Abstract

We present an enhanced method for synthesizing a novel compound, 1-(4-phenylquinolin-2-yl)propan-1-one (3), through the solvent-free Friedländer quinoline synthesis using poly(phosphoric acid) as an assisting agent. The crystal structure of compound 3 is analyzed using FT-IR, and the chemical shifts of its 1H- and 13C NMR spectra are measured and calculated using B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) basis sets in the gas phase. Additionally, the optimized geometry of quinoline 3 is compared with experimental X-ray diffraction values. Through density functional theory calculations, we explore various aspects of the compound’s properties, including noncovalent interactions, Hirshfeld surface analysis, nonlinear optical properties, thermodynamic properties, molecular electrostatic potential, and frontier molecular orbitals. These investigations reveal chemically active sites within this quinoline derivative that contribute to its chemical reactivity.
1. Introduction
The quinoline scaffold is an abundant heterocyclic structural core that exists in numerous naturally found quinoline alkaloids.1 The quinoline skeleton is used as a template for several synthetic compounds with various pharmacological properties, including antimalarial, anti-inflammatory, antiasthmatic, antibacterial, antiviral, and antihypertensive and tyrosine kinase inhibiting agents.1−8 Researchers continue to seek new methodologies and products for synthesizing quinoline-based moieties. Various classic techniques to synthesize quinoline moiety include the Skraup,9,10 Doebner–von Miller,11 Pfitzinger,12 Conrad–Limpach,13 Combes syntheses,14 and Povarov reactions.15,16 Among them, a notable approach for attaining quinolines and related poly-heterocycles involves Friedländer quinoline synthesis.17−19 Among these methods, the Friedländer synthesis is the most effective and prominent protocol for the synthesis of quinolines in recent years.19−23 It has become the evergreen synthetic approach to prepare quinoline derivatives by the condensation of easily accessible 2-aminoarylketones with carbonyl compounds possessing a reactive methylene group, followed by cyclodehydration.24−28 The most exciting method for improving organic synthesis is continuously reporting new methodologies and catalytic reactions. A solvent-free synthesis is an essential synthetic approach from the perspective of synthetic organic chemistry. Recently, sulfuric acid functionalized heterogeneous catalysts have developed as user-friendly catalysts due to their remarkable advantages over homogeneous catalytic systems, such as prolonged reaction times, enhanced selectivity, the comfort of product purification, ease of operation, low turnover frequency, and surplus amount of catalyst.29−31
Based on the above insight, quinoline derivatives were developed by condensing 2-aminoarylketones with carbonyl compounds owning a reactive methylene group, followed by cyclodehydration. Quantum chemical calculations were conducted at the B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels of theory in the gas phase to optimize the structure, determine vibrational frequencies, chemical shift values using NMR analysis, Hirshfeld surface analysis, highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO), molecular electrostatic potential (MEP) maps, nonlinear optical (NLO) properties, and thermodynamic properties.32−37 Here, we would like to report a convenient and straightforward workup method for the synthesis of quinoline molecule (3) using freshly prepared polyphosphoric acid (PPA)38−41 (P2O5 in H3PO4) under solvent-free conditions from 2-aminobenzophenone (1) and pentan-2,3-dione (2) through Friedländer quinoline synthesis.
2. Instrumentation Methods
2.1. FT-IR and NMR Analysis
Fourier transform infrared (FT-IR) BRUKER brand (VECTOR 22) was used to record the FT-IR with spectral data collected in the range 4000–400 cm–1. FT-Raman spectrum was recorded using a WiTec Alpha 300 RA Raman-AFM. BRUKER AVANCE III HD-400 [400 MHz (1H) and 100 MHz (13C)] spectrometers used tetramethyl silane (TMS) as an internal reference for 1H NMR and 13C NMR. The chemical shifts are observed in parts per million (ppm). Coupling constants (J) are reported in hertz (Hz). The terms Jo and Jm refer to the ortho coupling constant and meta coupling constant. The terms s, d, t, and dd refer to singlet, doublet, triplet, and doublet of doublet, respectively, and bs refers to a broad singlet. A liquid chromatography–mass spectrometry (LC–MS) experiment was conducted using a UHPLC Eksigent1 coupled with ABSciex1MS detector Triple Quad 4500 model equipment. The sample was injected using a syringe, and the data were acquired in a range of 100.0–600.0 Da, at 200 Da s–1 under positive polarity.
2.2. Single-Crystal XRD Analysis
The 1-(4-phenylquinolin-2-yl)propan-1-one (3) crystals were chosen for measurement. The diffraction data were collected at 296 K on a Bruker D8 Venture diffractometer equipped with a bidimensional CMOS Photon 100 detector. The monochromatic Mo Kα (λ = 0.71073 Å) radiation with a graphite monochromator was used for the data collection. The acquired diffraction frames were integrated using the APEX3 package,42 and it was corrected for absorptions with SADABS.43 The structure of 1-(4-phenylquinolin-2-yl)propan-1-one (3) was solved by intrinsic phasing44 using the OLEX2 program.45 Then, the obtained structure was refined with full-matrix least-squares methods based on F2 (SHELXL-2014).44 The non-hydrogen atoms were refined with anisotropic displacement parameters during the refinement process of the crystal structure. All hydrogen atoms were included in their calculated positions, assigned fixed isotropic thermal parameters, and confined to riding their parent atoms.
