Abstract
The reactions between seven fluorogenic substrates and different groups of enzymes, esterases, lipases, phosphatases, and dehydrogenases, were studied in a search for a new method for the detection of actinomycete spores. Fluorescence measurement was chosen as a fast and sensitive method for microbial analysis. The focus of the research was on the spores of important air contaminants: Streptomyces albus and Thermoactinomyces vulgaris. For the measurement of the enzymatic activity, the chosen fluorogenic substrate was added to a mixture of spores and nutrient media, and the resulting fluorescence was measured with a spectrofluorometer. Fluorogenic substrates were found to show enzymatic activities even for dormant spores. Comparison of the enzymatic activities of dormant spores with those of vegetative cells showed similarity of the enzymatic profiles but higher activity for vegetative cells. The increase of enzymatic activity from dormant spores to vegetative cells was not linear but fluctuating. The largest fluctuations were found after 4 to 5 h of incubation. The enzymatic activities of S. albus were 10 to 50 times lower than those of T. vulgaris, except for the dehydrogenase activity, which was seven times higher. These results indicate that analysis with fluorogenic substrates has the potential for becoming a fast and sensitive method for the enumeration and identification of airborne actinomycete spores.
Actinomycetes are recognized as important indoor air pollutants. Their appearance in indoor air can be used as an indicator of mold problems (3, 16). Similar to fungi, actinomycetes can grow on building materials in wet and warm places and spread their spores into the indoor air. Exposure to actinomycete spores can cause various adverse health effects (10, 14, 15). The physical diameter of actinomycete spores is about 1 μm, which makes their counting and identification from field samples difficult with optical microscopes due to masking by background particles. Therefore, airborne actinomycete spores have traditionally been analyzed by impaction onto special nutrient media and subsequent colony enumeration (3, 16). However, the forming of actinomycete colonies takes at least 1 week. In addition, microbial impaction may cause significant stress to viable microorganisms resulting from the mechanical interaction with a solid or semisolid medium, from desiccation, and from insufficient embedding in the nutrient medium (20). The viable spores of some species have dormant status and may need activation before the spore development can begin (5, 12). Thus, fast and reliable detection of these spores from air samples, especially without long preliminary growth, is highly desirable.
In a search for a reliable and faster method, actinomycete spores were analyzed in this study by measuring their enzymatic activity in the presence of artificial substrates. Enzymatic activity is an important feature of any live cell, and while the characteristics of enzymatic activities of vegetative cells are well known, less is known about that of spores (17, 18). Actinomycete spores have various enzymatic systems: enzymes involved in the central metabolism of carbon, nitrogen, and phosphorous compounds; enzymes catalyzing dehydrogenation reactions; cytochrome a, b, and c, and catalase (5). The enzymatic activity changes during the transformation of the spores from the dormant stage to emergence of germination tubes and appearance of the vegetative cells (11). These features were found by traditional biochemical methods, which are complicated and time-consuming.
The enzymatic activity of microorganisms can be detected and measured by using artificial substrates. Fluorogenic substrates engage directly with special enzymes of live cells through biochemical reactions and turn to fluorescent substances inside or outside the cell. The amount of the resulting fluorescence depends on the enzymatic activity of the cell. This feature is used in cyto- and histochemistry, as well as in microbiology for the analysis of vegetative cells (6, 7, 19). However, not much is known about the interaction between fluorogenic substrates and enzymes of airborne actinomycete spores. Furthermore, the penetration of large molecules of fluorogenic substrates through a thick and multilayered spore sheath and their involvement in spore metabolism need study.
In this study, we investigated the reactions between seven fluorogenic artificial substrates and the following groups of enzymes: esterases, lipases, phosphatases, and dehydrogenases. In these biochemical reactions, colorless, nonfluorescent fluorogenic substrates are cleaved by microbial enzymes into highly fluorescent substances (8). Because fluorescence analysis is one of the most sensitive methods for analytical purposes (6), it was believed that the fluorogenic substrate method could be developed into a sensitive, fast, and reliable method for actinomycete spore analysis. The first phase of this method development was focused on the following aspects: (i) investigation of the enzymatic activities of dormant actinomycete spores by using fluorogenic substrates and (ii) study of the changes in enzymatic activity of actinomycete spores during their development from dormant cells to emergence and to vegetative cells.
