ABSTRACT
Background
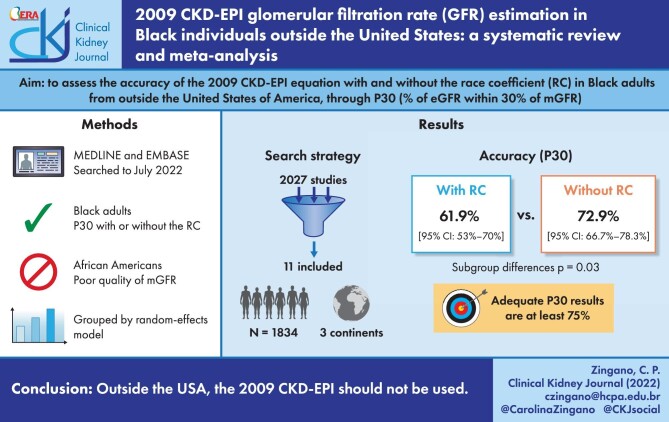
The 2009 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation is the most used equation to estimate glomerular filtration rate (GFR), with race being a factor thereof, increasing GFR by 16% in self-identified Black persons compared with non-Black persons. However, recent publications indicate that it might overestimate GFR for Black adults outside the USA. In this meta-analysis, we assessed the accuracy, evaluated by the percentage of estimated GFR within 30% of measured GFR (P30), of the 2009 CKD-EPI equation in estimating GFR with and without the race coefficient in Black individuals outside the United States of America (USA).
Methods
We searched MEDLINE and Embase from inception to 9 July 2022, with no language restriction, supplemented by manual reference searches. Studies that assessed the CKD-EPI P30 accuracy with or without the race coefficient in Black adults outside the USA with an adequate method of GFR measurement were included. Data were extracted by independent pairs of reviewers and were pooled using a random-effects model.
Results
We included 11 studies, with a total of 1834 Black adults from South America, Africa and Europe. The race coefficient in the 2009 CKD-EPI equation significantly decreased P30 accuracy {61.9% [95% confidence interval (CI) 53–70%] versus 72.9% [95% CI 66.7–78.3%]; P = .03}.
Conclusions
Outside the USA, the 2009 CKD-EPI equation should not be used with the race coefficient, even though the 2009 CKD-EPI equation is not sufficiently accurate either way (<75%). Thus we endorse the Kidney Disease: Improving Global Outcomes guidelines to use exogenous filtration markers when this may impact clinical conduct.
Keywords: 2009 CKD-EPI, Black people, creatinine, glomerular filtration rate, race coefficient
Graphical Abstract
Graphical Abstract.
INTRODUCTION
The glomerular filtration rate (GFR) is usually estimated in clinical practice by using serum creatinine values in validated equations, such as the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) [1], Modification of Diet in Renal Disease (MDRD) [2] and European Kidney Function Consortium (EKFC) [3] equations. Among these equations, the MDRD and 2009 CKD-EPI take into consideration race as a noteworthy coefficient, increasing the estimated GFR (eGFR) for Black people in roughly 21% and 16%, respectively [1, 2]. Accuracy values measured by the percentage of eGFR within 30% (P30) of measured GFR (mGFR) by a reference method that are >90% are ideally expected from these equations, although levels >75% are considered suitable [4].
The race coefficient (RC) was introduced into the CKD-EPI and MDRD equations because serum creatinine was higher in Black Americans than White adults for the same GFR level [2]. The reason for this higher serum creatinine in Black Americans remains unknown and debated [5–7].
The 2009 CKD-EPI equation was developed and mostly validated for the Black American population (among a total of 2969 Black individuals, roughly 3% were from Europe) [1]. Recently, in the USA, the use of the RC has been largely criticized as a potential source of discrimination [8–13]. For this reason, a new race-free equation has been proposed [14], yet the bias observed with this new equation in African Americans is not better than with the 2009 CKD-EPI equation (−3.7 to 3.6 ml/min/1.73 m2) and is slightly worse in White people (−0.5 to −3.9 ml/min/1.73 m2) [14]. Nonetheless, the absolute bias was similar between both populations. This new 2021 CKD-EPI equation was also mainly developed in the USA and is yet to be tested outside the USA.
Since Black individuals from other countries were not part of the development of the RC, the much-debated issue with the 2009 race-based equation in the USA is also present abroad. This is an important observation because it is also important to evaluate the recently developed 2021 CKD-EPI creatinine-based equation outside the USA. It was suggested that the 2009 CKD-EPI would provide more accurate GFR estimations by eliminating the RC [15–19], yet the studies were small. Hence a sounder answer requires compilation of all available data through meta-analysis. Thus we need to adequately evaluate the RC in this population before dismissing it by using the 2021 equation, given that this population has not been part of the development of either equation. In addition, removing the 1.159 RC from the 2009 CKD-EPI equation greatly impacts clinical conduct and alters the prevalence of CKD [20–22], which calls for careful consideration. Bearing in mind these major clinical implications, we conducted a systematic review and meta-analysis to assess the accuracy of the CKD-EPI equation with and without the RC in Black individuals from outside the USA.
MATERIALS AND METHODS
This article is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) [23] statement and was registered in the International Prospective Register of Systematic Reviews (identification number CRD42021236613).