2.3. DFT Computational Studies
Molecular structure of the compound in the ground state was optimized, from crystal structure as starting point, using three density functional theory (DFT) methods: B3LYP46 the long-range corrected version of B3LYP: CAM-B3LYP47 and the Minnesota 06 functional as M06-2X.48 All DFT calculations, such as geometrical parameters, energy, IR and NMR spectra, atomic charges, HOMO and LUMO energy, and dipole moment, etc., were performed with the 6-311G(d,p)49 basis set level. GaussView 5.0.950 and Gaussian 09AS64L-G09RevD.0151 package programs were used to do numerical calculations. ChemBioDraw Ultra Version (13.0.0.3015)52 was used as utility program. Energy gap (Egap) global hardness (η), global softness (S), global electronegativity (χ), and chemical potential (μ) were calculated to determine the reactivity of studied compound (3) with eqs 1–5,53−55 respectively
| 1 |
| 2 |
| 3 |
| 4 |
| 5 |
2.4. Hirshfeld Surface Analyses
Hirshfeld surface analysis56 was performed using CrystalExplorer 17.5.57 This analysis consists of dnorm surface plots and 2D (two-dimensional) fingerprint plots.58 The mapped electrostatic potentials on the Hirshfeld surfaces using the BLYP/6-31G(d,p) level of theory over a range of ±0.002 au using the TONTO computational package integrated into the program CrystalExplorer.59 The analysis was conducted using the crystallographic information file (CIF) of 1-(4-phenylquinolin-2-yl)propan-1-one (3) as the input. The normalized contact distance (dnorm) was described in terms of de, di, and vdW radii of the atoms was calculated using eq 6, where de and di are the lengths from the Hirshfeld isosurface to the nearest external and internal nucleus, respectively, and vdW corresponds to the van der Waals radii of atoms.60,61
| 6 |
3. Experimental Details
3.1. General
All reagents and chemicals used in the study were purchased from AKSci and Sigma-Aldrich. Unless otherwise indicated, other reagents were obtained from commercial suppliers. Relevant references were given when known compounds were prepared according to the literature procedures. The purity of the synthesized product was demonstrated by TLC silica gel 60 F254 25 aluminum foil 20 × 20 C (purchased from Merck) using petroleum ether and ethyl acetate in the ratio of 95:5 as preparing solvents. Melting points (Mp) were shown on a Kofler Thermogerate apparatus and were uncorrected. They are displayed in degrees of centigrade (°C).
3.2. Synthesis
3.2.1. General Procedure for Preparation of 1-(4-Phenylquinolin-2-yl)propan-1-one (3)
A mixture of the 2-aminobenzophenone (1, 1 mmol) and pentan-2,3-dione (2, 1.2 mmol) was heated for 1 h in the presence of freshly prepared PPA reagent (P2O5 in H3PO4) as a catalyst without any solvent at 90 °C. The completion of the reaction was analyzed by TLC. The reaction was quenched with an excess amount of saturated sodium carbonate solution (30 mL). Then, the obtained solid was filtered, washed with water, extracted with CH2Cl2 (3 × 10 mL), and dried over anhydrous sodium sulfate. Evaporation of the solvent was followed by purification via recrystallization from CH2Cl2 to yield the pure product 2-acetyl-4-arylquinoline (3).
White solid; yield 82%, mp 111–112 °C. FT-IR (KBr, cm–1) (Figure S1) νmax: 3055, 2947, 1697, 1558, 1435, 1373, 1172, 1010.
1H NMR (400 MHz, CDCl3) (Figure S5) (ppm): δ 1.30 (t, 3H, CH3, J = 7.20 Hz), 3.46 (q, 2H, CH2, J = 7.20 Hz), 7.47–7.56 (m, 5H, C2-, C3-, C4-, C5-, C6-H), 7.59 (t, 1H, C12-H, J = 8.00 Hz), 7.78 (t, 1H, C13-H, J = 8.00 Hz), 7.97 (d, 1H, C14-H, J = 8.00 Hz), 8.08 (s, 1H, C8-H), 8.26 (d, 1H, C11-H, J = 8.00 Hz).
1H NMR (400 MHz, DMSO-d6) (Figure S6) (ppm): δ 1.18 (t, 3H, CH3, J = 7.20 Hz), 3.41 (q, 2H, CH2, J = 7.20 Hz), 7.56–7.64 (m, 5H, C2-, C3-, C4-, C5-, C6-H), 7.75 (t, 1H, C12-H, J = 8.00 Hz), 7.89–7.95 (m, 3H, C8-, C13, C14-H), 8.26 (d, 1H, C11-H, J = 8.00 Hz).
