Abstract
A model procedure has been developed for the rapid extraction of five bacteriocins (nisin, pediocin RS2, leucocin BC2, lactocin GI3, and enterocin CS1) from concentrated freeze-dried crude culture supernatants by adsorption onto acid or alkaline rice hull ash (RHA) or silicic acid (SA). Bacteriocins were adsorbed onto RHA or SA by a pH-dependent method and desorbed by decreasing the pH to 2.5 or 3.0 and heating at 90°C for 5 min. The maximum adsorption and optimal pH range for different bacteriocins were as follows: nisin, 97% at pH 7.0; lactocin GI3, 94% at pH 6.0; pediocin RS2, 97% at pH 8.0 to 9.0; leucocin BC2, 88% at pH 9.0; and enterocin CS1, 94% at pH 5.0. The desorption level of lactocin GI3 or enterocin CS1 from the surfaces of both RHA and SA was 94%, while the desorption level of pediocin RS2 and leucocin BC2 was 50% or less. Nisin was desorbed readily from SA (91%) but not from RHA (50% or less). The adsorption of bacteriocins onto RHA and SA increased with the increasing concentration of bacteriocins. Analysis of the desorbed bacteriocins after dialysis and sodium dodecyl sulfate–16% polyacrylamide gel electrophoresis showed a single band that gave a single inhibition zone when overlaid with Lactobacillus plantarum for detection of lactocin GI3, enterocin CS1, and nisin. RHA appears useful for extraction, concentration, and partial purification of the five bacteriocins.
Yang et al. (16) purified bacteriocins (pediocin AcH, nisin, sakacin A, and leuconocin Lcm1) by adsorbing them to heat-killed producer bacteria. They found that the adsorption of all bacteriocins tested was pH dependent with maximum adsorption occurring at a pH near 6.0. By reducing the pH to 2.0 and adding 50 mM sodium dodecyl sulfate (SDS), they were able to release 93 to 100% of the bound bacteriocins from the producer cells. Daba et al. (5) also purified pediocin PA-1 by adsorption onto producer cells but desorbed the bacteriocin by acid treatment alone and recovered only 10% of the initial concentration. Bacteriocins (nisin, pediocin PO3, brevicin 286, and piscicolin 126) have also been removed from fermentation broths by adsorption onto diatomic calcium silicate (4). However, once adsorbed to diatomic calcium silicate, these bacteriocins could not be desorbed between a pH range of 1.0 to 12.0 except with the addition of 1% SDS. Methods therefore need to be devised to extract large quantities of purified bacteriocins from crude bacteriocin-containing culture supernatants for use in food preservation.
Rice hull ash (RHA) is a coproduct of rice milling and is composed primarily of silica and carbon (12). Analysis of rice hull silica by infrared spectroscopy indicated it was similar to silicic acid (13). Proctor and Palaniappan (11) found that heating RHA at 500°C for 10 h and treating it with sulfuric acid improved the adsorption of lutein from soy oil. However, the acid-activated RHA (aRHA) reduced the extraction of free fatty acids from crude soy oil while the heat-treated alkaline RHA (kRHA) effectively removed the free fatty acids from crude soy oil (14). However, under commercial bleaching conditions, kRHA was not effective in reducing the levels of pigments, free fatty acids, and peroxides from soy oil (13). In addition, aRHA was used to effectively immobilize a lipase produced by Candida cylindracea (15).
In this study, the optimum pHs for adsorption of five bacteriocins onto kRHA, aRHA and silicic acid (SA) were investigated. In addition, the optimum conditions for the desorption of bacteriocins from kRHA, aRHA, and SA and resulting specific activities were determined. The purity of the resulting bacteriocins after desorption was determined by SDS-polyacrylamide gel electrophoresis (PAGE).
MATERIALS AND METHODS
Cultures and growth conditions.
The sources of the five bacteriocin-producing strains used in this study are shown in Table 1. The Leuconostoc mesenteroides, Pediococcus acidilactici, and Lactococcus lactis species were grown for 20 h at 30°C. L. lactis subsp. diacetylactis GI3 and Enterococcus sp. strain CS1 were grown for 18 h at 30°C. The indicator organisms used were Listeria monocytogenes serotype 1/2a, (ATCC 15313) for leucocin BC2 and Lactobacillus plantarum NCDO 955, obtained from Mark Daeschel, Oregon State University, for the other four bacteriocins. Pure strains were stored at −70°C and subcultured twice at 30°C for 24 h in 4 ml of de Man, Rogosa, and Sharpe (MRS) broth or brain heart infusion (BHI) (Difco, Detroit, Mich.) before being used.