MATERIALS AND METHODS
Microorganisms and growth conditions.
Two actinomycete species were used in the experiments: Streptomyces albus (ATCC 3004) and Thermoactinomyces vulgaris (ATCC 43649). The test spores were grown according to the recommendations of the American Type Culture Collection (1). S. albus was grown on ISP medium 2 (ISP2; Difco Laboratories, Detroit, Mich.) at 37°C for 7 days. T. vulgaris was grown on Trypticase soy agar (TSA; Becton Dickinson Microbiological Systems, Cockeysville, Md.) at 50°C for 7 days. These conditions were sufficient for spore development.
Preparation of spores.
The spores were removed from the nutrient media and collected on filters for storage. First, the spores were washed off the ISP2- or TSA-filled petri plates with 10 to 15 ml of a 0.01% solution of Tween 80 (Fisher Scientific, Pittsburgh, Pa.). The resulting suspension consisted primarily of spores but also included some vegetative cells. The latter were then killed, as live cells may increase the background fluorescence during the enzymatic activity measurements of the spores and negatively affect the accuracy of the measurements. This was achieved by filtering the suspension through white cellulosic 47-mm-diameter filters (MSI, Westboro, Mass.) with a pore size of 0.45 μm and then treating the microorganisms on the filter with 5 ml of a 30% ethanol solution (in water) for 1 min. Our preliminary experiments with both species showed that the ethanol concentration and the treatment time were sufficient to kill the vegetative cells without killing the spores (data not shown). The filters with the live spores were stored in an airtight box containing dry silica gel at 4°C for at least 3 days. Storage at 4°C is appropriate for long-term storage of dormant spores (4) and for transformation of the spores from the activated to the dormant stage (12).
Prior to each experiment, the dormant spores were washed off the filters by shaking the filters for 10 min in a liquid mixture containing 20% autoclaved nutrient broth and 80% autoclaved NaCl solution in distilled water (9 g of NaCl in 1 liter). The ISP2 broth was used for S. albus, and TSA broth was used for T. vulgaris. As all repeats of the tests on the enzymatic activity needed to be performed at the same spore concentration, the concentration was adjusted to the same level for each repetition by measuring the optical transmission of the solution with a spectrophotometer at a wavelength of 600 nm (Spectronic 21D; Milton Roy Co., Rochester, N.Y.). Distilled water was used as the standard for 100% optical transmission. The mixture of spores and nutrient solution was diluted with nutrient solution until the optical transmission reached the desired value, as indicated below. After the appropriate spore concentration was achieved, the mixture was divided into two parts. The first part (25 to 30 ml) was used for measuring the enzymatic activity of dormant spores. The second part (180 to 200 ml) was activated and used for measuring the changes of the spore enzymatic activity during the development of the spores from the dormant stage to emergence and to the vegetative cell stage. The spores were activated by heating the suspension of S. albus for 10 min at 45°C and the suspension of T. vulgaris for 10 min at 70°C (13). The activated mixture was poured into eight petri plates, 25 ml in each, and was incubated at 37°C for S. albus and at 50°C for T. vulgaris. These petri plates were removed from the incubator one by one, at the time intervals indicated below, and the enzymatic activities of the spores were then determined.
The appearance of vegetative cells at the end of each experiment was monitored by observing the shape of the cells with a phase-contrast microscope (Labophot-2; Nikon Corp., Tokyo, Japan).
Enzymatic activity measurements.
The measurements of the fluorescence were performed with a spectrofluorometer (Model LS-5; Perkin-Elmer Corp., Oak Brook, Ill.). The enzymatic activities of the spores were measured with seven different fluorogenic substrates. We used four fluorogenic substrates based on 4-methylumbelliferone: 4-methylumbelliferyl phosphate disodium salt (MUPh), 4-methylumbelliferyl acetate (MUA), 4-methylumbelliferyl butyrate (MUB), and 4-methylumbelliferyl propionate (MUPr). Two fluorogenic substrates were based on fluorescein (fluorescein diacetate [FDA] and fluorescein dipropionate [FDPr]), and one was based on resorufin:resazurin (RSZ) (see Table 1). The concentration of all fluorogenic substrates in the storage solutions was 0.2 mg/ml. The solvent for the storage solutions was acetone optima (Fisher Scientific) for MUA, MUB, MUPr, FDA, and FDPr. The distilled water was utilized as the storage solution for RSZ and MUPh.
