Key Points
Question
What is the association of sex with the comparative efficacy and safety of an abbreviated or standard duration of dual antiplatelet therapy (DAPT) in patients with high bleeding risk?
Findings
In this prespecified comparative effectiveness analysis of the MASTER-DAPT trial including 4579 participants, ischemic and bleeding risks were comparable between sexes. There was no significant interaction for net adverse and bleeding events, while a significant heterogeneity was observed for major adverse cardiac or cerebral events with a benefit of abbreviated treatment in women but not in men.
Meaning
The effects of abbreviated DAPT on ischemic and bleeding outcomes after percutaneous coronary intervention may be different between sexes.
This comparative effectiveness study, a prespecified analysis from the MASTER DAPT trial, reports sex differences and the association of sex with outcomes among patients with high bleeding risk treated with an abbreviated vs standard dual antiplatelet therapy regimen.
Abstract
Importance
Abbreviated dual antiplatelet therapy (DAPT) reduces bleeding with no increase in ischemic events in patients at high bleeding risk (HBR) undergoing percutaneous coronary intervention (PCI).
Objectives
To evaluate the association of sex with the comparative effectiveness of abbreviated vs standard DAPT in patients with HBR.
Design, Setting, and Patients
This prespecified subgroup comparative effectiveness analysis followed the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated vs Standard DAPT Regimen (MASTER DAPT) trial, a multicenter, randomized, open-label clinical trial conducted at 140 sites in 30 countries and performed from February 28, 2017, to December 5, 2019. A total of 4579 patients with HBR were randomized at 1 month after PCI to abbreviated or standard DAPT. Data were analyzed from July 1 to October 31, 2022.
Interventions
Abbreviated (immediate DAPT discontinuation, followed by single APT for ≥6 months) or standard (DAPT for ≥2 additional months, followed by single APT for 11 months) treatment groups.
Main Outcomes and Measures
One-year net adverse clinical events (NACEs) (a composite of death due to any cause, myocardial infarction, stroke, or major bleeding), major adverse cardiac or cerebral events (MACCEs) (a composite of death due to any cause, myocardial infarction, or stroke), and major or clinically relevant nonmajor bleeding (MCB).
Results
Of the 4579 patients included in the analysis, 1408 (30.7%) were women and 3171 (69.3%) were men (mean [SD] age, 76.0 [8.7] years). Ischemic and bleeding events were similar between sexes. Abbreviated DAPT was associated with comparable NACE rates in men (hazard ratio [HR], 0.97 [95% CI, 0.75-1.24]) and women (HR, 0.87 [95% CI, 0.60-1.26]; P = .65 for interaction). There was evidence of heterogeneity of treatment effect by sex for MACCEs, with a trend toward benefit in women (HR, 0.68 [95% CI, 0.44-1.05]) but not in men (HR, 1.17 [95% CI, 0.88-1.55]; P = .04 for interaction). There was no significant interaction for MCB across sex, although the benefit with abbreviated DAPT was relatively greater in men (HR, 0.65 [95% CI, 0.50-0.84]) than in women (HR, 0.77 [95% CI, 0.53-1.12]; P = .46 for interaction). Results remained consistent in patients with acute coronary syndrome and/or complex PCI.
Conclusions and Relevance
These findings suggest that women with HBR did not experience higher rates of ischemic or bleeding events compared with men and may derive particular benefit from abbreviated compared with standard DAPT owing to these numerically lower rates of events.
Trial Registration
ClinicalTrials.gov Identifier: NCT03023020
Introduction
Dual antiplatelet therapy (DAPT) with aspirin and a platelet ADP P2Y12 receptor (P2Y12) inhibitor is the cornerstone of pharmacological treatment in patients with acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI) to reduce the risk of ischemic complications, such as stent thrombosis and myocardial infarction (MI).1,2 However, the superior ischemic protection ensured by prolonging DAPT duration is counterbalanced by higher risks of major bleeding, which carries similar or even worse prognostic impact than a recurrent MI.3 This is particularly noteworthy in the contemporary PCI era since the refinements of procedural techniques and technological improvements have led to a significant reduction of ischemic events after revascularization. Furthermore, a significant proportion (≤40%) of patients undergoing PCI are at high bleeding risk (HBR).4,5,6 Among the clinical features associated with enhanced bleeding risk, the association with sex remains controversial; some studies have demonstrated that female sex confers greater HBR,7,8,9,10 while others have not.11,12,13,14 This uncertainty is reflected in the fact that female sex is considered among the bleeding risk features in the US15 but not European guidelines.1
The Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated vs Standard DAPT Regimen (MASTER DAPT) trial is the largest clinical trial investigating an abbreviated compared with a standard DAPT in largely unselected patients with HBR at 1 month after biodegradable polymer-coated sirolimus-eluting stent (hereinafter referred to as sirolimus-eluting stent) implantation.16,17 We report a prespecified comparative effectiveness analysis from the MASTER DAPT trial, which investigated clinical outcomes among male and female patients and the treatment outcomes of abbreviated vs standard DAPT across the sexes in what is, to our knowledge, the largest contemporary cohort of patients with HBR to date.
Methods
Study Design and Population
This study was a prespecified subgroup comparative effectiveness analysis of the MASTER DAPT trial. The MASTER DAPT trial is an investigator-initiated, multicenter, randomized, open-label noninferiority clinical trial with sequential superiority testing in a large cohort of patients with HBR who underwent PCI with implantation of a sirolimus-eluting stent (Ultimaster; Terumo Corporation).16,17,18 The trial was performed at 140 sites in 30 countries across Europe, South America, the Middle East, Asia, and Australia. The trial was approved by the institutional review board at each participating site, and all patients gave written informed consent. Trial protocol, study organization, and participating sites are reported in Supplement 1.
Patients with HBR were considered for participation in the trial if they had undergone PCI of all planned coronary artery stenoses with implantation of the sirolimus-eluting stent for acute or chronic coronary syndromes and remained event free (including a new ACS, symptomatic restenosis, stent thrombosis, stroke, or any revascularization resulting in the prolonged use of DAPT) at 1 month after the index procedure. Key exclusion criteria were the implantation of a stent other than the sirolimus-eluting stent within 6 months before the index procedure, the implantation of a bioresorbable scaffold at any time before the index procedure, and treatment for in-stent restenosis or stent thrombosis. Data on sex and ethnicity were systematically collected in the case report form of the MASTER DAPT trial. Detailed inclusion and exclusion criteria are presented in the eMethods in Supplement 2.
Randomization and Follow-Up
Patients were centrally randomized (1:1 ratio) to an open-label abbreviated or standard DAPT regimen 30 to 44 days after the index procedure. Randomization was concealed using a web-based system. Randomization sequences were computer generated; blocked with randomly selected 10 block sizes of 2, 4, or 6; and stratified by site, history of acute MI within the past 12 months, and clinical indication for at least 12 months of oral anticoagulation (OAC) therapy. Three independent clinical research organizations (Cardiovascular European Research Center, Massy, France; Cardialysis, Rotterdam, the Netherlands; and CVQuest, Tokyo, Japan) performed on-site and remote monitoring visits, verified the source documents, and collected source material for event adjudication. All events were adjudicated by an independent adjudication committee that was unaware of the treatment allocations. All data were stored at a central database (Clinical Trials Unit, Bern, Switzerland).