TABLE 1.
Bacteriocins used in this study and their sources
| Bacteriocin | Producer species | Strain | Source | Reference |
|---|---|---|---|---|
| Nisin | Lactococcus lactis | ATCC 11454 | ATCCa | |
| Pediocin RS2 | Pediococcus acidilactici | RS2 | University of Arkansasb | 3 |
| Lactocin GI3 | Lactococcus lactis subsp. diacetylactis | GI3 | University of Arkansas | 7 |
| Enterocin CS1 | Enterococcus sp. | CS1 | University of Arkansas | 7 |
| Leucocin BC2 | Leuconostoc mesenteroides | BC2 | University of Arkansas | 7 |
a American Type Culture Collection, Rockville, Md. 20852.
b Food Science Department, University of Arkansas, Fayetteville.
RHA preparation.
The RHA (97% silica) was obtained from Comet Rice Ingredients Company, Los Angeles, Calif., and was further processed as described by Proctor and Palaniappan (13) by combustion in a muffle furnace at 500°C for 10 h to remove residual carbon. The RHA was ground with a mortar and pestle and was designated kRHA. aRHA was prepared by mixing 40 g of kRHA with 1 liter of 20% (vol/vol) sulfuric acid. The resulting mixture was stirred at 25°C for 5 h, washed with sterile distilled water (dH2O), collected onto a Whatman no. 1 filter paper, and dried at 65°C for 48 h. SA with a mesh size of 60 to 200 was obtained from Sigma, St. Louis, Mo.
Determination of bacteriocin activity by agar overlay method.
BHI and MRS soft agar (20 ml) were melted in a hot water bath (95°C) and tempered to 47°C before being mixed with 7 μl of a cell suspension of indicator species. The soft agar was then poured over the agar plates and cooled at room temperature for 30 min. After the plates were cooled and solidified, 5 μl of twofold bacteriocin dilutions were inoculated onto the indicator plates. After 24 to 48 h of incubation, the plates were examined for zones of inhibition. The bacteriocin activity, expressed as arbitrary units per milliliter (AU/ml), was calculated from the reciprocal of the highest dilution of a bacteriocin which gave a minimum of 2-mm-diameter zones of inhibition on the indicator plates. In this study, all reported data are means of three or more separate experiments.
Adsorption and desorption of bacteriocins.
Bacteriocin-producing strains (0.1% inoculation) were grown in 500 ml of casein glucose broth (CGB) at the time and temperature indicated above. CGB was used during this study because it produces more distinct clear protein bands when SDS-PAGE is performed (1, 2, 3). The bacterial suspension was heat killed at 90°C for 20 min, and the crude bacteriocin supernatant was collected by centrifugation (25 min at 22,100 × g), freeze-dried, and then reconstituted with 50 ml of dH2O. The pH of concentrated bacteriocin supernatant in 5-ml fractions was adjusted from 2.0 to 9.0 by the addition of 1 M HCl or 1 M NaOH. After pH adjustment, the concentrated supernatant was brought up to a volume of 10 ml with sterile dH2O, and 2% kRHA, aRHA, or SA (wt/vol) was added.
The samples were stirred overnight at 4°C. After bacteriocin adsorption, RHA or SA was separated from the crude supernatant by centrifugation (20 min at 1,470 × g), washed with sterile dH2O, and resuspended to the original volume of 10 ml. For desorption of the bound bacteriocins, the pH of RHA or SA was adjusted by the addition of 5% phosphoric acid to pH 2.5 for enterocin CS1 and lactocin GI3. Nisin, pediocin RS2, and leucocin BC2 lost activity when heated at a pH of 2.5, and so the pH of these three was adjusted to 3.0. The samples were either stirred for 30 min at 25°C or stirred for 25 min and heated for 5 min at 90°C. The supernatant containing released bacteriocin was collected by centrifugation for 20 min at 1,470 × g, and the pH was adjusted to 4.0 with 1 M NaOH. The bacteriocin activity of all fractions collected at different stages of adsorption and desorption was assayed by the agar overlay method. The following terms were used to determine the percentages of adsorbed and desorbed bacteriocins: O = the bacteriocin activity (AU/ml) in the concentrated freeze-dried supernatant before adsorption; PA = the activity of bacteriocin (AU/ml) adsorbed to 2% RHA or SA (wt/vol) when adsorbent was resuspended in dH2O; SA = the activity of bacteriocin (AU/ml) remaining in concentrated freeze-dried supernatant after the adsorption step and removal of RHA or SA by centrifugation; PD = the bacteriocin activity (AU/ml) remaining adsorbed to 2% RHA or SA (wt/vol) after the desorption step and when adsorbent was resuspended in dH2O; SD = the bacteriocin activity (AU/ml) released into dH2O after desorption by acid and/or heat and the removal of RHA or SA by centrifugation. The formulae used are as follows: the percentage of adsorption onto RHA or SA = {PA/[(O + PA + SA)/2]} × 100; and the percentage of desorption from RHA or SA = {SD/[(O + PD + SD)/2]} × 100.