TABLE 1.
Fluorogenic substrates used to detect enzymatic activities of actinomycete spores with a spectrofluorometer with the indicated wavelengths
| Fluorogenic substratea | Enzymes detected with substrate | Fluorescence measurement
|
|
|---|---|---|---|
| Excitation wavelength (nm) | Emission wavelength (nm) | ||
| MUA | Esterases | 320 | 450 |
| MUB | Lipases | 320 | 450 |
| MUPr | Lipases | 320 | 450 |
| FDA | Esterases | 480 | 515 |
| FDPr | Lipases | 480 | 515 |
| RSZ | Dehydrogenases | 560 | 590 |
| MUPh | Phosphatases | 320 | 450 |
a MUA, MUB, MUPr, and MUPh were from Sigma Chemical Co., St. Louis, Mo.; FDA, FDPr, and RSZ were from ICN Biochemicals Inc., Aurora, Ohio.
The measurements of the enzymatic activities were performed for dormant spores (time zero) and for activated spores. In the first set of experiments, the activated spores were analyzed at different stages by incubating the mixture of spores and nutrient solution on petri plates at the specified temperature for 2, 4, and 6 h before the measurement. In this set of experiments, the optical transmission of the suspension containing the spores and nutrient solution was 95.5%, which corresponded to a concentration of approximately 2 · 106 CFU/ml for both actinomycete species, as determined by cultivating dilutions on agar medium. For the measurements, each of seven glass tubes, one for each fluorogenic substrate, received 2.5 ml of the incubated mixture of spores and nutrient media. Then, 0.25 ml of the fluorogenic substrate solution was added to each tube and the fluorescence was measured immediately. This first measurement gave the background fluorescence. After this, the solutions were incubated for 30 min at 40°C and the fluorescence was measured again. This measurement gave the total fluorescence, which includes the background fluorescence and the fluorescence resulting from the enzymatic activity. The enzymatic activity of each substrate was determined as the difference between total and background fluorescence.
In the second set of experiments, the time changes in enzymatic activity of T. vulgaris spores were measured every 30 min with two of the fluorogenic substrates, FDPr and RSZ. In these experiments, a higher spore concentration was used to reduce the time of incubation with substrates from 30 to 10 min, and thus, the concentration permitted more frequent fluorescence measurements. The optical transmission of the mixture of spores and diluted nutrient media was 92%.
All measurements were performed under the same conditions of the spectrofluorometer: the excitation slit was set to the minimum not exceeding 0.1 nm, and the emission slit was set to the minimum of 3 nm. The fluorescence wavelengths used in this study have been presented in Table 1. The results are expressed as arbitrary units because the focus of this study was to investigate relative changes and differences in fluorescence, not the absolute fluorescence values. Each experiment was repeated four times.
Statistical analysis.
All statistical tests were conducted with the analysis of variance procedures of the Statistical Analysis System (SAS Institute, Inc., Cary, N.C.). Scheffe’s test was used to locate the difference that the analysis of variance indicated. The acceptance level for statistical significance was P < 0.05.