Randomized Treatments
Patients who were randomized to the abbreviated treatment group immediately discontinued DAPT and continued single antiplatelet therapy (SAPT) until the study completion except for those receiving OAC, who continued SAPT up to 6 months after the index procedure. Patients who were randomized to the standard treatment group continued DAPT for at least 5 additional months (6 months after the index procedure) or, among those receiving OAC, for at least 2 additional months (3 months after the index procedure) and continued thereafter to receive SAPT. Antiplatelet and anticoagulant treatments were dosed according to authorizations for use and locally approved regimens.16,17
Study End Points
The 3 ranked coprimary outcomes were 11-month net adverse clinical events (NACEs) (a composite of death due to any cause, MI, stroke, or major bleeding), major adverse cardiac or cerebral events (MACCEs) (a composite of death due to any cause, MI, or stroke), and major or clinically relevant nonmajor bleeding (MCB) (a composite of Bleeding Academic Research Consortium [BARC] type 2, 3, or 5 bleeding). The secondary outcomes included the individual components of the 3 coprimary outcomes, the composite of stroke and transient ischemic attack, definite or probable stent thrombosis, and all BARC bleeding events.
Statistical Analysis
The data were analyzed according to the intention-to-treat principle from July 1 to October 31, 2022. Outcomes were assessed separately for male and female patients by calculating hazard ratios (HRs) with 95% CIs. For patients with a primary outcome, time to event was calculated as the difference between the date of occurrence of the outcome event and the date of randomization plus 1. For patients with incomplete clinical follow-up, time to censoring was defined as the difference between the dates of the last known clinical status and randomization plus 1.
Associations of sex with bleeding and ischemic outcomes were evaluated using Cox proportional hazards regression and adjusted in 2 models: one extended also used for censor weights (model 1) and one simplified including only baseline differences (model 2). Kaplan-Meier calculations included all (first) adjudicated outcome events that occurred between randomization and 335 days thereafter according to the randomized treatment assignment, irrespective of the DAPT regimen received at the time of the outcome event. Hazard ratios and 95% CIs were generated for primary and secondary outcomes with the use of Cox proportional hazards regression analysis with censoring at the end of the study and at the time of death. Two-sided P values for testing homogeneity of the HR in subgroups of patients were derived in Cox proportional hazards regression models, with the interaction terms for treatment group (abbreviated vs standard) and male or female sex tested using 1 degree of freedom. The 95% CI and P values for interaction were not adjusted for multiplicity and should not be used to infer definitive treatment effects. The Com-Nogue method19 was used to calculate differences in the cumulative incidence of events at 335 days. The analyses used Stata, release 17.0 (StataCorp LLC).
Results
Study Population
Of the 4579 patients enrolled in the MASTER DAPT trial from February 28, 2017, through December 5, 2019, 3171 (69.3%) were men and 1408 (30.7%) were women (mean [SD] age, 76.0 [8.7] years). At a median of 34 (IQR, 32-39) days after stenting, 2295 patients were randomized to an abbreviated DAPT regimen (1590 men and 705 women) and 2284 to a standard DAPT regimen (1581 men and 703 women) (eFigure 1 in Supplement 2). Composition of DAPT and type of SAPT did not differ between sexes, with the only exception being a slightly higher use of ticagrelor monotherapy in women than men (eTable 1 in Supplement 2). Detailed information on antiplatelet use in male and female patients is shown in eFigures 2 and 3 in Supplement 2.
Baseline and Procedural Characteristics
Baseline and procedural characteristics according to sex are reported in eTables 2 and 3 in Supplement 2. Compared with men, women were older; had higher prevalence of arterial hypertension, chronic kidney disease, and hematological or coagulation disorders; and were more likely treated with corticosteroids or nonsteroidal anti-inflammatory drugs. Women had a lower prevalence of previous ischemic events (peripheral arterial disease, prior MI or PCI) and concomitant comorbidities, including history of heart failure or active cancer and clinical indication for 12-month OAC. The mean (SD) PRECISE DAPT (Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Anti Platelet Therapy) score was higher in women (28.96 [10.40]) compared with men (25.78 [11.10]) (scores range from 0 to 35, with higher scores indicating greater risk of bleeding). The proportion of patients with chronic coronary syndrome was comparable between the 2 groups, whereas ST-segment elevation MI at presentation was more common in women. Total contrast volume, total stent length, and mean stent diameter per lesion were higher in men than women (eTable 3 in Supplement 2). Other angiographic and procedural characteristics were similar between sexes.
Baseline and procedural characteristics according to sex and randomized treatment regimens were well balanced between the groups (Table 1 and eTable 4 in Supplement 2) except for a slightly higher prevalence of diabetes, hematological or coagulation disorders, and increased PRECISE DAPT score in women randomized to the standard compared with the abbreviated DAPT group. Treated lesion characteristics were well balanced between groups (eTable 5 in Supplement 2).
Table 1. Baseline Characteristics by Randomized DAPT Regimen and Sexa.