The above formulae take into account the inherent inability of the twofold dilutions to narrowly distinguish close differences of bacteriocin activities by the agar overlay method. To address this variability, the total bacteriocin activity of the original crude culture supernatant was derived in two different ways: (i) direct measurement of bacteriocin activity present in the crude culture supernatant; and (ii) by combining the bacteriocin activities of the two subfractions resulting from the crude culture supernatant when treated with adsorbent for purification. The latter refers to the sum of the bacteriocin activities in the two subfractions and in the adsorbed fraction (PA) and unadsorbed bacteriocin remaining in the supernatant (SA). The sum of the PA and SA was always greater than the value of O due to inability of the twofold dilution method to distinguish between sample differences. Therefore, we derived an average of the initial bacteriocin activity of the original samples using (O + PA + SA)/2 in the formulae above.
SDS-PAGE.
The adsorption and desorption procedures described above were followed, except the concentrated crude supernatant was resuspended to 30 ml with sterile dH2O. After desorption, the resulting bacteriocin suspension containing supernatant was concentrated by freeze-drying, reconstituted to 4 ml with sterile dH2O, and dialyzed against dH2O for 24 h with a 2-kDa-molecular-mass cutoff membrane (Spectrum, Houston, Tex.). The dialysis resulted in the formation of a precipitate that was collected by centrifugation (6 min at 16,000 × g), washed three times in sterile dH2O, and suspended with 400 μl of sterile dH2O before being assayed for bacteriocin activity. The protein concentrations of the samples were determined with the protein assay kit (Bio-Rad, Hercules, Calif.). For the SDS-PAGE analysis, 20 μl of the purified bacteriocin samples was mixed with an equal volume of the sample solvent (62.5 mM Tris-HCl [pH 6.8], 10% glycerol, 2% SDS, 5% β-mercaptoethanol) and heated at 60°C for 15 min. The denatured samples were loaded onto Tris-Tricine SDS–16% polyacrylamide gels (14) with 10 to 20 μl of sample and solvent per well along with the polypeptide molecular weight standards (Bio-Rad). Electrophoresis was carried out for 6 h at 40 mA. The gel was either stained with Coomassie brilliant blue or prepared for an agar overlay as described by Bhunia et al. (1, 3).
RESULTS
Adsorption of bacteriocins.
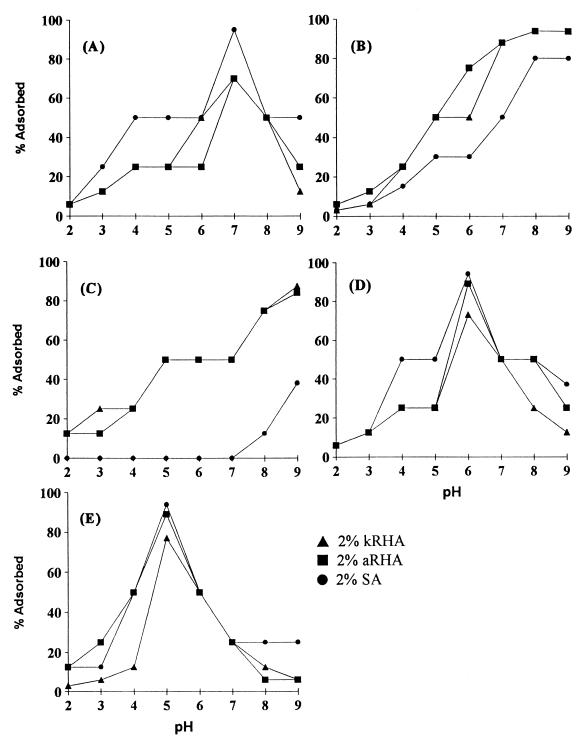