Data sources and searches
We included observational studies that tested the performance of the 2009 CKD-EPI equation, measured by P30, in estimating GFR in Black individuals from outside the USA with or without the RC. We considered participants as Black according to the definition of each included study. Only studies that measured GFR by a gold standard method such as plasma clearance of iohexol, technetium-99m diethylene triamine pentaacetic acid (99mTc-DTPA) or chromium-51 ethylenediaminetetraacetic acid (51Cr-EDTA) or urinary clearance of iothalamate or 51Cr-EDTA were included. No restrictions were placed on language or date of publication.
We searched MEDLINE (through PubMed) and Embase from inception through 9 July 2021 using a combination of MeSH or EmTree terms that are detailed in the supplementary material. Additionally, we reviewed reference lists of the selected articles and searched all papers co-cited at least two times by using the CoCites tool [24, 25] to achieve a thorough search.
Study selection
Two pairs of researchers (C.P.Z. and B.M.R.; G.M.E. and I.F.P.) independently screened titles and abstracts of retrieved records on Endnote software (version 20; Clarivate Analytics, Philadelphia, PA, USA), after removal of duplicates, to exclude studies that clearly did not meet the inclusion criteria. In case of divergence, discussions were held to achieve consensus in periodic group meetings.
Data extraction and quality assessment
One pair of reviewers (C.P.Z. and B.M.R.) independently extracted data from the included studies using a predefined standardized spreadsheet, resolving divergences in a similar fashion to the selection phase of the study. Information retrieved included the following: digital object identifier (DOI) of the main publication, author name, year, country, sample size, age, race, sex distribution, mean serum creatinine values, mean mGFR, gold standard method used for GFR measurement, P30, mean bias and mean eGFR. We used published articles, supplements and additional data provided by the corresponding authors of the selected studies by e-mail as sources for this meta-analysis.
The risk of bias and the quality of the studies were assessed by the Quality of Diagnostic Studies-2 (QUADAS-2) tool [26].
Data synthesis and analysis
The statistical analyses were performed and described in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [27]. General linear mixed models were applied to calculate the overall proportion of the single proportion (P30) reported and to calculate the mean difference of mean mGFR and creatinine extracted from the studies using the free version of R version 3.6 and R Studio version 1.2 for MacOS (R Foundation for Statistical Computing, Vienna, Austria), with the package ‘meta’ and random-effects model.
Eggers's analysis and funnel plots were used to assess publication bias. I2 and the Cochran Q statistic were used to evaluate the heterogeneity. Mixed models of meta-regression were stepwise built and applied to evaluate the influence of the location, mean mGFR, gold standard method used for GFR measurement, age, creatinine level and prevalence of diabetic individuals in the obtained results. The assumptions of mixed models were tested with bubble plots for linearity, the QQnorm test for the normality of the residues and residual scatter plot for homoscedasticity, all of which showed the model was appropriate.
A statistically significant heterogeneity was considered for P < .05 or I2 > 50% were obtained. P-values <.05 in our meta-analysis were considered statistically significant.
RESULTS
Study selection
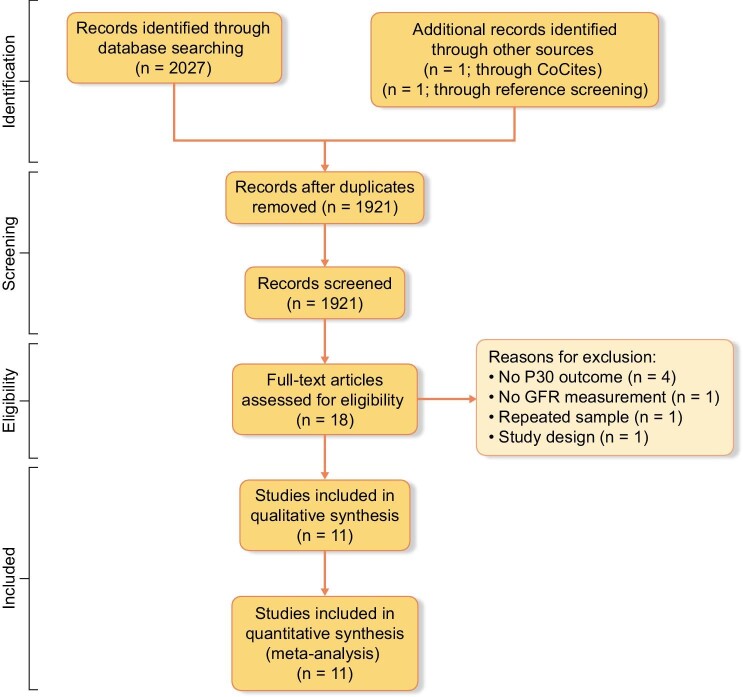
The research strategy identified 2027 publications, of which 1921 remained after removal of duplicates. One study was identified in the references of included articles and one study was identified through CoCites. We excluded 1903 studies after title and abstract screening, thus 18 articles remained for full-text evaluation. Most articles were excluded in this phase because they evaluated different outcomes. Eleven articles were selected for this systematic review, comprising 1834 Black adults. A flow diagram of the study screening and selection is presented in Fig. 1.
Figure 1:
PRISMA flow diagram of studies.