| Characteristic | Male patients | Female patients | ||||
|---|---|---|---|---|---|---|
| Abbreviated DAPT (n = 1590) | Standard DAPT (n = 1581) | P value | Abbreviated DAPT (n = 705) | Standard DAPT (n = 703) | P value | |
| Age, mean (SD), y | 75.3 (8.9) | 75.1 (9.2) | .68 | 78.0 (7.9) | 77.8 (7.5) | .60 |
| BMI, mean (SD) | 27.3 (4.5) | 27.5 (4.5) | .15 | 27.2 (5.2) | 27.3 (5.2) | .73 |
| Family history of coronary artery disease | 349 (21.9) | 360 (22.8) | .58 | 207 (29.4) | 193 (27.5) | .44 |
| Known arterial hypertension | 1190 (74.8) | 1207 (76.3) | .34 | 576 (81.7) | 580 (82.5) | .73 |
| Diabetes | 539 (33.9) | 523 (33.1) | .65 | 38 (5.4) | 45 (6.4) | .01 |
| Known hyperlipidemia | 1070 (67.3) | 1067 (67.5) | .91 | 215 (30.5) | 261 (37.1) | .33 |
| Current smoking | 184 (11.6) | 147 (9.3) | .04 | 46 (6.5) | 37 (5.3) | .37 |
| Known peripheral and/or vascular disease | 181 (11.4) | 183 (11.6) | .87 | 62 (8.8) | 59 (8.4) | .85 |
| History of heart failure | 329 (20.7) | 323 (20.4) | .86 | 100 (14.2) | 115 (16.4) | .27 |
| LVEF, mean (SD), % | 52.46 (11.49) | 52.26 (12.09) | .63 | 55.77 (11.00) | 54.50 (10.88) | .04 |
| Prior myocardial infarction | 333 (20.9) | 327 (20.7) | .86 | 101 (14.3) | 103 (14.7) | .88 |
| Prior PCI | 450 (28.3) | 441 (27.9) | .81 | 144 (20.4) | 153 (21.8) | .56 |
| Prior stroke | 134 (8.4) | 160 (10.1) | .11 | 59 (8.4) | 57 (8.1) | .92 |
| Prior coronary artery bypass grafting | 143 (9.0) | 142 (9.0) | >.99 | 27 (3.8) | 29 (4.1) | .79 |
| Prior bleeding before or after qualifying PCI | 133 (8.4) | 123 (7.8) | .56 | 51 (7.2) | 52 (7.4) | .92 |
| Known chronic pulmonary disease | 191 (12.0) | 201 (12.7) | .55 | 64 (9.1) | 82 (11.7) | .12 |
| Known liver disease | 22 (1.4) | 19 (1.2) | .75 | 7 (1.0) | 13 (1.8) | .19 |
| Atrial fibrillation | 567 (35.7) | 531 (33.6) | .23 | 203 (28.8) | 189 (26.9) | .44 |
| Known active cancer | 90 (5.7) | 97 (6.1) | .60 | 20 (2.8) | 29 (4.1) | .19 |
| Known hematological or coagulation disorders | 185 (11.6) | 154 (9.7) | .09 | 105 (14.9) | 134 (19.1) | .04 |
| Long-term treatment with corticosteroids or NSAIDs | 129 (8.1) | 156 (9.9) | .09 | 73 (10.4) | 83 (11.8) | .40 |
| Prior vitamin K antagonist treatment | 258 (16.2) | 233 (14.7) | .26 | 69 (9.8) | 66 (9.4) | .86 |
| Clinical indication for 12-mo OAC | 636 (40.0) | 612 (38.7) | .47 | 212 (30.1) | 206 (29.3) | .77 |
| PRECISE DAPT score, mean (SD)b | 26.12 (11.33) | 25.45 (10.85) | .09 | 28.37 (9.74) | 29.54 (11.00) | .04 |
| Prior bleeding | 127 (8.0) | 111 (7.0) | .31 | 38 (5.4) | 44 (6.3) | .50 |
| Hemoglobin level, mean (SD), g/dL | 1.3 (0.2) | 1.4 (0.2) | .45 | 1.3 (0.2) | 1.2 (0.2) | .006 |
| WBC count, cells/μLb | 8030 (3660) | 8030 (3500) | .98 | 8870 (19 890) | 8120 (3170) | .32 |
| Creatinine clearance MDRD, mean (SD), mL/min/1.73 m2 | 71.81 (24.53) | 73.04 (24.08) | .15 | 68.26 (22.55) | 66.41 (23.51) | .13 |
| Clinical presentationc | ||||||
| Stable angina | 637 (40.1) | 650 (41.1) | .56 | 285 (40.4) | 277 (39.4) | .70 |
| Silent ischemia | 195 (12.3) | 212 (13.4) | .34 | 50 (7.1) | 62 (8.8) | .24 |
| NSTEMI | 405 (25.5) | 376 (23.8) | .28 | 190 (27.0) | 182 (25.9) | .67 |
| STEMI | 170 (10.7) | 176 (11.1) | .73 | 103 (14.6) | 89 (12.7) | .31 |
| Unstable angina | 183 (11.5) | 167 (10.6) | .40 | 77 (10.9) | 93 (13.2) | .19 |
| Killip class II, III, or IV | 183 (11.5) | 168 (10.6) | .43 | 69 (9.8) | 86 (12.2) | .15 |
| Cardiac arrest | 21 (1.3) | 20 (1.3) | >.99 | 5 (0.7) | 12 (1.7) | .09 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); DAPT, dual antiplatelet therapy; LVEF, left ventricular ejection fraction; MDRD, modification of diet in kidney disease equation; NSAIDs, nonsteroidal anti-inflammatory drugs; NSTEMI, non–ST-segment elevation myocardial infarction; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; PRECISE DAPT, Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent DAPT; STEMI, ST-segment elevation myocardial infarction; WBC, white blood cell.
SI conversion factors: To convert hemoglobin to g/L, multiply by 10.0; WBC count to 109/L, multiply by 0.001.
Unless otherwise indicated, data are expressed as No. (%) of patients.
Calculated at screening visit; 1 PRECISE DAPT score was calculated without risk caused by white blood cell count. Scores range from 0 to 35, with higher scores indicating greater risk of bleeding.
Indicates data from first PCI only.
Clinical Outcomes by Sex
At 12 months (eTable 6 in Supplement 2), NACEs occurred in 243 of 3171 men (7.7%) and in 111 of 1408 women (7.9%) (unadjusted HR, 1.02 [95% CI, 0.82-1.28]; P = .83). MACCEs (unadjusted HR, 0.96 [95% CI, 0.75-1.25]; P = .78) and MCB (unadjusted HR, 0.98 [95% CI, 0.78-1.23]; P = .86) did not differ between men and women. There were no significant differences in the individual components of the coprimary and secondary outcomes. At multivariable adjustment for baseline confounders (eTable 6 in Supplement 2), the risk of NACEs, MACCEs, and MCB remained similar between the sexes.