The influence of pH on the adsorption patterns of all five bacteriocins onto aRHA, kRHA, and SA is shown in Fig. 1. The adsorption of bacteriocins onto aRHA, kRHA, and SA was pH dependent. The minimum adsorption occurred at a pH of 2.0 to 3.0 for all bacteriocins. Maximum adsorption of nisin occurred at a pH of 7.0 for aRHA, kRHA, and SA. The SA adsorbed 97% of nisin from the crude culture supernatant, while aRHA and kRHA adsorbed only 70% of nisin (Fig. 1A). The optimum pH for the maximum adsorption of pediocin RS2 was between 8.0 and 9.0 (Fig. 1B). The adsorption of pediocin RS2 onto SA, aRHA, and kRHA were 80, 97, and 97% respectively. Leucocin BC2 had a maximum adsorption rate of 84 to 88% at a pH of 9.0 for aRHA and kRHA. However, maximum adsorption of leucocin BC2 onto SA was only 38% (Fig. 1C). The maximum adsorption of lactocin GI3 occurred at a pH of 6 with 89 to 94% adsorbed onto aRHA and SA and 70% adsorbed onto kRHA (Fig. 1D). Maximum adsorption of enterocin CS1 occurred at a pH of 5.0 with 89 to 94% adsorbed onto aRHA and SA but only 70% adsorption onto kRHA.
FIG. 1.
The influence of pH on the adsorption of bacteriocins onto aRHA, kRHA, and SA. Bacteriocins: (A) nisin; (B) pediocin RS2; (C) leucocin BC2; (D) lactocin GI3; (E) enterocin CS1.
Desorption of bacteriocins.
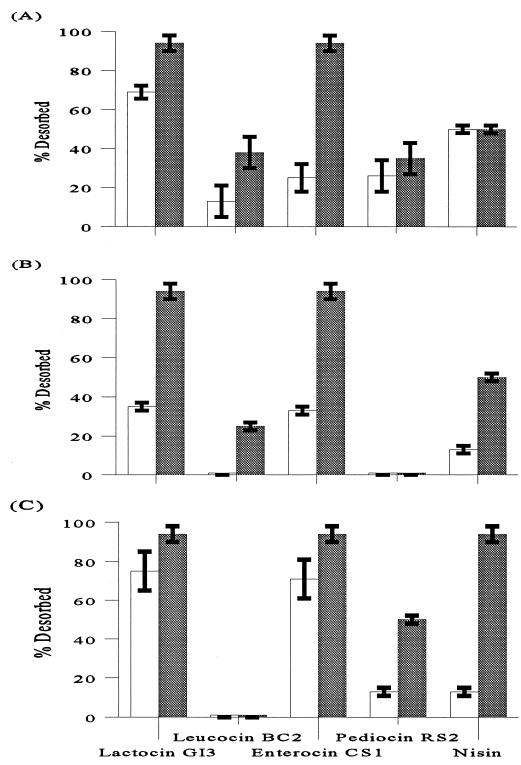
Upon decreasing the pH to 2.5 or 3.0, the desorption of bacteriocins was improved by heating at 90°C for 5 min in comparison with 30 min of incubation at 25°C (Fig. 2 and 3). Under these heating conditions, nisin, lactocin GI3, and enterocin CS1 were desorbed readily, while pediocin RS2 and leucocin BC2 had the least desorption. Maximum desorption of lactocin GI3 and enterocin CS1 was 94% at 90°C for 5 min. With all adsorbents, this rate was 25 to 60% greater than that for the treatment of 25°C for 30 min. Pediocin RS2 had a maximum desorption of 50% from SA at 90°C for 5 min, but only 26 to 32% from aRHA and no detectable amount of activity desorbed from kRHA treatments. Nisin had 91% desorption at 90°C for 5 min for SA compared to 25 to 50% desorption in the absence of heat treatment for all three adsorbents tested. Leucocin BC2 had a maximum desorption of 38% from aRHA at 90°C for 5 min, and no detectable amount was desorbed from kRHA and SA without heating.
FIG. 2.
Desorption of bacteriocins from aRHA (A), kRHA (B), and SA (C) by acid treatment with or without heat. Open bars, desorption at 25°C for 30 min; shaded bars, desorption at 90°C for 5 min. The means were calculated from three values, and the error bars indicate standard deviations.