Study characteristics
The selected articles were all observational studies published from 2010 to 2021 in multiple countries that tested the 2009 CKD-EPI equation's estimating performance in Black adults, with or without the RC, by comparison with a reference method via P30. Race was self-reported in five studies [15, 16, 18, 19, 28, 29] and one defined race according to the research team's assignment [30]. Six studies were conducted in Africa [15, 16, 18, 19, 28, 29]—four in South Africa, one in Kenya and one in Congo and Ivory Coast—two in Europe [17, 31], two in Brazil [30, 32] and one in multiple countries [33]. The latter, conducted by Stevens et al. [33], was subdivided according to the origin of the population (namely, USA and Europe or South Africa). We only included data from the South African population in this review (Table 1).
Table 1:
General characteristics of the included studies.
| Reference | Population | Reference method | Participants, n | Age (years), median (range) or mean (SD) |
BMI (kg/m2), median (range) or mean (SD) |
DM, n (%) |
Women, n (%) | mGFR (ml/min/1.73 m2), median (range) or mean (SD) |
Creatinine (mg/dl), median (range) or mean (SD) |
|---|---|---|---|---|---|---|---|---|---|
| Bukabau et al., 2019 [19] | Congo and Ivory Coast | Iohexol PC | 494 | 38 (30–53) | 24 (21–28) | 0 (0) | 229 (46) | 88 (74–100) | 0.9 (0.8–1.2) |
| Flamant et al., 2013 [31] | Europe | 51Cr-EDTA UC | 302 | 48 (SD 14) | 26 (SD 4.7) | NA | 92 (30) | 57.6 (SD 28.4) | 1.9 (SD 1.3) |
| Gama et al., 2021 [17] | UK | 51Cr-EDTA PC | 266 | 47 (SD 11) | 27 (SD 5.5) | NA | 125 (47) | 78.9 (SD 21.3) | 0.9 (0.7–1.1) |
| Holness et al., 2020 [16] | South Africa | 99mTc-DTPA PC | 80 | 39 (range NA) | NA | NA | 50 (63) | 59 (10–126) | 1 (0.5–7.9) |
| Moodley et al., 2018 [29] | South Africa | 99mTc-DTPA PC | 188 | 48 (SD 16) | NA | 84 (45) | 107 (57) | 66.2 (SD 38.6) | 2.1 (SD 2.8) |
| Rocha et al., 2020 [30] | Brazil | 51Cr-EDTA PC | 61 | 60 (SD 14) | NA | 18 (30) | 51 (31) | 49.8 (SD 32.2) | NA |
| Seape et al., 2016 [15] | South Africa | 51Cr-EDTA PC | 97 | 37 (SD 10) | 21 | 0 (0) | 40 (41) | 92.5 (23–165) | 0.8 (IQR 0.5) |
| Stevens et al., 2011 [33] | Multiple countriesa | Iothalamate UC | 99 | 47 (SD 17) | 26 (SD 5) | 6 (6) | 49 (49) | 61 (SD 32) | 1.8 (SD 1.7) |
| van Deventer et al., 2010 [28] | South Africa | 51Cr-EDTA PC | 100 | 47 (SD 26) | NA | 25 (25) | 49 (49) | 61.5 (3–132) | NA |
| Veronese et al., 2014 [32] | Brazil | 51Cr-EDTA PC | 48 | 53 (SD 17) | 29 (SD 5) | 26 (54) | 28 (58) | 94.2 (SD 39) | 1.1 (SD 0.9) |
| Wyatt et al., 2013 [18] | Kenya | Iohexol PC | 99 | 35 | 22 | 0 (0) | 60 (61) | 115 (102–137) | 0.7 (0.6–0.8) |
n, number of Black adults; SD, standard deviation; IQR, interquartile range; BMI, body mass index; DM, diabetes mellitus; NA, not available; PC, plasma clearance; UC, urinary clearance.
SI conversion: to convert serum creatinine level to μmol/L, multiply by 88.4.
aAll data retrieved from this study are from the subpopulation of 99 South Africans, excluding the data on Black Americans, which was also studied in this publication.
One (9%) study used urinary clearance of iothalamate as a reference method to calculate P30 [33], two (18%) used plasma clearance of iohexol [18, 19], five (45%) used plasma clearance [15, 17, 28, 30, 32], one (9%) used urinary clearance [31] of 51Cr-EDTA and two (18%) used plasma clearance of 99mTc-DTPA [16, 29]. The mean mGFR in these studies was 67 ± 32 ml/min/1.73 m2, obtained from the six studies that provided mean mGFR and standard deviation (five studies only supplied median and interquartile range values). Three (27%) studies had either mean or median mGFR values <60 ml/min/1.73 m2 [16, 30, 31]. The main characteristics of the included studies are summarized in Table 1.
CKD-EPI accuracy with and without the RC
The 2009 CKD-EPI equation calculated with the RC was significantly less accurate than without it for Black adults outside the USA {61.9% [95% confidence interval (CI) 53–70] versus 72.9% [95% CI 66.7–78.3%]; P = .03}, as shown in Fig. 2A. Even when analysing only studies that provided a P30 both with and without the RC, a significant decrease in accuracy was still found [60.8% (95% CI 50.3–70.3)–73% (95% CI 66.1–79); P = .04; k = 8], as shown in Fig. 2B. This pattern is also seen when eliminating the studies with the lowest P30 results (see Supplementary Fig. 1).
Figure 2:
(A) Mean accuracy (P30) of GFR estimation by the 2009 CKD-EPI equation with and without the RC using random-effects models. (B) Mean accuracy (P30) of GFR estimation by the 2009 CKD-EPI equation with and without the RC using random-effects models including only studies that provided both accuracy values. P30 values are considered sufficiently accurate when >75%. n, number of Black adults in each study.