RESULTS
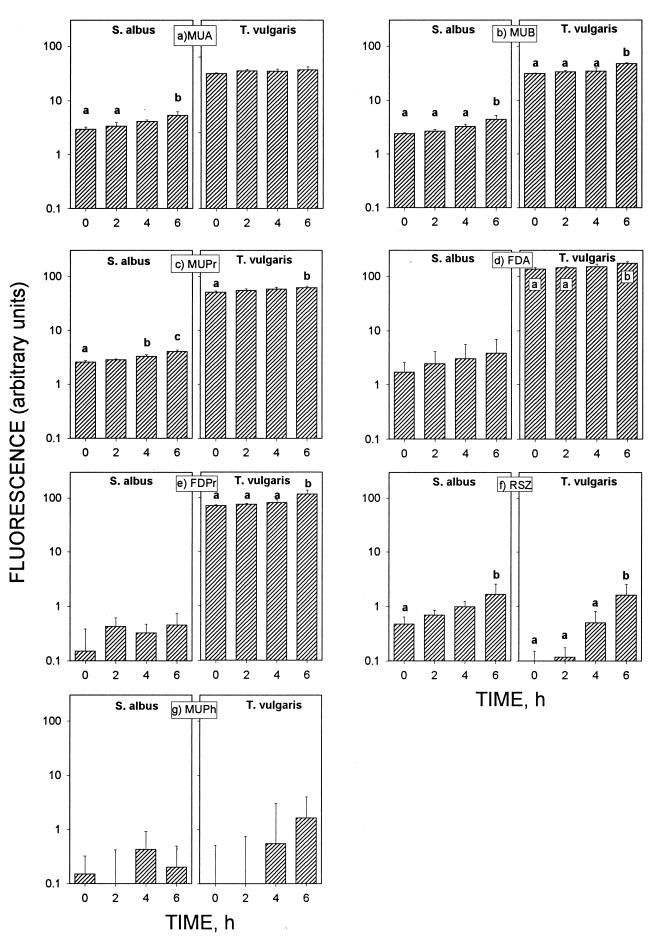
The data on the enzymatic activity of spores obtained at t = 0, 2, 4, and 6 h are presented in Fig. 1. These measurements cover the time needed for the spore development from the dormant stage to the emergence of the vegetative cell. Enzymatic activity is shown in dormant spores with fluorogenic substrates. Both S. albus and T. vulgaris showed esterase, lipase, and dehydrogenase activity with MUA, MUB, MUPr, FDA, FDPr, and RSZ (Fig. 1a to f). Phosphatase activity (MUPh) was found to be very weak for both S. albus and T. vulgaris in the dormant stage (Fig. 1g). With most of the substrates, the fluorescence, i.e., the enzymatic activities of spores, increased with increasing incubation time. However, the magnitude of enzymatic activity changes was different for different fluorogenic substrates. Esterase and lipase activities (MUA, MUB, MUPr, FDA, and FDPr) showed modest increases, about 1.1 to 3.0 times, from the dormant stage until the emergence stage (Fig. 1a to e), whereas dehydrogenase (RSZ) activity increased 3.5 times in S. albus spores and 17.3 times in T. vulgaris spores (Fig. 1f). Some measurable amounts of phosphatase activity (MUPh) were found only after 4 and 6 h of incubation of T. vulgaris. All enzymatic activities, except dehydrogenase activity (RSZ), were higher in T. vulgaris spores than in S. albus spores.
FIG. 1.
Enzymatic activities of S. albus and T. vulgaris spores with seven different fluorogenic substrates during spore development. Measurements at time zero were with dormant spores; other measurements were with activated spores after 2, 4, and 6 h of incubation. Error bars indicate the standard deviations from the means of four repeats. Statistical analysis with analysis of variance and Scheffe’s test indicated that a < b < c (P < 0.05).
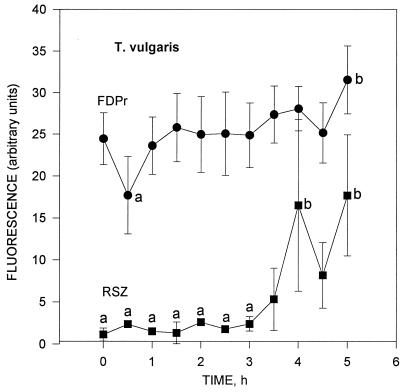
The enzymatic activity changes measured every 0.5 h with T. vulgaris by using FDPr (lipase activity) and RSZ (dehydrogenase activity) are presented in Fig. 2. Activation of the dormant T. vulgaris spores done at t = 0.5 h (Fig. 2) slightly increased the dehydrogenase activity. After that, the activities remained fairly constant between 1 and 3.5 h. Large fluctuations appeared between t = 3.5 and 5 h, especially with RSZ (dehydrogenase activity).
FIG. 2.