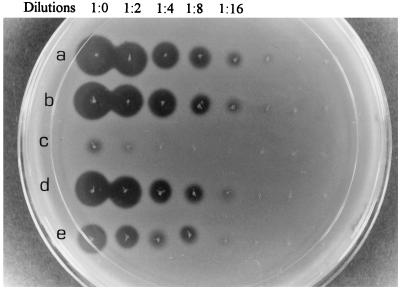
FIG. 3.
The inhibition of L. plantarum by enterocin CS1 from different desorption fractions with or without heat treatment. (a) PA, enterocin CS1 adsorbed to SA with an activity of 3,200 AU/ml; (b) SD enterocin CS1 desorbed from SA at 90°C with an activity 3,200 AU/ml; (c) PD, enterocin CS1 remaining on SA after desorption at 90°C with an activity of 200 AU/ml; (d) SD, enterocin CS1 desorbed from SA at 25°C with an activity of 1,600 AU/ml; (e) PD, enterocin CS1 remaining on SA after desorption at 25°C with an activity of 1,600 AU/ml.
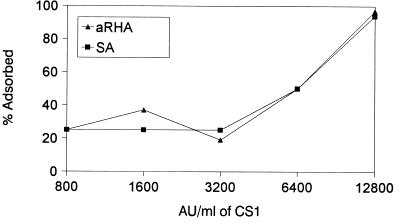
Influence of initial bacteriocin concentration on the adsorption pattern.
Different dilutions of freeze-dried enterocin CS1 directly and freshly rehydrated at activity levels between 12,800 AU/ml and 800 AU/ml were tested for their adsorption onto 2% aRHA and SA (Fig. 4). The adsorption of enterocin CS1 onto aRHA or SA increased steadily with an increasing concentration of this bacteriocin. The highest adsorption level (up to 94 to 95%) was achieved by using 12,800 AU of enteriocin CS1/ml while the least amount of adsorption, 20 to 30%, occurred for both adsorbents when the enterocin CS1 concentrations were lower, in the range of 800 to 3,200 AU/ml (Fig. 4).
FIG. 4.
The effect of different concentrations of enterocin CS1 on bacteriocin adsorption onto 2% aRHA or 2% SA.
Purification of bacteriocins.
Three bacteriocins, nisin, enterocin CS1, and lactocin GI3, were tested through all stages of the purification process involving adsorption and desorption by aRHA or SA in order to determine the resulting bacteriocin activities and recovery yields at different stages (Table 2). Of the total original activity of 400,000 AU/ml present within the crude culture supernatant for enterocin CS1, about 336,000 AU/ml was recovered by adsorption and desorption by using aRHA, yielding an 84% recovery rate of the total bacteriocin by this method. Subsequent dialysis resulted in the loss of 75% of enterocin CS1 activity (Table 2). For nisin, about 672,000 AU/ml of the original activity of 800,000 AU/ml was recovered by the SA method, yielding an 84% rate of recovery. The loss of nisin activity as a result of dialysis was close to 87% (Table 2). Similarly for lactocin GI3, about 168,000 AU/ml of the original activity of 200,000 AU/ml was recovered by the aRHA method, yielding an 84% recovery rate. The loss of lactocin GI3 activity was 50% due to subsequent dialysis (Table 2). The loss of bacteriocin activity during the dialysis step was due to incomplete precipitation of the bacteriocins. More than half of the lost activity was accounted for in the liquid portion of the dialysis bag.
TABLE 2.
Recovery of enterocin CS1, nisin, and lactocin GI3 by using RHA or SA
| Bacteriocin and purification step | Fraction vol (ml) | Total protein (mg)a | Total activity (AU) | Sp act (AU/mg protein) | Activity recovered (%) | Fold purification |
|---|---|---|---|---|---|---|
| Enterocin CS1 | ||||||
| Culture supernatant | 500 | 50 | 400,000 | 8,000 | 100 | 1 |
| Desorption from aRHAb | 30 | 7.8 | 336,000 | 43,077 | 84 | 5.3 |
| Dialysisc | 0.4 | 0.21 | 102,400 | 487,619 | 26 | 61 |
| Nisin | ||||||
| Culture supernatant | 500 | 30.74 | 800,000 | 26,025 | 100 | 1 |
| Desorption from SAb | 30 | 9 | 672,000 | 74,667 | 84 | 2.9 |
| Dialysisc | 0.4 | 0.11 | 102,400 | 930,909 | 13 | 36 |
| Lactocin GI3 | ||||||
| Culture supernatant | 500 | 43.4 | 200,000 | 4,608 | 100 | 1 |
| Desorption from aRHAb | 30 | 6 | 168,000 | 28,000 | 84 | 6.0 |
| Dialysisc | 0.4 | 0.32 | 102,400 | 320,000 | 51 | 69.4 |
a Protein concentration was determined by Bio-Rad protein assay.
b Total activity was determined by subtracting the loss in the supernatant after adsorption (SA) and the loss on the pellet after desorption (PD) from the bacteriocin desorbed from SA or aRHA (SD).
c Dialysis was carried out for 24 h against sterile water and resulted in the formation of a precipitate that was collected and tested.