We analysed whether the mGFR level had an influence on our findings. Thus we subdivided the studies by their mGFR, gathering publications with mean mGFRs above or below 60 ml/min/1.73 m2. Respectively, the values of P30 obtained with the RC were similar for both groups [60.5% (95% CI 51.1–69.2) versus 64.1% (95% CI 45.7–79.1); P = .72; k =10]. For more details, see Fig. 3.
Figure 3:
Mean accuracy (P30) of eGFR by the 2009 CKD-EPI equation with RC, subdividing studies by mGFR status. P30 values are considered sufficiently accurate when >75%. There was no statistically significant difference between the two groups (P = .84), which indicated that the different mean of the median mGFR values of each study did not influence our findings.
In addition, we performed a meta-regression analysis to explore potential sources for the high heterogeneity found in our meta-analysis (I2 = 85% without the RC and 90% with the RC; see Fig. 2A) using known variables that interfere in GFR, such as the percentage of diabetic individuals, mean mGFR, creatinine level and age in single and multiple models. The results showed that the prevalence of diabetes (R2 = 11%, P = .51), mean mGFR (R2 = 9.38%, P = .09) and age (R2 = 12.79%, P = .88) did not seem to contribute as sources of heterogeneity, although data about the prevalence of diabetes was missing in ≈25% of the studies. On the other hand, a multiple meta-regression including both significant characteristics alone, i.e. the location and the gold standard method used, found a substantial contribution to heterogeneity (R2 = 88.84%, P < .001). We then analysed if eliminating studies based on 99mTc-DTPA plasma clearance for GFR measurement (both performed in South Africa) would change the results, as it greatly contributed to the heterogeneity of our results, yet subgroup analysis still showed significant improvement by eliminating the RC [from 65.5% (95% CI 56.2–73.7)–75.6% (95% CI 71.1–79.6); P = .03; k = 9], as seen in Supplementary Fig. 2. We also tested for subgroup differences among studies subdivided by continent and found no significant differences (see Supplementary Fig. 3).
Assessment of bias
The analysis of bias using the QUADAS-2 tool showed that the data regarding the 2009 CKD-EPI equation's accuracy in Black individuals presented a low risk of bias (see Appendix Table 1 in the supplementary material). We performed a linear regression test of funnel plot asymmetry that showed no evidence of publication bias (t = 0.03, P = .980; see Appendix Fig. 4 in the supplementary material).
DISCUSSION
The major contribution of this systematic review and meta-analysis of 11 observational studies comprising 1834 Black individuals is that it is the first of its kind dedicated to questioning the accuracy of the RC of the 2009 CKD-EPI equation outside the USA. Recently a solution to the RC conundrum in the USA has been proposed through a new race-free 2021 CKD-EPI equation [14]. However, this equation barely included Black individuals from beyond the USA, as was the case for the 2009 CKD-EPI equation. Due to the smaller size of all studies that tested the 2009 equation outside the USA, no single observational study could clearly grasp this matter to settle if there was a similar (or perhaps more pronounced) issue regarding use of the RC worldwide, making this question only properly addressable through data compilation by meta-analysis. Thus in this systematic review and meta-analysis, the overall accuracy measured by P30 of the 2009 CKD-EPI equation was significantly higher when eliminating the RC [61.9% (95% CI 53–70) versus 72.9% (95% CI 66.7–78.3); P = .03]. Nonetheless, both equations failed to reach the optimal threshold of 75% [4].
It is worth noting that participants’ race was mostly classified as ‘Black’ or ‘White’ among included studies by self-reporting (which is in accordance with current recommendations [34]), with only one study [30] defining race by the study team. In addition, the P30 values may appear much less accurate than when this equation is used in the USA (95% CI values range from 77.2 to 100% with the RC and 72.8 to 96.7% without the RC among validation sets) [14]. This pattern may also be observed in the multicentric study by Stevens et al. [33], included in this review, in which the original 2009 CKD-EPI equation (i.e. the equation calculated with the RC) performs far below acceptable levels of P30 in the South African population [55.6% (95% CI 46.5–64.6)], whereas it reaches optimal overall accuracy levels within Black adults in the USA [84% (95% CI 83–86)].
Even though our search was not designed to find studies conducted on African Americans, the data we found favoured maintenance of the RC in the 2009 CKD-EPI equation [6, 14], even when race is assigned by ancestry markers or when accounting for body mass area and fat-free mass estimated by bioelectrical impedance analysis [6]. When testing for subgroup differences between African Americans and the rest of the world, we also found significant differences between groups both with and without the RC (see Supplementary Fig. 5). These findings underpin the matter addressed in this meta-analysis, since these results point towards the lack of external validation of the 2009 CKD-EPI RC. There are several possible contributors to this phenomenon, including genetic and lifestyle variations. African American ancestry is mostly from West Africa, whereas Brazilians are more mixed race, predominantly of Angolan and Mozambican roots, for instance [35]. Furthermore, there are significant cultural, social and environmental factors that might equally come into play in this matter.
It is worth noting that eliminating the RC significantly increases accuracy (measured by P30) statistically and is also clinically relevant. Indeed, in a cohort with a median follow-up of 4 years conducted in 1658 Black adults with CKD in the USA, it was estimated that keeping the RC delayed the achievement of an eGFR <20 ml/min/1.73 m2 by 1.9 years [21]. Important clinical implications of removing the RC were also seen in another observational study, with significant implications for drug-related recommendations (absolute difference of 3.5%) and eligibility for the kidney transplant waiting list (absolute difference of 0.05%) [22]. In contrast, wrongly eliminating the RC is equally harmful, to the point of excluding patients from anticancer therapies [36]. Thus the decision to include or exclude the RC has major clinical implications and should be made based on sound evidence.