Clinical Outcomes by Sex and Randomly Allocated DAPT Regimen
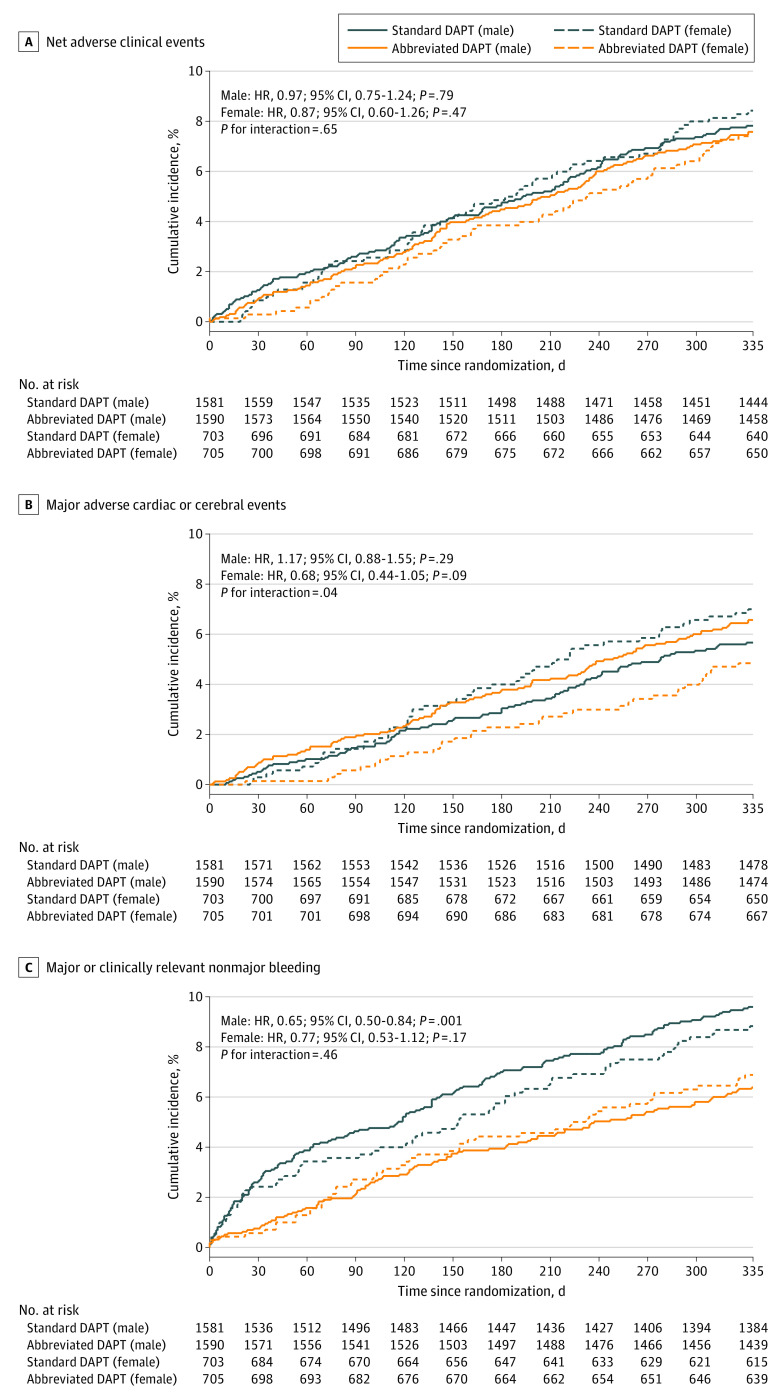
Clinical outcomes at 12 months in male or female patients stratified by DAPT regimen are shown in Figure 1 and Figure 2. NACEs did not differ by abbreviated and standard DAPT groups among male (120 [7.6%] vs 123 [7.8%]; HR, 0.97 [95% CI, 0.75-1.24]; P = .79) or female (52 [7.4%] vs 59 [8.4%]; HR, 0.87 [95% CI, 0.60-1.26]; P = .47) patients, with no heterogeneity at interaction testing (P = .65 for interaction) (Table 2). A significant interaction between randomized DAPT regimens by sex (P = .04 for interaction) was observed for MACCEs. When compared with standard DAPT, abbreviated DAPT was associated with numerically higher MACCE rates among male patients (89 [5.6%] vs 104 [6.5%]; HR, 1.17 [95% CI, 0.88-1.55]; P = .29) and lower rates among female patients (49 [7.0%] vs 34 [4.8%]; HR, 0.68 [95% CI, 0.44-1.05]; P = .09) (Table 2). Major or clinically relevant nonmajor bleeding was numerically lower with abbreviated DAPT in female patients (48 [6.8%] vs 61 [8.7%]; HR, 0.77 [95% CI, 0.53-1.12]; P = .17) and significantly lower among male patients (100 [6.3%] vs 150 [9.5%]; HR, 0.65 [95% CI, 0.50-0.84]; P = .001), with no interaction of treatment allocation by sex (P = .46 for interaction) (Table 2).
Figure 1. Net Adverse Clinical Events, Major Adverse Cardiac or Cerebral Events, and Major or Clinically Relevant Nonmajor Bleeding.
DAPT indicates dual antiplatelet therapy; HR, hazard ratio.
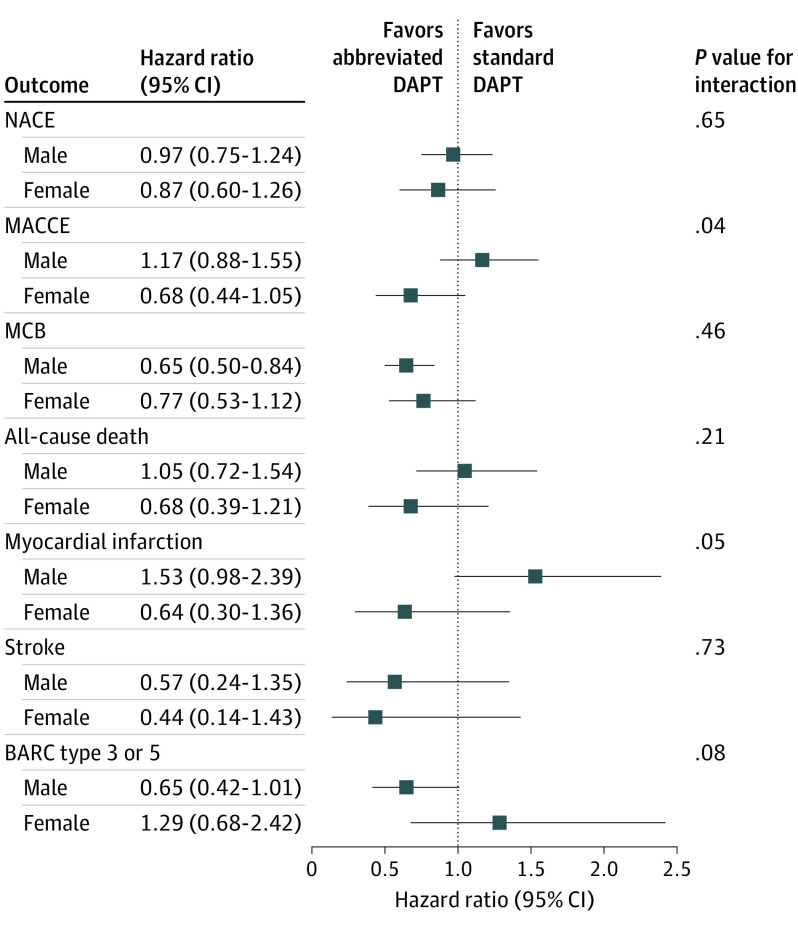
Figure 2. Main Outcomes of Abbreviated vs Standard Dual Antiplatelet Therapy (DAPT) in Male and Female Patients .
Abbreviated and standard DAPT were compared by sex subgroups, with hazard ratios and 95% CIs for the 3 coprimary outcomes and their components (all-cause death, myocardial infarction, stroke, and Bleeding Academic Research Consortium [BARC] type 3 or 5). MACCE indicates major adverse cardiac and cerebral event; MCB, major or clinically relevant nonmajor bleeding; and NACE, net adverse clinical event.