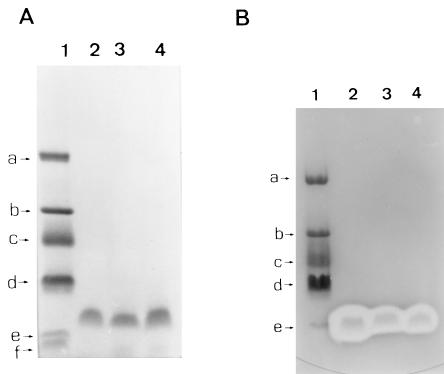
The SDS-polyacrylamide gel stained with Coomassie blue showed only one polypeptide band for nisin, enterocin CS1, and lactocin GI3 compared to the molecular mass protein standards which ranged from approximately 26 to 1.4 kDa (Fig. 5A). The other half of the gel overlaid with an indicator organism (L. plantarum) shows clear zones of inhibition for nisin at 3.5 kDa, enterocin CS1 at 4.0 kDa, and lactocin GI3 at 3.9 kDa (Fig. 5B).
FIG. 5.
SDS-PAGE analysis of enterocin CS1, nisin, and lactocin GI3 purified by aRHA or SA. (A) SDS-polyacrylamide gel half stained with Coomassie blue. Lanes: 1, molecular mass standard [(a) 26,625 Da; (b) 16,950 Da; (c) 14,437 Da; (d) 6,512 Da; (e) 3,496 Da; (f) 1,423 Da; 2, enterocin CS1; 3, nisin; 4, lactocin GI3. (B) SDS-polyacrylamide gel overlaid with L. plantarum showing the inhibitory effects of the bacteriocins. Lanes: 1, molecular mass standard; 2, enterocin CS1; 3, nisin; 4, lactocin GI3.
DISCUSSION
The model procedure described here uses one of three adsorbents, aRHA, kRHA, or SA, for recovery of the total bacteriocin from their crude culture supernatants. This new extraction procedure has proven suitable for five bacteriocins, including nisin, pediocin RS2, enterocin CS1, lactocin GI3, and leucocin BC2, which exhibit different biochemical and antimicrobial properties as characterized previously (2, 7, 9).
Our studies have shown that concentrating bacteriocins by removal of excess water by freeze-drying increased their adsorption onto RHA and SA. These results suggest that the adsorption of bacteriocins onto RHA and SA was due to a multilayer of protein-protein weak hydrogen bonds that were influenced by electrostatic interactions. It is well known that the intensities of electrostatic attractions are reduced by the presence of water molecules surrounding the charged ions and thus contribute to the lower percentages of adsorption of bacteriocins when diluted. The adsorption of bacteriocins onto aRHA, kRHA, and SA was primarily dependent on the pH of the crude supernatant. Maximum adsorption of pediocin RS2 and leucocin BC2 occurred at a pH of 9.0. The maximum adsorption of lactocin GI3, enterocin CS1, and nisin occurred at pHs of 5.0, 6.0, and 7.0, respectively. The optimal pH values we observed for bacteriocin adsorption onto RHA and SA differ slightly from the optimal pH of 6.0 reported for adsorption of bacteriocins (pediocin ACH, nisin, sakacin A, and leucocin Lcm1) onto producer cells (16). The method of adsorption onto the cells of producer organisms relies on the cationic nature of bacteriocins at pHs below 7.0 (6). In addition, the optimal adsorption of bacteriocins onto diatomic calcium silicate occurs at the fermentation pH of 4.5 (4). Coventry et al. (4) suggested that hydrophobic and electrostatic interactions were involved in the adsorption of bacteriocins onto the diatomic calcium silicate they tested.
