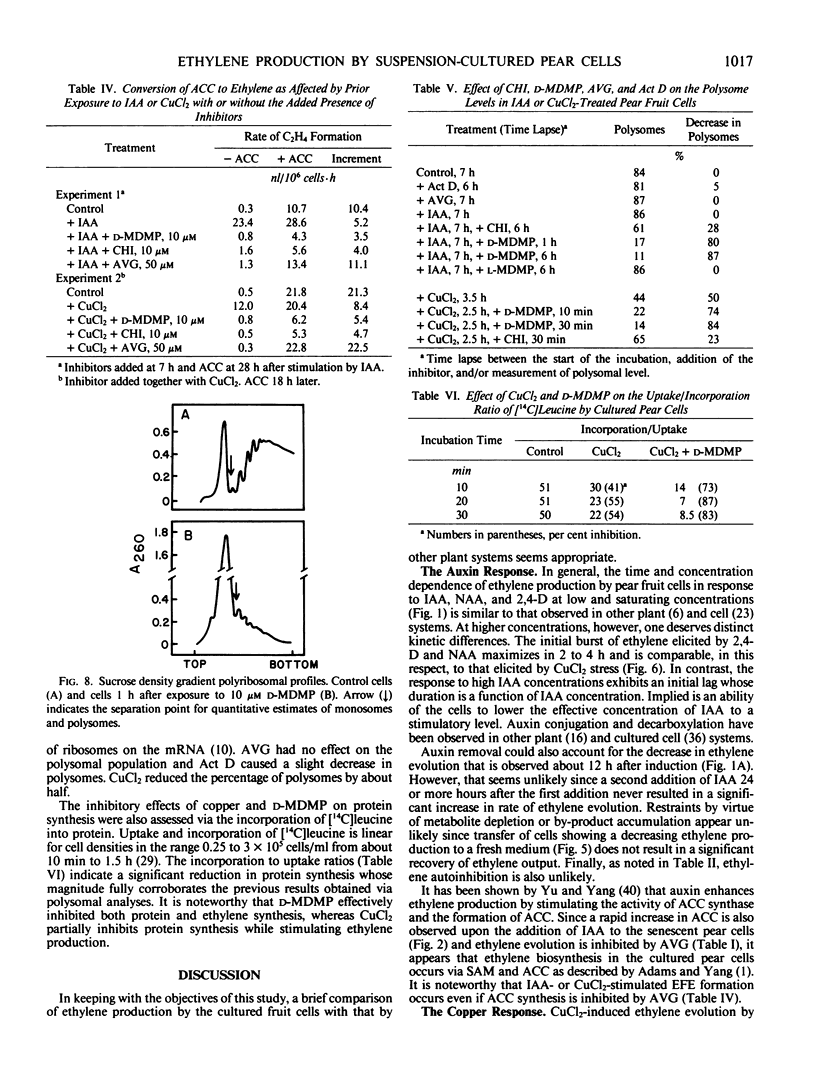
Auxin-deprived, mannitol-supplemented, suspension-cultured pear (Pyrus communis L. Passe Crassane) fruit cells produce large quantities (20-40 nanoliters ethylene per 106 cells per hour) of ethylene in response to auxins, CuCl2 or 1-amino-cyclopropane-1-carboxylic acid (ACC). Maximum rates of production are achieved about 12 hours after the addition of optimal amounts of indoleacetic acid (IAA), naphthalene acetic acid (NAA), 2,4-dichlorophenoxyacetic acid (2,4-D), 4 to 5 hours after the addition of CuCl2 and 1 to 2 hours after the addition of ACC. Supraoptimal concentrations of IAA result in a lag phase followed by a normal response. High concentrations of NAA and 2,4-D result in an early (4-5 hours) stress response and injury.