Table 2. Clinical Outcomes by Sex and Randomized Treatment Assignment at 11 mo After Randomization (12-mo Follow-Up)a.
| Outcome | Male patients | Female patients | P value for interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviated DAPT (n = 1590) | Standard DAPT (n = 1581) | HR (95% CI) | Com-Nogue risk difference (95% CI) | P value | Abbreviated DAPT (n = 705) | Standard DAPT (n = 703) | HR (95% CI) | Com-Nogue risk difference (95% CI) | P value | ||
| NACE | 120 (7.6) | 123 (7.8) | 0.97 (0.75 to 1.24) | −0.24 (−2.10 to 1.62) | .79 | 52 (7.4) | 59 (8.4) | 0.87 (0.60 to 1.26) | −1.01 (−3.84 to 1.81) | .47 | .65 |
| MACCE | 104 (6.5) | 89 (5.6) | 1.17 (0.88 to 1.55) | 0.91 (−0.76 to 2.58) | .29 | 34 (4.8) | 49 (7.0) | 0.68 (0.44 to 1.05) | −2.15 (−4.62 to 0.32) | .09 | .04 |
| MCB | 100 (6.3) | 150 (9.5) | 0.65 (0.50 to 0.84) | −3.21 (−5.11 to −1.31) | .001 | 48 (6.8) | 61 (8.7) | 0.77 (0.53 to 1.12) | −1.94 (−4.77 to 0.89) | .17 | .46 |
| Death | 55 (3.5) | 52 (3.3) | 1.05 (0.72 to 1.54) | 0.17 (−1.10 to 1.43) | .80 | 20 (2.8) | 29 (4.1) | 0.68 (0.39 to 1.21) | −1.29 (−3.21 to 0.63) | .19 | .22 |
| Cardiovascular | 26 (1.6) | 28 (1.8) | 0.92 (0.54 to 1.57) | −0.14 (−1.05 to 0.78) | .77 | 11 (1.6) | 16 (2.3) | 0.68 (0.32 to 1.47) | −0.73 (−2.18 to 0.72) | .33 | .52 |
| Noncardiovascular | 21 (1.3) | 20 (1.3) | 1.04 (0.57 to 1.93) | 0.06 (−0.74 to 0.86) | .89 | 8 (1.1) | 8 (1.1) | 0.99 (0.37 to 2.63) | −0.01 (−1.13 to 1.12) | .98 | .93 |
| Cerebrovascular accident | 11 (0.7) | 19 (1.2) | 0.57 (0.27 to 1.21) | −0.52 (−1.20 to 0.17) | .14 | 6 (0.9) | 13 (1.8) | 0.46 (0.17 to 1.20) | −1.03 (−2.26 to 0.20) | .11 | .71 |
| Strokeb | 8 (0.5) | 14 (0.9) | 0.57 (0.24 to 1.35) | −0.39 (−0.98 to 0.20) | .20 | 4 (0.6) | 9 (1.3) | 0.44 (0.14 to 1.43) | −0.74 (−1.76 to 0.28) | .17 | .73 |
| Ischemic | 7 (0.5) | 9 (0.6) | 0.77 (0.29 to 2.08) | −0.13 (−0.63 to 0.37) | .61 | 4 (0.6) | 9 (1.3) | 0.44 (0.14 to 1.43) | −0.74 (−1.76 to 0.28) | .17 | .47 |
| Hemorrhagic | 1 (0.1) | 5 (0.3) | 0.20 (0.02 to 1.70) | −0.26 (−0.57 to 0.05) | .14 | 0 | 0 | NA | NA | NA | NA |
| TIA | 3 (0.2) | 5 (0.3) | 0.60 (0.14 to 2.49) | −0.13 (−0.49 to 0.23) | .48 | 2 (0.3) | 4 (0.6) | 0.50 (0.09 to 2.70) | −0.29 (−0.98 to 0.40) | .42 | .87 |
| Myocardial infarction | 49 (3.1) | 32 (2.0) | 1.53 (0.98 to 2.39) | 1.08 (−0.04 to 2.19) | .06 | 11 (1.6) | 17 (2.4) | 0.64 (0.30 to 1.36) | −0.88 (−2.37 to 0.61) | .24 | .051 |
| Definite or probable stent thrombosis | 11 (0.7) | 5 (0.3) | 2.19 (0.76 to 6.31) | 0.38 (−0.12 to 0.89) | .15 | 3 (0.4) | 4 (0.6) | 0.74 (0.17 to 3.31) | −0.15 (−0.90 to 0.60) | .70 | .25 |
| Definite stent thrombosis | 9 (0.6) | 4 (0.3) | 2.24 (0.69 to 7.27) | 0.32 (−0.13 to 0.77) | .18 | 2 (0.3) | 3 (0.4) | 0.66 (0.11 to 3.93) | −0.15 (−0.79 to 0.49) | .65 | .26 |
| Probable stent thrombosis | 2 (0.1) | 1 (0.1) | 1.99 (0.18 to 21.94) | 0.06 (−0.16 to 0.28) | .57 | 1 (0.1) | 1 (0.1) | 0.99 (0.06 to 15.90) | 0.00 (−0.40 to 0.39) | >.99 | .71 |
| Bleeding BARC classification | |||||||||||
| Type 1 | 45 (2.8) | 76 (4.8) | 0.58 (0.40 to 0.84) | −2.00 (−3.35 to −0.65) | .004 | 20 (2.8) | 33 (4.7) | 0.59 (0.34 to 1.03) | −1.89 (−3.90 to 0.12) | .07 | .97 |
| Type 2 | 74 (4.7) | 104 (6.6) | 0.70 (0.52 to 0.94) | −1.95 (−3.57 to −0.32) | .02 | 28 (4.0) | 48 (6.8) | 0.57 (0.36 to 0.90) | −2.94 (−5.33 to −0.54) | .02 | .47 |
| Type 3 | 31 (1.9) | 42 (2.7) | 0.73 (0.46 to 1.16) | −0.70 (−1.76 to 0.35) | .18 | 22 (3.1) | 17 (2.4) | 1.29 (0.68 to 2.42) | 0.70 (−1.04 to 2.44) | .44 | .16 |
| Type 3a | 18 (1.1) | 22 (1.4) | 0.81 (0.44 to 1.51) | −0.25 (−1.04 to 0.53) | .51 | 8 (1.1) | 8 (1.1) | 0.99 (0.37 to 2.64) | −0.01 (−1.13 to 1.12) | .98 | .74 |
| Type 3b | 11 (0.7) | 12 (0.8) | 0.91 (0.40 to 2.06) | −0.06 (−0.66 to 0.54) | .82 | 10 (1.4) | 8 (1.1) | 1.24 (0.49 to 3.14) | 0.28 (−0.92 to 1.47) | .65 | .62 |
| Type 3c | 3 (0.2) | 8 (0.5) | 0.37 (0.10 to 1.40) | −0.32 (−0.74 to 0.09) | .14 | 4 (0.6) | 1 (0.1) | 3.96 (0.44 to 35.46) | 0.43 (−0.20 to 1.06) | .22 | .07 |
| Type 4 | 0 | 0 | NA | NA | NA | 0 | 0 | NA | NA | NA | NA |
| Type 5 | 2 (0.1) | 8 (0.5) | 0.25 (0.05 to 1.17) | −0.39 (−0.79 to 0.01) | .08 | 0 | 0 | NA | NA | NA | NA |
| Type 5a | 0 | 2 (0.1) | 0.20 (0.01 to 4.16) | −0.13 (−0.31 to 0.05) | .25 | 0 | 0 | NA | NA | NA | NA |
| Type 5b | 2 (0.1) | 6 (0.4) | 0.33 (0.07 to 1.64) | −0.26 (−0.62 to 0.10) | .18 | 0 | 0 | NA | NA | NA | NA |
| Type 3 or 5 | 33 (2.1) | 50 (3.2) | 0.65 (0.42 to 1.01) | −1.09 (−2.21 to 0.04) | .06 | 22 (3.1) | 17 (2.4) | 1.29 (0.68 to 2.42) | 0.70 (−1.04 to 2.44) | .44 | .08 |
Abbreviations: BARC, Bleeding Academic Research Consortium; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACCE, major adverse cardiac and cerebral event; MCB, major or clinically relevant nonmajor bleeding; NA, not applicable; NACE, net adverse clinical event; TIA, transient ischemic attack.