These results were found to have high levels of heterogeneity. Although we cannot definitely exclude all tested factors as contributors because of the limited number of included studies, the locations and gold standard methods of each study substantially explained these results (R2 = 88.84%, P < .001). Indeed, this finding is plausible even within a single well conducted study [33], as location seems to be responsible for prominent variability (28.4% between the USA and South Africa and 54.6% between the USA and Japan). For this reason, we also conducted a subgrouped forest plot by continent (see Supplementary Fig. 3) in which we also noticed high levels in heterogeneity in Africa and Europe; all regions failed to achieve minimally acceptable accuracy levels. No subgroup difference was found between continents. Regarding the gold standard methods, there is indeed evidence suggesting that plasma clearance of 99mTc-DTPA should be avoided [37], yet there are also studies that indicate this exogenous marker may be as accurate and precise as plasma clearance of 51Cr-EDTA [38–40]. Nevertheless, when eliminating the studies that used this marker, our overall results were not altered (see Supplementary Fig. 2). These findings led us to include the studies that used this tracer in our main analyses.
It is also worth noting that the prevalence of diabetes mellitus did not seem to contribute to heterogeneity levels, suggesting that even though the 2009 CKD-EPI equation tends to underestimate GFR in people with diabetes [41, 42], this did not seem to influence our results, even though it is striking that the only study [32] that showed a tendency of favouring the RC was the one with the highest prevalence of DM (54%).
The RC first appeared in eGFR equations in the MDRD study [2], based on the finding that creatinine was higher in Black Americans compared with White Americans with the same mGFR. In this study, this was attributed to African Americans’ increased muscle mass, referencing three small studies [43–45] that did not adequately provide a substantial biological rationale for this claim. Only four studies included in this review included White people from outside the USA [17, 30–32]. We performed a meta-analysis of these studies comparing the P30 accuracy of the 2009 CKD-EPI equation (with and without the RC) and found that either way the equation performs significantly better in White people (Supplementary Fig. 6). Even though the 2009 CKD-EPI study [1] included a considerable proportion of Black people, they did not account for the confounding biases in their analysis [46]. The majority of the Black adults included had CKD and were mostly from the African American Study of Kidney Disease and Hypertension [47], which has received criticism regarding its serum creatinine calibration technique [48, 49]. In addition, it is well established that different races do not necessarily translate into genetic differences [50–52], which has anchored the current approach to eliminating race algorithms from medicine by aiming towards race-free equations in GFR estimation.
As previously hinted by several authors [7–9, 11–13, 53, 54], the best long-term solution will most likely arise from the development of a carefully considered equation that is tested in several different countries and is precise and accurate without race in the equation. Race deeply reflects social and cultural characteristics that go beyond ancestry and genetic differences. Increasing studies show, especially in mixed-race countries such as Brazil, that skin colour does not adequately reflect African ancestry [55, 56]. It is also a very unclear concept, given that race is self-assessed by the patient and can be wrongly designated by the physician. These characteristics lead to the risk of race-based algorithms not being reproducible at an individual level, resulting in bias and potential harm to a population that is already at increased risk of kidney disease.
Limitations
This systematic review and meta-analysis has some limitations. We were unable to obtain P30 without the RC in two publications [31, 33] and P30 with the RC from one publication [28], which could have provided valuable data, as the literature in this field is limited. Moreover, our study was limited by the high heterogeneity observed across studies that was not fully explained by subgroup analysis or meta-regressions. Additionally, due to the small number of included studies [27], a lack of statistical significance of tested factors in meta-regression analysis does not necessarily imply that these factors did not contribute to heterogeneity. Nevertheless, high heterogeneity is a common occurrence in meta-analyses of diagnostic tests by a combination of factors that may well be present, such as different tests or laboratory techniques used, different patient selection or clinical settings [57]. Thus the plurality of standard references to obtain mGFR is a potential limitation for our article, although this is expected when the object of study is estimating GFR. This does not seem to be compromising, since there is moderately strong evidence demonstrating that plasma clearance of iohexol and 51Cr-EDTA and urinary clearance of 51Cr-EDTA and iothalamate are accurate ways of measuring GFR and may therefore offer comparable mGFR results. However, due to the lack of mGFR standardization, possible cohort effects cannot be ruled out.
We were also unable to meta-analyse mean bias, due to the lack of available data.
CONCLUSION
In conclusion, the 2009 CKD-EPI equation calculated with the RC had a poorer performance than without the RC for Black adults outside the USA, although neither equation is sufficiently accurate and therefore the 2009 CKD-EPI equation should not be used. Moreover, these results underpin the use of race-free creatinine-based equations for the future, which should be tested in large multicentred studies. More studies are also essential to test the recently proposed race-free revised creatinine and creatinine–cystatin C–based 2021 CKD-EPI equations in countries outside the USA, as well as the recently developed EKFC equation. Hence we endorse the Kidney Disease: Improving Global Outcomes guidelines [58] and strongly recommend clinicians measure GFR by other exogenous filtration markers when a more accurate estimation of GFR can clearly impact clinical decisions in these patients.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Prof. Strogoff, Dr Wyatt and Dr Moodley for replying to our e-mails asking for missing data from their original articles, which are included in this review.