Lipase (FDPr) and dehydrogenase (RSZ) enzymatic activities of T. vulgaris measured every 30 min. The enzymatic activity of dormant spores is seen at t = 0; that of activated spores is seen at t = 0.5 h. The measurements between 1 and 5 h show the enzymatic activity during the spore development from activated spore status toward the vegetative cell status. Error bars indicate the standard deviations from the means of four repeats. Statistical analysis with analysis of variance and Scheffe’s test indicated that a < b (P < 0.05).
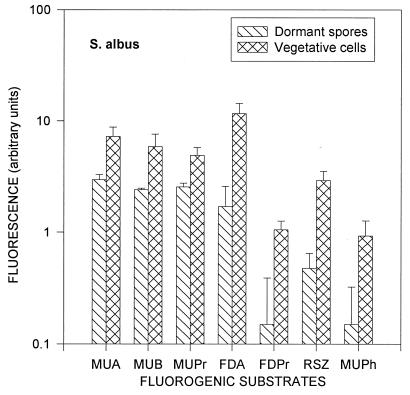
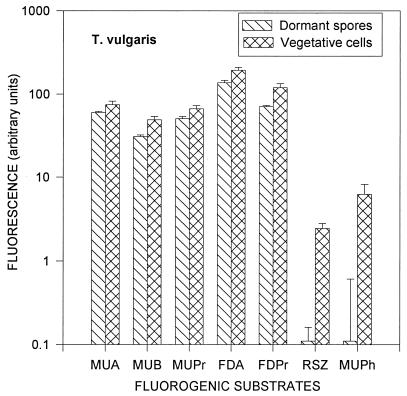
After 7 to 7.5 h of incubation, all spores were transformed into vegetative cells, which was confirmed by examining the shape of the cells under a phase-contrast microscope. Differences in enzymatic activities between dormant spores and vegetative cells are shown in Fig. 3 for S. albus and in Fig. 4 for T. vulgaris. The enzymatic activities of S. albus vegetative cells were two to seven times higher than those of dormant spores. The same ratio for T. vulgaris ranged from 1 to 25, except for the phosphatase activity, which was almost absent in the dormant stage. S. albus had the largest increases from dormant spore to vegetative cell in esterase (FDA), lipase (FDPr), dehydrogenase (RSZ), and phosphatase (MUPh) activities. T. vulgaris had the largest increases in lipase (FDPr), dehydrogenase (RSZ), and phosphatase (MUPh) activities. Similar to the spores, all enzymatic activities of the S. albus vegetative cells, except for the dehydrogenase activity (RSZ), were lower than those of T. vulgaris.
FIG. 3.
Enzymatic activities of dormant spores and vegetative cells of S. albus. Error bars indicate the standard deviations from the means of four repeats.
FIG. 4.
Enzymatic activities of dormant spores and vegetative cells of T. vulgaris. Error bars indicate the standard deviations from the means of four repeats.
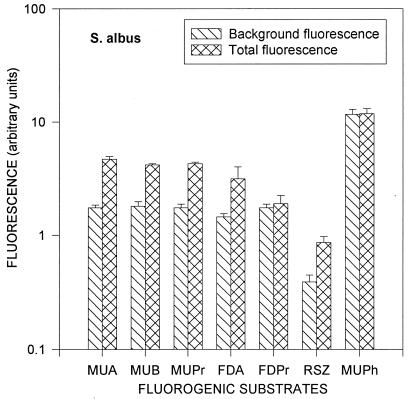
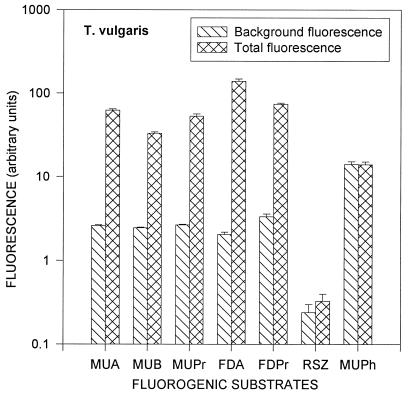
Information about the difference between background fluorescence and the fluorescence caused by enzymatic activity is very important for creating a method of actinomycete spore enumeration based on fluorescence measurements. This difference is shown for dormant spores of S. albus in Fig. 5 and dormant spores of T. vulgaris in Fig. 6. These figures suggest that the background fluorescence is caused mainly by the nutrient solution and that the difference between the total fluorescence and the background fluorescence is caused by the enzymatic activity of the dormant spores. Except for MUPh, the total fluorescence was 1.1 to 3 times higher than the background for S. albus and 1.4 to 70 times higher for T. vulgaris. The largest increases of the total fluorescence compared to the background fluorescence were observed with MUA, MUB, MUPr, and FDA for both species, as well as with RSZ for S. albus and with FDPr for T. vulgaris (Fig. 5 and 6).