Data are expressed as No. of first events of each type (Kaplan-Meier failure %). Hazard ratios (95% CI) are calculated using Cox proportional hazards regression time-to–first event analyses in the intention-to-treat population. Continuity-corrected risk ratios (95% CI) were calculated in case of zero events with a Fisher exact test P value. An interaction P value was tested for a modifying effect of sex (male or female) on the HR scale.
Includes undetermined strokes.
There was no evidence of heterogeneity of the treatment effects by sex for any of the secondary end points (eFigure 4 in Supplement 2) except for MI (P = .05 for interaction), with a trend toward higher MI risk with abbreviated DAPT in male (HR, 1.53 [95% CI, 0.98-2.39]; P = .06) but not in female (HR, 0.64 [95% CI, 0.30-1.36]; P = .24) patients. The results remained entirely consistent with abbreviated vs standard DAPT regimens among patients with ACS (n = 2211) (eTable 7 in Supplement 2) or those with ACS and/or complex PCI (n = 2836) (eTable 8 in Supplement 2).
Outcomes in Male and Female Patients With or Without Clinical Indication for OAC
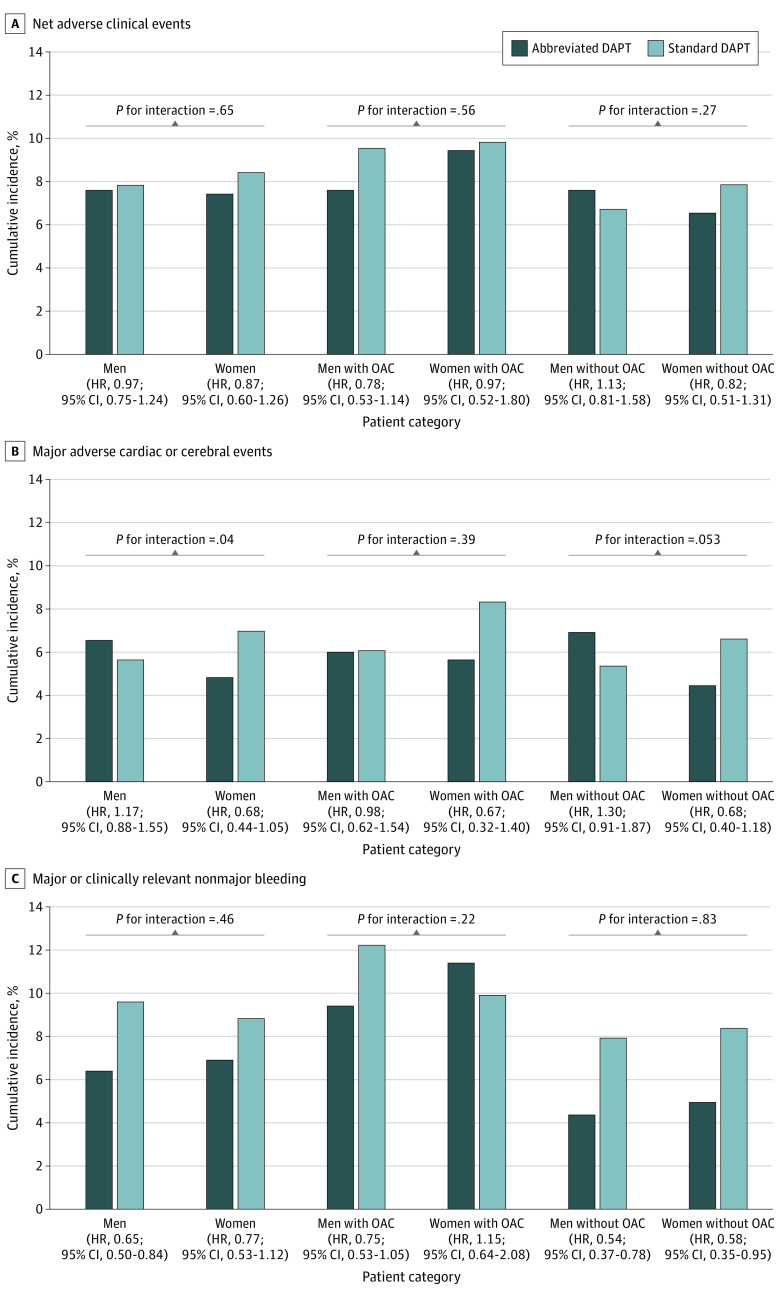
Among male and female patients with clinical indication for OAC (Figure 3 and eTable 9 in Supplement 2), NACEs, MACCEs, and MCB did not differ with abbreviated vs standard DAPT. Clinical outcomes at 12 months in patients without an indication for OAC are shown in Figure 3 and eTable 10 in Supplement 2. NACEs and MACCEs did not differ with abbreviated vs standard DAPT regimens among male and female patients without clinical indication for OAC, with no positive interaction (P = .27 for interaction) or borderline positive interaction (P = .053 for interaction) testing. Major or clinically relevant nonmajor bleeding was significantly and consistently reduced in male (HR, 0.54 [95% CI, 0.37-0.78]; P = .001) and female (HR, 0.58 [95% CI, 0.35-0.95]; P = .03) patients (P = .83 for interaction).
Figure 3. Interaction Between Sex and Dual Antiplatelet Therapy (DAPT) on Coprimary Efficacy Outcomes in the Overall Cohort and Stratified by Clinical Indication for Oral Anticoagulation (OAC).
The x-axis shows the categories of the patients according to sex and clinical indication for OAC; the y-axis shows event rates of the coprimary efficacy outcomes. HR indicates hazard ratio.