Biography
Rocha et al., 2020 [30]
Contributor Information
Carolina Pires Zingano, Graduate Program in Medical Sciences: Endocrinology, Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Diabetes and Metabolism Group, Centro de Pesquisa Clínica, Hospital de Clínicas de Porto Alegre, Porto, Alegre.
Gustavo Monteiro Escott, Graduate Program in Medical Sciences: Endocrinology, Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Diabetes and Metabolism Group, Centro de Pesquisa Clínica, Hospital de Clínicas de Porto Alegre, Porto, Alegre.
Bruna Martins Rocha, Graduate Program in Medical Sciences: Endocrinology, Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Diabetes and Metabolism Group, Centro de Pesquisa Clínica, Hospital de Clínicas de Porto Alegre, Porto, Alegre.
Indianara Franciele Porgere, Graduate Program in Medical Sciences: Endocrinology, Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Diabetes and Metabolism Group, Centro de Pesquisa Clínica, Hospital de Clínicas de Porto Alegre, Porto, Alegre.
Candice Cristine Moro, Graduate Program in Medical Sciences: Endocrinology, Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Diabetes and Metabolism Group, Centro de Pesquisa Clínica, Hospital de Clínicas de Porto Alegre, Porto, Alegre.
Pierre Delanaye, Department of Nephrology, Dialysis, Transplantation, University of Liège, CHU Sart Tilman, Liège, Belgium; Department of Nephrology-Dialysis-Apheresis, Hôpital Universitaire Carémeau, Nîmes, France.
Sandra Pinho Silveiro, Graduate Program in Medical Sciences: Endocrinology, Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; Diabetes and Metabolism Group, Centro de Pesquisa Clínica, Hospital de Clínicas de Porto Alegre, Porto, Alegre; Division of Endocrinology and Metabolism, Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil.
FUNDING
Funding was received from the Hospital de Clínicas de Porto Alegre Research Fund (FIPE-HCPA), the Division of Research of Universidade Federal do Rio Grande do Sul (PROPESQ-UFRGS), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS; process number 16/2551-0000-476-5), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Hospital de Clínicas de Porto Alegre (HCPA) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES; finance code 001).
AUTHORS’ CONTRIBUTIONS
C.P.Z. and G.M.E. planned the project and wrote the article. S.P.S. and P.D. revised and supervised this work. C.P.Z., G.M.E., B.M.R. and I.F.P. screened the data. C.P.Z. and B.M.R. extracted the data. G.M.E. was responsible for data analysis and for the forest plot figures. C.P.Z., G.M.E., S.P.S. and C.C.M. helped develop the search strategy. All authors revised the final text and made suggestions.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the articles included in the meta-analysis, within its supplementary materials or upon request to the authors.
CONFLICT OF INTEREST STATEMENT
P.D. is member of the CKJ Editorial Board. All other authors have no conflicts of interest. The opinions, results and conclusions presented in this article are those of the authors and do not reflect the position of the funding sources.
REFERENCES
- 1.Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey AS, Bosch JP, Lewis JBet al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 1999;130:461–70. 10.7326/0003-4819-130-6-199903160-00002 [DOI] [PubMed] [Google Scholar]
- 3.Pottel H, Björk J, Courbebaisse Met al. Development and validation of a modified full age spectrum creatinine-based equation to estimate glomerular filtration rate: a cross-sectional analysis of pooled data. Ann Intern Med 2021;174:183–91. 10.7326/M20-4366 [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266. https://www.ncbi.nlm.nih.gov/pubmed/11904577 [PubMed] [Google Scholar]
- 5.Eneanya ND, Boulware LE, Tsai Jet al. Health inequities and the inappropriate use of race in nephrology. Nat Rev Nephrol 2022;18:84–94. https://www.ncbi.nlm.nih.gov/pubmed/33324009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu CY, Yang W, Parikh RVet al. Race, genetic ancestry, and estimating kidney function in CKD. N Engl J Med 2021;385:1750–60. 10.1056/NEJMoa2103753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delanaye P, Pottel H, Glassock RJ.. Americentrism in estimation of glomerular filtration rate equations. Kidney Int 2022;101:856–8. 10.1016/j.kint.2022.02.022 [DOI] [PubMed] [Google Scholar]
- 8.Sehgal AR. Race and the false precision of glomerular filtration rate estimates. Ann Intern Med 2020;173:1008–9. 10.7326/M20-4951 [DOI] [PubMed] [Google Scholar]
- 9.Norris KC, Eneanya ND, Boulware LE.. Removal of race from estimates of kidney function: first, do no harm. JAMA 2021;325:135–7. https://www.ncbi.nlm.nih.gov/pubmed/33263722 [DOI] [PubMed] [Google Scholar]
- 10.Vyas DA, Eisenstein LG, Jones DS.. Hidden in plain sight—reconsidering the use of race correction in clinical algorithms. N Engl J Med 2020;383:874–82. 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 11.Grubbs V. Precision in GFR reporting: let's stop playing the race card. Clin J Am Soc Nephrol 2020;15:1201–2. 10.2215/CJN.00690120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powe NR. Black kidney function matters: use or misuse of race? JAMA 2020;324:737–8. 10.1001/jama.2020.13378 [DOI] [PubMed] [Google Scholar]