FIG. 5.
Background fluorescence and total fluorescence (background plus fluorescence resulting from enzymatic activity) of dormant spores of S. albus. Error bars indicate the standard deviations from the means of four repeats.
FIG. 6.
Background fluorescence and total fluorescence (background plus fluorescence resulting from enzymatic activity) of dormant spores of T. vulgaris. Error bars indicate the standard deviations from the means of four repeats.
DISCUSSION
The data presented in Fig. 1a to g show that all fluorogenic substrates reacted with spore enzymes. For these experiments, only those fluorogenic substrates that react with large groups of enzymes were used: esterases, lipases, phosphatases, and dehydrogenases. Each group contains tens of specific enzymes that react with the substrate and create the average esterase, lipase, phosphatase, or dehydrogenase activity.
Unexpectedly, one of the largest and most diverse enzyme groups, phosphatases, showed very weak activity in the dormant stage of both species. There are several possible explanations for this phenomenon. The nutrient solutions used for spore incubation could have contained many more available soluble phosphates than phosphate of MUPh. The pH conditions may not have been favorable for MUPh acceptance by phosphatases, or soluble MUPh joined another reaction(s) before contacting phosphatases. It is also possible that the spores of S. albus and T. vulgaris do not contain phosphatases in the dormant stage and start to exhibit phosphatase activity only after 3 to 4 h of incubation with nutrient solution. The last postulate suggests that the phosphatase activity could be used as a marker between dormant spores and vegetative cells. Almost all groups of enzymes were more active in T. vulgaris spores than in S. albus spores. We can connect this phenomenon with the high level of T. vulgaris thermoresistance. It is known that organisms developed under a high temperature have enhanced levels of metabolism (2), i.e., enhanced enzymatic activity. This feature suggests that it may be used to differentiate thermoresistant from mesophilic microorganisms. However, further experiments with other thermoresistant microorganisms are needed to prove this hypothesis.
Only dehydrogenase activity (RSZ) was higher in spores of S. albus than in spores of T. vulgaris, but only in the beginning of incubation. This may be due to the smaller amount of cytochrome a in thermophilic than in mesophilic spores in the dormant stage (21), which leaves less opportunity for respiration and activity of the dehydrogenase system. The dehydrogenase activity increased faster in T. vulgaris than in S. albus spores during incubation and after 6 h of incubation reached the same level for both species. We attribute the faster increase of the dehydrogenase activity in thermophilic T. vulgaris spores than in S. albus spores to the fast increase in the amount of cytochrome a and c during the transformation of thermophilic spores into vegetative cells (21).
The fluctuations in enzymatic activity during spore development, measured every 0.5 h (Fig. 2), can be attributed to the substantial biochemical changes in the spores during their transformation from dormant spores to vegetative cells. The sequence of events in germination includes endogenous metabolism, excretion of calcium-dipicolinic acid, loss of the spore cortex, lipid turnover, active RNA synthesis, DNA synthesis, and other events caused by lipases, proteases, and dehydrogenases (8, 11, 18). Conversion from one phase to another significantly changes the enzyme composition. In our tests, each fluorogenic substrate represented a large group of enzymes; therefore, changes inside each group cannot be distinguished. Nevertheless, increases in the lipase activity are probably caused by the transformation of spores into more metabolically active vegetative cells, and increases in the dehydrogenase activity after 3.5 to 5 h (Fig. 2) can be explained through the transformation of endogenous into exogenous respiration.