Continuous protein and RNA synthesis are essential for elaboration of the full IAA response; only protein synthesis is necessary for the response to CuCl2 and ACC. Based on polysomal states and rates of amino acid incorporation, CuCl2 partially inhibits protein synthesis while nonetheless stimulating ethylene production. In general, ethylene production by the pear cells resembles that of other plant systems. Some differences may reflect the sensitivity of the cells and are discussed. The relatively high levels of ethylene produced and the experimental convenience of the cultured cells should make them especially suitable for further investigations of ethylene production and physiology.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apelbaum A., Yang S. F. Biosynthesis of stress ethylene induced by water deficit. Plant Physiol. 1981 Sep;68(3):594–596. doi: 10.1104/pp.68.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford K. J., Yang S. F. Stress-induced Ethylene Production in the Ethylene-requiring Tomato Mutant Diageotropica. Plant Physiol. 1980 Feb;65(2):327–330. doi: 10.1104/pp.65.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Ethylene formation in pea seedlings; its relation to the inhibition of bud growth caused by indole-3-acetic Acid. Plant Physiol. 1968 Jul;43(7):1069–1074. doi: 10.1104/pp.43.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Widholm J. M. A simple, rapid, and sensitive method for estimation of DNA, RNA, and protein synthesis in carrot cell cultures. Anal Biochem. 1973 Dec;56(2):346–352. doi: 10.1016/0003-2697(73)90200-5. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Adamson S. D., Herbert E. Effects of cycloheximide on polyribosome function in reticulocytes. J Mol Biol. 1967 Jul 14;27(1):57–72. doi: 10.1016/0022-2836(67)90351-8. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Biosynthesis of wound ethylene in morning-glory flower tissue. Plant Physiol. 1976 Apr;57(4):538–541. doi: 10.1104/pp.57.4.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyodo H., Nishino T. Wound-induced Ethylene Formation in Albedo Tissue of Citrus Fruit. Plant Physiol. 1981 Mar;67(3):421–423. doi: 10.1104/pp.67.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue T. A., Gamborg O. L. Ethylene Production by Plant Cell Cultures: Variations in Production during Growing Cycle and in Different Plant Species. Plant Physiol. 1971 Oct;48(4):394–398. doi: 10.1104/pp.48.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Mechanism of a Synergistic Effect of Kinetin on Auxin-induced Ethylene Production: Suppression of Auxin Conjugation. Plant Physiol. 1973 Jun;51(6):1011–1014. doi: 10.1104/pp.51.6.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau O. L., Yang S. F. Stimulation of ethylene production in the mung bean hypocotyls by cupric ion, calcium ion, and kinetin. Plant Physiol. 1976 Jan;57(1):88–92. doi: 10.1104/pp.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Wang S. Y., Owens L. D. Ethylene production by callus and suspension cells from cortex tissue of postclimacteric apples. Plant Physiol. 1979 May;63(5):811–815. doi: 10.1104/pp.63.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- McGlasson W. B., Pratt H. K. Effects of Wounding on Respiration and Ethylene Production by Cantaloupe Fruit Tissue. Plant Physiol. 1964 Jan;39(1):128–132. doi: 10.1104/pp.39.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech J. C., Romani R. J. Senescence of Pear Fruit Cells Cultured in a Continuously Renewed, Auxin-deprived Medium. Plant Physiol. 1979 Nov;64(5):814–817. doi: 10.1104/pp.64.5.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riov J., Yang S. F. Effects of exogenous ethylene on ethylene production in citrus leaf tissue. Plant Physiol. 1982 Jul;70(1):136–141. doi: 10.1104/pp.70.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R. J., Bos T. J., Pech J. C. Cycloheximide stimulation of cyanide-resistant respiration in suspension cultures of senescent pear fruit cells. Plant Physiol. 1981 Oct;68(4):823–826. doi: 10.1104/pp.68.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romani R., French K. Temperature-dependent Changes in the Polysomal Population of Senescent (Ripening) Pear Fruit. Plant Physiol. 1977 Dec;60(6):930–932. doi: 10.1104/pp.60.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltveit M. E., Dilley D. R. Rapidly Induced Wound Ethylene from Excised Segments of Etiolated Pisum sativum L., cv. Alaska: I. Characterization of the Response. Plant Physiol. 1978 Mar;61(3):447–450. doi: 10.1104/pp.61.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. A., Reider M. L., Fletcher J. S. Relationship between Vital Staining and Subculture Growth during the Senescence of Plant Tissue Cultures. Plant Physiol. 1982 Oct;70(4):1228–1230. doi: 10.1104/pp.70.4.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle J. C., Kende H. Ethylene and senescence in petals of tradescantia. Plant Physiol. 1978 Aug;62(2):267–271. doi: 10.1104/pp.62.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. Regulation of Auxin-induced Ethylene Production in Mung Bean Hypocotyls: Role of 1-Aminocyclopropane-1-Carboxylic Acid. Plant Physiol. 1979 Mar;63(3):589–590. doi: 10.1104/pp.63.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]