- 13.Diao JA, Inker LA, Levey ASet al. In search of a better equation—performance and equity in estimates of kidney function. N Engl J Med 2021;384:396–9. 10.1056/NEJMp2028243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inker LA, Eneanya ND, Coresh Jet al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021;385:1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seape T, Gounden V, van Deventer HEet al. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem 2016;53:58–66. 10.1177/0004563215579695 [DOI] [PubMed] [Google Scholar]
- 16.Holness JL, Bezuidenhout K, Davids MRet al. Validation of equations to estimate glomerular filtration rate in South Africans of mixed ancestry. S Afr Med J 2020;110:229–34. 10.7196/SAMJ.2020.v110i3.13995 [DOI] [PubMed] [Google Scholar]
- 17.Gama RM, Clery A, Griffiths Ket al. Estimated glomerular filtration rate equations in people of self-reported black ethnicity in the United Kingdom: inappropriate adjustment for ethnicity may lead to reduced access to care. PLoS One 2021;16:e0255869. 10.1371/journal.pone.0255869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wyatt CM, Schwartz GJ, Owino Ong'or Wet al. Estimating kidney function in HIV-infected adults in Kenya: comparison to a direct measure of glomerular filtration rate by iohexol clearance. PLoS One 2013;8:e69601. 10.1371/journal.pone.0069601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bukabau JB, Yayo E, Gnionsahé Aet al. Performance of creatinine- or cystatin C-based equations to estimate glomerular filtration rate in sub-Saharan African populations. Kidney Int 2019;95:1181–9. 10.1016/j.kint.2018.11.045 [DOI] [PubMed] [Google Scholar]
- 20.Bragg-Gresham J, Zhang X, Le Det al. Prevalence of chronic kidney disease among black individuals in the US after removal of the black race coefficient from a glomerular filtration rate estimating equation. JAMA Netw Open 2021;4:e2035636. 10.1001/jamanetworkopen.2020.35636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zelnick LR, Leca N, Young Bet al. Association of the estimated glomerular filtration rate with vs without a coefficient for race with time to eligibility for kidney transplant. JAMA Netw Open 2021;4:e2034004. 10.1001/jamanetworkopen.2020.34004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diao JA, Wu GJ, Taylor HAet al. Clinical implications of removing race from estimates of kidney function. JAMA 2021;325:184–6. https://www.ncbi.nlm.nih.gov/pubmed/33263721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McInnes MDF, Moher D, Thombs BDet al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA 2018;319:388–96. 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 24.Janssens AC, Gwinn M.. Novel citation-based search method for scientific literature: application to meta-analyses. BMC Med Res Methodol 2015;15:84. 10.1186/s12874-015-0077-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Longstocking . CoCites 2022. https://www.cocites.com (10 November 2022, date last accessed). [Google Scholar]
- 26.Whiting PF, Rutjes AW, Westwood MEet al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 27.Higgins J, Thomas J, Chandler Jet al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0. www.training.cochrane.org/handbook (10 November 2022, date last accessed). [Google Scholar]
- 28.van Deventer HE, Paiker JE, Katz IJet al. A comparison of cystatin C- and creatinine-based prediction equations for the estimation of glomerular filtration rate in black South Africans. Nephrol Dial Transplant 2011;26:1553–8. 10.1093/ndt/gfq621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moodley N, Hariparshad S, Peer Fet al. Evaluation of the CKD-EPI creatinine based glomerular filtration rate estimating equation in Black African and Indian adults in KwaZulu-Natal, South Africa. Clin Biochem 2018;59:43–9. 10.1016/j.clinbiochem.2018.06.014 [DOI] [PubMed] [Google Scholar]
- 30.Rocha AD, Garcia S, Santos ABet al. No race-ethnicity adjustment in CKD-EPI equations is required for estimating glomerular filtration rate in the Brazilian population. Int J Nephrol 2020;2020:2141038. 10.1155/2020/2141038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flamant M, Vidal-Petiot E, Metzger Met al. Performance of GFR estimating equations in African Europeans: basis for a lower race-ethnicity factor than in African Americans. Am J Kidney Dis 2013;62:182–4. 10.1053/j.ajkd.2013.03.015 [DOI] [PubMed] [Google Scholar]
- 32.Veronese FV, Gomes EC, Chanan Jet al. Performance of CKD-EPI equation to estimate glomerular filtration rate as compared to MDRD equation in South Brazilian individuals in each stage of renal function. Clin Chem Lab Med 2014;52:1747–54. 10.1515/cclm-2014-0052 [DOI] [PubMed] [Google Scholar]
- 33.Stevens LA, Claybon MA, Schmid CHet al. Evaluation of the Chronic Kidney Disease Epidemiology Collaboration equation for estimating the glomerular filtration rate in multiple ethnicities. Kidney Int 2011;79:555–62. 10.1038/ki.2010.462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Department of Health and Human Services, Food and Drug Administration, Office of the Commissioner et al. Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. Silver Spring, MD: Office of Minority Health, 2016. [Google Scholar]