Comparison of the enzymatic activities in spores and vegetative cells shows higher activity of the vegetative cells (Fig. 3 and 4). This can be explained by the more active metabolism of vegetative cells, by the exchange of nutrients with the surrounding environment, and by reproduction. It appears that the detected large difference between dormant spores and vegetative cells in dehydrogenase (RSZ) and phosphatase (MUPh) activities can be used in the future to distinguish the spores from vegetative cells. Except for the phosphatase and dehydrogenase activities, the enzymatic activities of dormant spores followed the pattern of activities of vegetative cells, but at lower levels. This finding may also be useful in the development of future identification methods for spore species.
The accuracy and reliability of the fluorescent method strongly depend on the amount and fluctuations of the background fluorescence. Figure 5 and 6 show comparisons of the background fluorescence with the total fluorescence after 0.5 h of incubation with the substrates. The total fluorescence has to be sufficiently higher than the background fluorescence for enzymatic activity measurements. Spores of T. vulgaris show a greater difference between the background and total fluorescence levels than do spores of S. albus, with the exception of RSZ and MUPh. Background and total fluorescence levels for phosphatase activity (MUPh) were almost the same for both S. albus and T. vulgaris, indicating that the dormant spores have very low phosphatase activity.
It appears that the background fluorescence may be decreased, thus increasing the sensitivity of the measurements. In our experiments, the background fluorescence was mainly caused by the nutrient solution (20% nutrient broth in NaCl solution). It included different fluorescent substances such as proteins and vitamins and had maximum fluorescence at 420 nm when excited at 320 nm and at 530 nm when excited at 480 nm, i.e., close to the wavelengths used in this study (fluorescence at 450, 515, and 590 nm and excitation at 320, 480, 560 nm, respectively). If the fluorescence measurements are performed for only dormant spores, there is no need for incubation with a nutrient solution. Therefore, a buffer that does not give any background fluorescence could substitute for the nutrient solution.
According to our study, MUA, MUB, MUPr, and FDA appear to be the most suitable fluorogenic substrates for the analysis of the live spores of both tested species. These substrates can be used with macroanalytical instruments, such as fluorometers and microplate readers, or with microanalytical instruments, such as luminescence microscopes or flow cytometer systems. The development of this methodology needs further research. For example, the possible interferences due to environmental debris in field samples have to be studied. However, the results of this study show that measurements of the enzymatic activities with fluorogenic substrates can be used for spore analysis without long preliminary growth. It is hoped that our data will be useful for the development of fast and sensitive methods for enumeration and identification of indoor air spores.
Conclusions.
The results of this study indicate that enzymatic activities of actinomycete spores can be measured by using artificial fluorogenic substrates. With this method, even dormant spores show enzymatic activities. The enzymatic activities of spores were found to increase when the spores develop from the dormant stage into vegetative cells. This increase with time is not linear and reflects biochemical changes that occur in spores during their development. The increase in enzymatic activity is different for different substrates. The enzymatic profiles of dormant spores significantly differ from those of vegetative cells. The enzymatic activities of S. albus and T. vulgaris were found to have some special characteristics that can be used to differentiate them. Our results indicate that measurements with fluorogenic substrates have the potential for development into a fast and sensitive method for the enumeration and identification of airborne actinomycete spores.
ACKNOWLEDGMENT
This work was supported by the U.S. Center for Indoor Air Research (CIAR) through a postdoctoral fellowship to Sergey V. Gazenko. We are thankful for this support.