Yang et al. (16) calculated the percentage of adsorption of bacteriocins onto the producer cells by determining the amount of activity (in AU/ml) that remained in the cell-free supernatant. However, they did not directly measure the activity bound to the producer cells. Their formula does not account for losses of bacteriocin activity due to pH. Conversely, the formulae we developed account for all the fractions obtained during the purification procedure and would account for any such losses of activity. For example, nisin, pediocin RS2, and leucocin BC2 lost activity at a pH of 2.5 with heat but not at a pH of 3.0 with heat during our study. Furthermore, the sum of activities of the bacteriocins adsorbed to the silica compounds plus the amount of bacteriocin activity remaining in the culture supernatant was sometimes greater than the original bacteriocin activity, reflecting the quantitative limitation of the agar overlay method. The formulae we developed also account for these limitations.
Infrared analysis has shown that the binding of oleic acid onto kRHA occurs by the removal of the hydrogen ion from the carboxyl group of oleic acid by potassium oxide, a strong base that initiates acid-base reactions found in RHA. The reaction caused by potassium oxide results in the formation of a bond between the carboxylate ion of oleic acid and silanol groups of kRHA (13). Furthermore, the removal of the potassium oxide from kRHA by acid wash decreased the adsorption of oleic acid. An opposite effect was seen with enterocin CS1 and lactocin GI3, which had maximum adsorption rates of 94% with SA, 89% with aRHA, and only 70% with kRHA. These results suggest that the absence of potassium and metal oxides increased the adsorption of enterocin CS1 and lactocin GI3 to RHA.
Based on infrared analysis, Proctor et al. (13) proposed that three bonding strengths could occur when oleic acid was adsorbed onto RHA: weaker hydrogen bonds, intermediate hydrogen bonds, and strong ionic bonds. The adsorption of bacteriocins to RHA and SA presumably involved similar binding interactions. Leucocin BC2 and pediocin RS2 each showed 50% or less desorption from each silica compound tested, suggesting that a mixture of intermediate bonds and stronger bonds might be involved in the adsorption process. In addition, both of these bacteriocins showed no detectable amount of desorption from kRHA, which contains potassium oxides that promote the formation of the stronger ionic bonds. Lactocin GI3 and enterocin CS1 showed close to 100% desorption following the addition of heat, implying that the weaker and intermediate bonds could possibly be involved in the adsorption of both these bacteriocins. Infrared analysis also revealed that RHA has more surface sites exposed than does SA, which caused oleic acid lying parallel to the surface of SA to be adsorbed. This could explain why leucocin BC2 lost activity when bound to silicic acid due to the blockage of the active receptor site of this bacteriocin (10). The addition of heat (90°C for 5 min) increased the desorption of bacteriocins, presumably by disrupting the weak and intermediate bonds between the bacteriocins and silica compounds.
Nisin had a 91% desorption rate from SA by heat treatment but only a 50% rate of desorption with all other treatments. The adsorption of nisin onto SA most probably involved the weaker and intermediate hydrogen bonds while aRHA and kRHA involved all three bond strengths. Preliminary work (results not shown) indicated that the desorption of nisin from RHA could be improved by using RHA treated by heating for 10 h at 800°C prior to use for nisin adsorption. The longer combustion of RHA would remove most of the impurities and produce a purer sample that is similar to SA.
The method described in this study for extraction of bacteriocins from the cell-free CGB supernatant recovered 84% of original bacteriocin activity after desorption from RHA and SA with specific activities of 28,000 to 74,667 AU/mg of protein. We have also found (data not shown) that MRS and milk-based media can be used for bacteriocin production without interfering with this adsorption and desorption method. After dialysis the specific activity of the bacteriocins increased 10-fold due to removal of contaminating proteins. However, the final recovery rate of bacteriocins after dialysis decreased to 50% or lower. Most of the lost bacteriocin activity was due to incomplete precipitation of the protein molecules during dialysis. Additional losses after dialysis when the precipitate was washed could have been due to their adsorption onto pipette tips, microfuge tubes, and glassware (8). Bhunia et al. (3) partially purified pediocin AcH by dialysis of the concentrated freeze-dried supernatant. They recovered twice as much total protein than observed in our method. However, Bhunia et al. (3) observed by SDS-PAGE at least three other protein bands besides pediocin AcH, while we obtained single protein bands that showed antimicrobial activities. Dialysis is a simple method to produce pure bacteriocin samples for further characterization of the biochemical properties of the proteins.