Discussion
To the best of our knowledge, this is the largest analysis to date investigating sex-based differences in patients with HBR and the association of sex with the comparative efficacy and safety of abbreviated vs standard DAPT. The main findings of this prespecified analysis from the MASTER DAPT trial are 3-fold. First, ischemic and bleeding events at 1 year after coronary revascularization did not differ between sexes before and after adjustment despite substantial differences in baseline characteristics between sexes. Second, there was no evidence of heterogeneity across sexes with respect to the 2 coprimary outcomes of NACEs and MCB, suggesting consistent treatment effects with abbreviated compared with prolonged DAPT in both sexes. Third, we observed a significant interaction between randomized treatment and sex (P = .04 for interaction) for MACCEs. Abbreviated DAPT was associated with a nominal 1% increase of MACCE rates in male patients and a more than 2% decrease of MACCE rates in female patients. However, these findings should be interpreted with caution since the 95% CI of the Com-Nogue risk difference included the null effect. The significant interaction for MACCEs was mainly due to different rates of MI. These results remained consistent in patients with ACS and/or complex PCI and accrued entirely from patients without clinical indication for OAC. Although these results come from subgroup analysis and should therefore be interpreted with caution (especially considering that randomization was not stratified by sex), the significant interaction between randomized DAPT and sex for MACCEs deserves further consideration. Our findings suggest for the first time that abbreviated DAPT should be considered for women with HBR in particular because they derive not only bleeding benefit, similarly to men, but also no discernible incremental ischemic risk compared with standard DAPT.
Increased bleeding risk in women compared with men has been reported in different studies of patients with ACS or PCI.7,8,9,10 This has been attributed to the higher prevalence of concomitant comorbidities such as advanced age, chronic kidney disease, and lower body mass index in women. In the contemporary PROMETHEUS10 and TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention) trials,8 women had an increased risk of bleeding compared with men, which was no longer significant after adjustment for baseline differences. At variance with the TWILIGHT trial, which randomized patients at 3 months to ticagrelor monotherapy or to aspirin and ticagrelor, we did not observe differences in the bleeding risk between sexes. The selection of patients with HBR, the shorter DAPT duration in the experimental group, and the inclusion of patients with OAC (who were excluded from that former study) in the MASTER DAPT trial may account for these differences. In a large and consecutive cohort of patients with PCI,13 only access site bleeding was shown to be higher in women, whereas overall and non–access site bleeding did not differ between sexes after adjustment. These findings suggest that the increased bleeding risk among women is mostly attributed to the different distribution of comorbid conditions rather than other indep6endent biological features.
Evidence on the independent association of sex with bleeding risk in patients with HBR is limited. The LEADERS FREE (Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent versus the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk) trial14 observed higher access site but not overall BARC type 3 or 5 bleeding risk in women with HBR after multivariable adjustment. At variance with the previous studies, inclusion of patients with HBR occurred at 1 month after PCI in the MASTER DAPT trial, thus excluding access site–related bleeding events.
The apparent different treatment effect of abbreviated DAPT in female as opposed to male patients does not seem justified by a differential bleeding benefit that, albeit consistent, was numerically less relevant in female patients than in male patients. One possible explanation might reside in the choice of antiplatelet agent (left at the investigator’s discretion) after DAPT discontinuation in the abbreviated DAPT group. Ticagrelor monotherapy was more frequently used in female vs male patients, although the difference was small. A prespecified analysis of the GLOBAL LEADERS trial20 did not find any difference based on sex in the primary or secondary outcomes with P2Y12 inhibitor monotherapy after 1 month of DAPT. However, a sex-based analysis from the TWILIGHT trial8 suggested that women, but not men, had lower mortality risk with ticagrelor monotherapy compared with DAPT.
Our results are consistent with a recent individual patient–level meta-analysis encompassing 24 096 patients who were randomized to P2Y12 inhibitor monotherapy (aspirin withdrawal 1 to 3 months after revascularization) vs standard DAPT after coronary revascularization.21 A significant heterogeneity of the treatment effect between treatment and sex was observed (P = .02 for interaction), suggesting that P2Y12 inhibitor monotherapy lowers the risk of the primary composite outcome of all-cause death, MI, and stroke in women (HR, 0.64 [95% CI, 0.46-0.89]) but not in men (HR, 1.00 [95% CI, 0.83-1.19]). Although the observed difference in this study was mainly attributable to cardiovascular death, P2Y12 inhibitor monotherapy resulted in a numerical reduction in MI risk in female but not in male patients. Our current results, largely driven by the different rates of MI, seem to extend to patients with HBR.
Limitations
Some limitations of this study should be considered. First, the MASTER DAPT was an open-label study, and randomization was performed at 1 month after PCI in patients who adhered to a DAPT regimen without ischemic and (active) bleeding events. Therefore, our results are not generalizable to patients with a complicated 30-day course after PCI. Second, randomization was not stratified by sex, and the lower proportion of female patients suggests a cautious interpretation of the results given the chance of a type II error. Third, DAPT duration was heterogeneous in the standard DAPT group and longer than currently recommended in both study groups among patients with clinical indication for 12-month OAC. Given the number of prespecified subgroups of interest and the lack of correction for multiplicity, our results remain exploratory and hypothesis generating. The type of SAPT also varied in the abbreviated DAPT group, with a higher use of ticagrelor monotherapy in female than male patients. Finally, our results were exclusively generated in patients with HBR and PCI treated with a sirolimus-eluting stent implantation; consequently, our results may not apply to an unselected population of patients undergoing PCI or those who receive other stent types.
Conclusions
In this prespecified comparative effectiveness analysis of the MASTER DAPT trial, female patients with HBR did not experience higher risks of bleeding and ischemic events compared with male patients despite several differences in baseline characteristics. The benefits of abbreviated over standard DAPT remained generally consistent in both sexes, albeit women may derive enhanced benefit from an abbreviated DAPT regimen owing to numerically lower rates of both bleeding and ischemic events. The latter findings should be regarded as exploratory and require prospective validation.