REFERENCES
- 1.American Type Culture Collection. Catalogue of bacteria and bacteriophages. Rockville, Md: American Type Culture Collection; 1992. [Google Scholar]
- 2.Bubela B, Holdworth E S. Protein synthesis in Bacillus stearothermophilus. Biochim Biophys Acta. 1966;123:364–375. doi: 10.1016/0005-2787(66)90290-5. [DOI] [PubMed] [Google Scholar]
- 3.Cole E C, Foarde K K, Leese K E, Green D A, Franke D L, Berry M A. Assessment of fungi in carpeted environments. Air Qual Monogr. 1994;2:103–128. [Google Scholar]
- 4.Dufrenne J, Tatini S, Notermans S. Stability of spores of Bacillus cereus stored on silicagel. Int J Food Microbiol. 1994;23:111–116. doi: 10.1016/0168-1605(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 5.Ensign J C. Formation, properties and germination of actinomycete spores. Annu Rev Microbiol. 1978;32:185–219. doi: 10.1146/annurev.mi.32.100178.001153. [DOI] [PubMed] [Google Scholar]
- 6.Guilbault G G, editor. Practical fluorescence. 2nd ed. New York, N.Y: Marcel Dekker; 1973. pp. 20–22. [Google Scholar]
- 7.Haugland R P. Detecting enzymatic activity in cells using fluorogenic substrates. Biotech Histochem. 1995;70:243–251. doi: 10.3109/10520299509108201. [DOI] [PubMed] [Google Scholar]
- 8.Haugland R P. Handbook of fluorescent probes and research chemicals. 6th ed. Eugene, Oreg: Molecular Probes Inc.; 1996. pp. 202–244. [Google Scholar]
- 9.Hirsch C F, Ensign J C. Germination of Streptomyces viridochromogenes spores. In: Gerhardt P, Costilow R N, Sadoff H L, editors. Spores. Washington, D.C: American Society for Microbiology; 1975. pp. 28–35. [Google Scholar]
- 10.Hirvonen M-R, Nevalainen A, Makkonen M, Mönkkönen J, Savolainen K. Streptomyces spores from moldy houses induce nitric oxide, TNFX and IL-6 secretion from RAW264.7 macrophage cell line without causing subsequent cell death. Environ Toxicol Pharmacol. 1997;3:57–63. doi: 10.1016/s1382-6689(96)00140-8. [DOI] [PubMed] [Google Scholar]
- 11.Kalakoutskii L V, Agre N S. Comparative aspects of development and differentiation in actinomycetes. Bacteriol Rev. 1976;40:469–524. doi: 10.1128/br.40.2.469-524.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keynan A, Evenchik Z. Activation. In: Gould G W, Hurst A, editors. The bacterial spore. London, United Kingdom: Academic Press; 1969. pp. 359–394. [Google Scholar]
- 13.Kirillova I P, Agre N S, Kalakoutskii L V. Conditions for initiation of Thermoactinomyces vulgaris spores. Microbiologija. 1973;42:867–872. [PubMed] [Google Scholar]
- 14.Lacey J, Crook B. Fungal and actinomycete spores as pollutants of the work place and occupational allergens. Ann Occup Hyg. 1988;32:515–533. doi: 10.1093/annhyg/32.4.515. [DOI] [PubMed] [Google Scholar]
- 15.Lacey J, Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci. 1994;25:1371–1404. [Google Scholar]
- 16.Nevalainen A, Pasanen A L, Niininen M, Reponen T, Jantunen M J, Kalliokoski P. The indoor air quality in Finnish homes with mold problems. Environ Int. 1991;17:1299–1302. [Google Scholar]
- 17.Sadoff H L. Spore enzymes. In: Gould G W, Hurst A, editors. The bacterial spore. London, United Kingdom: Academic Press; 1969. pp. 275–297. [Google Scholar]
- 18.Setlow P. Biochemistry of bacterial forespore development and spore germination. In: Levinson H L, Sonenshein A L, Tipper D J, editors. Sporulation and germination. Washington, D.C: American Society for Microbiology; 1981. pp. 13–29. [Google Scholar]
- 19.Stewart A, Deacon J W. Vital fluorochromes as tracers for fungal growth studies. Biotech Histochem. 1995;70:57–65. doi: 10.3109/10520299509108318. [DOI] [PubMed] [Google Scholar]
- 20.Stewart S L, Grinshpun S A, Willeke K, Terzieva S, Ulevicius V, Donnelly J. Effect of impact stress on microbial recovery on an agar surface. Appl Environ Microbiol. 1995;61:1232–1239. doi: 10.1128/aem.61.4.1232-1239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taptikova S D, Kalakoutskii L V, Agre N S. Cytochromes in spores of actinomycetes. J Gen Appl Microbiol. 1969;15:383–386. [Google Scholar]