- 35.Stefflova K, Dulik MC, Barnholtz-Sloan JSet al. Dissecting the within-Africa ancestry of populations of African descent in the Americas. PLoS One 2011;6:e14495. 10.1371/journal.pone.0014495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casal MA, Ivy SP, Beumer JHet al. Effect of removing race from glomerular filtration rate-estimating equations on anticancer drug dosing and eligibility: a retrospective analysis of National Cancer Institute phase 1 clinical trial participants. Lancet Oncol 2021;22:1333–40. 10.1016/S1470-2045(21)00377-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soveri I, Berg UB, Björk Jet al. Measuring GFR: a systematic review. Am J Kidney Dis 2014;64:411–24. 10.1053/j.ajkd.2014.04.010 [DOI] [PubMed] [Google Scholar]
- 38.Andersen TB, Jødal L, Nielsen NSet al. Comparison of simultaneous plasma clearance of 99mTc-DTPA and 51Cr-EDTA: can one tracer replace the other? Scand J Clin Lab Invest 2019;79:463–7. 10.1080/00365513.2019.1658217 [DOI] [PubMed] [Google Scholar]
- 39.Rehling M, Møller ML, Thamdrup Bet al. Simultaneous measurement of renal clearance and plasma clearance of 99mTc-labelled diethylenetriaminepenta-acetate, 51Cr-labelled ethylenediaminetetra-acetate and inulin in man. Clin Sci (Lond) 1984;66:613–9. 10.1042/cs0660613 [DOI] [PubMed] [Google Scholar]
- 40.Vidal-Petiot E, Courbebaisse M, Livrozet Met al. Comparison of 51Cr-EDTA and 99mTc-DTPA for glomerular filtration rate measurement. J Nephrol 2021;34:729–37. 10.1007/s40620-020-00932-9 [DOI] [PubMed] [Google Scholar]
- 41.Silveiro SP, Araújo GN, Ferreira MNet al. Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation pronouncedly underestimates glomerular filtration rate in type 2 diabetes. Diabetes Care 2011;34:2353–5. 10.2337/dc11-1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwandt A, Denkinger M, Fasching Pet al. Comparison of MDRD, CKD-EPI, and Cockcroft-Gault equation in relation to measured glomerular filtration rate among a large cohort with diabetes. J Diabetes Complications 2017;31:1376–83. 10.1016/j.jdiacomp.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 43.Cohn SH, Abesamis C, Zanzi Iet al. Body elemental composition: comparison between black and white adults. Am J Physiol 1977;232:E419–22. [DOI] [PubMed] [Google Scholar]
- 44.Worrall JG, Phongsathorn V, Hooper RJet al. Racial variation in serum creatine kinase unrelated to lean body mass. Br J Rheumatol 1990;29:371–3. 10.1093/rheumatology/29.5.371 [DOI] [PubMed] [Google Scholar]
- 45.Harsha DW, Frerichs RR, Berenson GS.. Densitometry and anthropometry of black and white children. Hum Biol 1978;50:261–80. https://www.ncbi.nlm.nih.gov/pubmed/721084 [PubMed] [Google Scholar]
- 46.Delanaye P, Mariat C, Cavalier Eet al. The « race » correction in estimating glomerular filtration rate: an European point of view. Curr Opin Nephrol Hypertens 2021;30:525–30. 10.1097/MNH.0000000000000739 [DOI] [PubMed] [Google Scholar]
- 47.Agodoa LY, Appel L, Bakris GLet al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 2001;285:2719–28. 10.1001/jama.285.21.2719 [DOI] [PubMed] [Google Scholar]
- 48.Delanaye P, Cohen EP.. Formula-based estimates of the GFR: equations variable and uncertain. Nephron Clin Pract 2008;110:c48–53; discussion c54. 10.1159/000151436 [DOI] [PubMed] [Google Scholar]
- 49.Delanaye P, Cavalier E, Cristol JPet al. Calibration and precision of serum creatinine and plasma cystatin C measurement: impact on the estimation of glomerular filtration rate. J Nephrol 2014;27:467–75. 10.1007/s40620-014-0087-7 [DOI] [PubMed] [Google Scholar]
- 50.Cooper RS, Kaufman JS, Ward R.. Race and genomics. N Engl J Med 2003;348:1166–70. 10.1056/NEJMsb022863 [DOI] [PubMed] [Google Scholar]
- 51.Tishkoff SA, Kidd KK.. Implications of biogeography of human populations for ‘race’ and medicine. Nat Genet 2004;36(11 Suppl):S21–7. 10.1038/ng1438 [DOI] [PubMed] [Google Scholar]
- 52.Sankar P, Cho MK.. Toward a new vocabulary of human genetic variation. Science 2002;298:1337–8. 10.1126/science.1074447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eneanya ND, Yang W, Reese PP. Reconsidering the consequences of using race to estimate kidney function. JAMA 2019;322:113–4. 10.1001/jama.2019.5774 [DOI] [PubMed] [Google Scholar]
- 54.Delanaye P, Mariat C, Maillard Net al. Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin J Am Soc Nephrol 2011;6:906–12. 10.2215/CJN.10931210 [DOI] [PubMed] [Google Scholar]
- 55.Haas Pizarro M, Conte Santos D, Gomes Nunes Melo Let al. Glomerular filtration rate estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation in type 1 diabetes based on genomic ancestry. Diabetol Metab Syndr 2020;12:71. 10.1186/s13098-020-00578-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parra FC, Amado RC, Lambertucci JRet al. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA 2003;100:177–82. 10.1073/pnas.0126614100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lijmer JG, Bossuyt PM, Heisterkamp SH.. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525–37. 10.1002/sim.1185 [DOI] [PubMed] [Google Scholar]
- 58.Kidney Disease: Improving Global Outcomes CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013:3:1–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the articles included in the meta-analysis, within its supplementary materials or upon request to the authors.