Trial Protocol and Statistical Analysis Plan
eAppendix. Committees and Investigators
eMethods. Additional Information
eTable 1. Details on DAPT and SAPT at 1-, 3-, 6- and 12-mo study visit
eTable 2. Baseline Characteristics According to Sex
eTable 3. Procedural Characteristics According to Sex
eTable 4. Procedural Characteristics by Randomized Antiplatelet Regimen and Sex
eTable 5. Treated Lesion Characteristics According to Sex and Randomly Allocated APT Regimen
eTable 6. Unadjusted and Adjusted Clinical Outcomes at 11 mo Post Randomization (12-mo Follow-Up) in Male and Female Patients
eTable 7. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients Presenting With ACS
eTable 8. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients Presenting With ACS or Who Had Complex PCI
eTable 9. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients With Clinical Indication for 12-mo OAC
eTable 10. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients Without Clinical Indication for 12-mo OAC
eFigure 1. CONSORT Diagram of the MASTER DAPT Study
eFigure 2. Antiplatelet Therapy Since Randomization in Male Patients Without and With Clinical Indication for Oral Anticoagulation (OAC) Therapy
eFigure 3. Antiplatelet Therapy Since Randomization in Female Patients Without and With Clinical Indication for Oral Anticoagulation (OAC) Therapy
eFigure 4. Kaplan Meier Curves for All-Cause Mortality, Myocardial Infarction, Stroke, and BARC Type 3 or 5 Bleeding
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Valgimigli M, Bueno H, Byrne RA, et al. ; ESC Scientific Document Group . 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS. Eur J Cardiothorac Surg. 2018;53(1):34-78. doi: 10.1093/ejcts/ezx334 [DOI] [PubMed] [Google Scholar]
- 2.Collet JP, Thiele H, Barbato E, et al. ; ESC Scientific Document Group . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 3.Valgimigli M, Costa F, Lokhnygina Y, et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur Heart J. 2017;38(11):804-810. doi: 10.1093/eurheartj/ehw525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueki Y, Bär S, Losdat S, et al. Validation of the Academic Research Consortium for High Bleeding Risk (ARC-HBR) criteria in patients undergoing percutaneous coronary intervention and comparison with contemporary bleeding risk scores. EuroIntervention. 2020;16(5):371-379. doi: 10.4244/EIJ-D-20-00052 [DOI] [PubMed] [Google Scholar]
- 5.Corpataux N, Spirito A, Gragnano F, et al. Validation of high bleeding risk criteria and definition as proposed by the academic research consortium for high bleeding risk. Eur Heart J. 2020;41(38):3743-3749. doi: 10.1093/eurheartj/ehaa671 [DOI] [PubMed] [Google Scholar]
- 6.Cao D, Mehran R, Dangas G, et al. Validation of the Academic Research Consortium high bleeding risk definition in contemporary PCI patients. J Am Coll Cardiol. 2020;75(21):2711-2722. doi: 10.1016/j.jacc.2020.03.070 [DOI] [PubMed] [Google Scholar]
- 7.Hess CN, McCoy LA, Duggirala HJ, et al. Sex-based differences in outcomes after percutaneous coronary intervention for acute myocardial infarction: a report from TRANSLATE-ACS. J Am Heart Assoc. 2014;3(1):e000523. doi: 10.1161/JAHA.113.000523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel B, Baber U, Cohen DJ, et al. Sex differences among patients with high risk receiving ticagrelor with or without aspirin after percutaneous coronary intervention: a subgroup analysis of the TWILIGHT randomized clinical trial. JAMA Cardiol. 2021;6(9):1032-1041. doi: 10.1001/jamacardio.2021.1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, Mehran R, Grinfeld L, et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85(3):359-368. doi: 10.1002/ccd.25630 [DOI] [PubMed] [Google Scholar]
- 10.Baber U, Sartori S, Aquino M, et al. Use of prasugrel vs clopidogrel and outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention in contemporary clinical practice: results from the PROMETHEUS study. Am Heart J. 2017;188:73-81. doi: 10.1016/j.ahj.2017.02.013 [DOI] [PubMed] [Google Scholar]
- 11.Chandiramani R, Cao D, Claessen BE, et al. Sex-related differences in patients at high bleeding risk undergoing percutaneous coronary intervention: a patient-level pooled analysis from 4 postapproval studies. J Am Heart Assoc. 2020;9(7):e014611. doi: 10.1161/JAHA.119.014611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Husted S, James SK, Bach RG, et al. ; PLATO Study Group . The efficacy of ticagrelor is maintained in women with acute coronary syndromes participating in the prospective, randomized, Platelet Inhibition and Patient Outcomes (PLATO) trial. Eur Heart J. 2014;35(23):1541-1550. doi: 10.1093/eurheartj/ehu075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spirito A, Gragnano F, Corpataux N, et al. Sex-based differences in bleeding risk after percutaneous coronary intervention and implications for the Academic Research Consortium high bleeding risk criteria. J Am Heart Assoc. 2021;10(12):e021965. doi: 10.1161/JAHA.121.021965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehran R, Chandrasekhar J, Urban P, et al. ; LEADERS FREE Investigators . Sex-based outcomes in patients with a high bleeding risk after percutaneous coronary intervention and 1-month dual antiplatelet therapy: a secondary analysis of the LEADERS FREE randomized clinical trial. JAMA Cardiol. 2020;5(8):939-947. doi: 10.1001/jamacardio.2020.0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123-e155. doi: 10.1161/CIR.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 16.Valgimigli M, Frigoli E, Heg D, et al. ; MASTER DAPT Investigators . Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. 2021;385(18):1643-1655. doi: 10.1056/NEJMoa2108749 [DOI] [PubMed] [Google Scholar]
- 17.Smits PC, Frigoli E, Tijssen J, et al. ; MASTER DAPT Investigators . Abbreviated antiplatelet therapy in patients at high bleeding risk with or without oral anticoagulant therapy after coronary stenting: an open-label, randomized, controlled trial. Circulation. 2021;144(15):1196-1211. doi: 10.1161/CIRCULATIONAHA.121.056680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frigoli E, Smits P, Vranckx P, et al. Design and rationale of the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen (MASTER DAPT) Study. Am Heart J. 2019;209:97-105. doi: 10.1016/j.ahj.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 19.Com-Nougue C, Rodary C, Patte C. How to establish equivalence when data are censored: a randomized trial of treatments for B non-Hodgkin lymphoma. Stat Med. 1993;12(14):1353-1364. doi: 10.1002/sim.4780121407 [DOI] [PubMed] [Google Scholar]
- 20.Chichareon P, Modolo R, Kerkmeijer L, et al. Association of sex with outcomes in patients undergoing percutaneous coronary intervention: a subgroup analysis of the GLOBAL LEADERS randomized clinical trial. JAMA Cardiol. 2020;5(1):21-29. doi: 10.1001/jamacardio.2019.4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valgimigli M, Gragnano F, Branca M, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ. 2021;373(1332):n1332. doi: 10.1136/bmj.n1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eAppendix. Committees and Investigators
eMethods. Additional Information
eTable 1. Details on DAPT and SAPT at 1-, 3-, 6- and 12-mo study visit
eTable 2. Baseline Characteristics According to Sex
eTable 3. Procedural Characteristics According to Sex
eTable 4. Procedural Characteristics by Randomized Antiplatelet Regimen and Sex
eTable 5. Treated Lesion Characteristics According to Sex and Randomly Allocated APT Regimen
eTable 6. Unadjusted and Adjusted Clinical Outcomes at 11 mo Post Randomization (12-mo Follow-Up) in Male and Female Patients
eTable 7. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients Presenting With ACS
eTable 8. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients Presenting With ACS or Who Had Complex PCI
eTable 9. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients With Clinical Indication for 12-mo OAC
eTable 10. Clinical Outcomes at 11 mo After Randomization (12-mo Follow-Up) in Male and Female Patients Without Clinical Indication for 12-mo OAC
eFigure 1. CONSORT Diagram of the MASTER DAPT Study
eFigure 2. Antiplatelet Therapy Since Randomization in Male Patients Without and With Clinical Indication for Oral Anticoagulation (OAC) Therapy
eFigure 3. Antiplatelet Therapy Since Randomization in Female Patients Without and With Clinical Indication for Oral Anticoagulation (OAC) Therapy
eFigure 4. Kaplan Meier Curves for All-Cause Mortality, Myocardial Infarction, Stroke, and BARC Type 3 or 5 Bleeding
Nonauthor Collaborators
Data Sharing Statement