Extraction of bacteriocins with aRHA and SA produced relatively pure protein samples as indicated by SDS-PAGE. The contaminating proteins adsorbed and then desorbed from the RHA and SA were removed during dialysis as indicated by the decrease in the specific activity of bacteriocin samples desorbed. The amount of other proteins adsorbed can be primarily eliminated by adjusting the concentration of the adsorbent. Preliminary work has indicated that the duration of heating and the temperature at which RHA was treated can reduce the amount of nonspecific protein binding. Our studies demonstrated that RHA and SA provide a simple, rapid means of purifying and concentrating bacteriocins from crude broth culture supernatants. Further investigations are under way for improving the desorption of nisin, pediocin, and leucocin BC2 after adsorption to RHA and SA.
ACKNOWLEDGMENTS
This research was supported by a grant from the Food Safety Consortium.
We gratefully acknowledge the assistance of M. Davis, W. Huff, and N. Hettiarachchy for their suggestions and critical review of the manuscript.
Footnotes
Published with the approval of the director of the Arkansas Agricultural Experiment Station; manuscript 98015.
REFERENCES
- 1.Bhunia A K, Johnson M C, Ray B. Direct detection of an antimicrobial peptide of Pediococcus acidilactici in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Ind Microbiol. 1987;2:319–322. [Google Scholar]
- 2.Bhunia A K, Johnson M C, Ray B. Purification, characterization and antimicrobial spectrum of a bacteriocin produced by Pediococcus acidilactici. J Appl Bacteriol. 1988;65:261–268. doi: 10.1111/j.1365-2672.1988.tb01893.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhunia A K, Johnson M G. A modified method to directly detect in SDS-PAGE the bacteriocin of Pediococcus acidilactici. Lett Appl Microbiol. 1992;15:5–7. [Google Scholar]
- 4.Coventry M J, Gordon J B, Alexander M, Hickey M W, Wan J. A food-grade process for isolation and partial purification of bacteriocins of lactic acid bacteria that uses diatomite calcium silicate. Appl Environ Microbiol. 1996;62:1764–1769. doi: 10.1128/aem.62.5.1764-1769.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daba H, Lacroix C, Huang J, Simard R E, Lemieux L. Simple method of purification and sequencing of a bacteriocin produced by Pediococcus acidilactici UL5. J Appl Bacteriol. 1994;77:682–688. doi: 10.1111/j.1365-2672.1994.tb02819.x. [DOI] [PubMed] [Google Scholar]
- 6.Jack R W, Tagg J R, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janes M E, Ahmed N M, Johnson M G. Abstracts of the IFT Annual Meeting, New Orleans, La. 1996. Identification of two bacteriocin-producing bacteria from garlic and ginger root active against food borne pathogens, abstr. 140-11; p. 31. [Google Scholar]
- 8.Joosten H M L J, Nunez M. Adsorption of nisin and enterocin 4 to polypropylene and glass surfaces and its prevention by Tween 80. Lett Appl Microbiol. 1995;21:389–392. [Google Scholar]
- 9.Liu W, Hansen J N. Some chemical and physical properties of nisin, a small protein antibiotic produced by Lactococcus lactis. Appl Environ Microbiol. 1990;56:2551–2558. doi: 10.1128/aem.56.8.2551-2558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maftah A, Renault D, Vignoles C, Héchard Y, Bressollier P, Ratinaud M H, Cenatiempo Y, Julien R. Membrane permeabilization of Listeria monocytogenes and mitochondria by the bacteriocin mesentericin Y105. J Bacteriol. 1993;175:3232–3235. doi: 10.1128/jb.175.10.3232-3235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proctor A, Palaniappan S. Soy oil lutein adsorption by rice hull ash. J Am Oil Chem Soc. 1989;66:1618–1621. [Google Scholar]
- 12.Proctor A, Palaniappan S. Adsorption of soy oil free fatty acids by rice hull ash. J Am Oil Chem Soc. 1990;67:15–17. [Google Scholar]
- 13.Proctor A, Adhikari C, Blyholder G D. Mode of oleic acid adsorption on rice hull ash cristobalite. J Am Oil Chem Soc. 1995;72:331–335. [Google Scholar]
- 14.Schagger H, Von Jagow G. Tricine-sodium dodecyl sulfate polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 15.Tantrakulsiri J, Jeyashoke N, Krisanangkura K. Utilization of rice hull ash as a support material for immobilization of Candida cylindracea lipase. J Am Oil Chem Soc. 1997;74:173–175. [Google Scholar]
- 16.Yang R, Johnson M C, Ray B. Novel method to extract large amounts of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1992;58:3355–3359. doi: 10.1128/aem.58.10.3355-3359.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]