13C NMR (100 MHz, CDCl3) (Figure S10) (ppm): δ 8.14, 30.91, 118.36, 125.85, 128.09, 128.44, 128.58, 128.63, 129.58, 129.73, 130.93, 137.83, 147.83, 149.39, 152.61, 203.29.
13C NMR (100 MHz, DMSO-d6) (Figure S11) (ppm): δ 8.42, 30.72, 118.09, 125.93, 127.73, 129.34, 129.65, 129.84, 130.92, 130.98, 137.52, 147.59, 149.40, 152.64, 202.51. LC–MS for (C18H15NO) m/z (%): 261.9 (M + 1, 100).
4. Results and Discussion
4.1. Synthesis
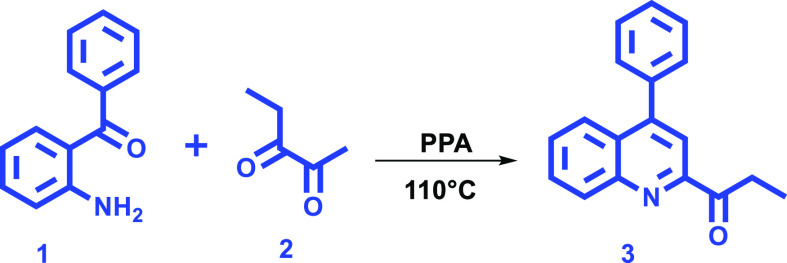
The 2-aminobenzophenone (1) was treated with pentan-2,3-dione (2) in the presence of freshly prepared PPA as a catalyst without any solvent at 90 °C for 1 h to yield 82% of 1-(4-phenylquinolin-2-yl)propan-1-one (3) (Scheme 1).
Scheme 1. Synthesis of 1-(4-Phenylquinolin-2-yl)propan-1-one (3).
4.2. Computational Methods
4.2.1. Full Optimizations
The studied compound is fully optimized at each computational level and the optimized structure of molecule 3 is obtained at B3LYP/6-311G(d,p) in the gas phase are given in Figure 1.
Figure 1.

Optimized structure of molecule 3 at the B3LYP/6-311G(d,p) level in vacuum.
4.2.2. FT-IR Analysis
FT-IR spectrum of molecule 3 is calculated at B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels of theory, and experimental stretching frequency values are represented as a distribution graph in Figure 2. Furthermore, correlation coefficient for each level. The calculated and experimental stretching frequencies are harmonic frequencies. Some stretching frequencies and correlation coefficients (R2) are shown in Table 1.
Figure 2.

Distribution graphs between experimental and calculated stretching frequencies for molecule 3.
Table 1. FT-IR Frequencies (cm–1) (Figures S1–S4).
| assignments | experimental | B3LYP | CAM-B3LYP | M06-2X |
|---|---|---|---|---|
| νCH asymmetric | 3055.24 | 3209.2–3163.7 | 3227.9–3182.1 | 3219.3–3170.3 |
| νCH symmetric | 2947.23 | 3108.9–3104.4 | 3128.0–3123.2 | 3150.8–3139.7 |
| –C=O | 1697.36 | 1764.6 | 1779.9 | 1844.9 |
| –C=N | 1558.48 | 1592.2 | 1610.7 | 1646.0 |
| =C–H | 1435.04 | 1443.1 | 1450.2 | 1458.7 |
| H–C–H | 1373.20 | 1392.9 | 1412.3 | 1427.2 |
| N–C–C=O | 1172.72 | 1215.3 | 1228.6 | 1230.4 |
| Ph ring | 1010.70 | 967.5 | 975.4 | 978 |
| R2 | 0.9849 | 0.9838 | 0.9715 |
The B3LYP/6-311G(d,p) level is the best calculation level in Table 1 and Figure 2 due to the fact that the highest R2 is obtained in this level. After those computational results in the B3LYP/6-311G(d,p) level are given for other computational parts. In the FT-IR (KBr, cm–1) spectrum (Figure S1) of molecule 3, the C=O stretching at 1697 cm–1 and C=N stretching at 1558 cm–1 were found. The theoretical values of FT-IR, which are given by B3LYP/6-311G(d,p) (Figure S2), CAM-B3LYP/6-311G(d,p) (Figure S3), and M06-2X/6-311G(d,p) (Figure S4) levels of theory are mostly in good agreement, as shown in Table 1.
4.2.3. FT-NMR Analysis
The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra of molecule 3 were recorded in CDCl3 and DMSO-d6 with TMS as the internal standard. Simulated 1H and 13C NMR spectra were calculated for each level. Experimental and calculated results in the B3LYP/6-311G(d,p) level for molecule 3 are given in Tables 2 and 3. For other computational results, they are given in Tables S1 and S2. Within 1H NMR by using CDCl3 (Figure S5) or DMSO-d6 (Figure S6) solvents, aliphatic proton peaks appeared in the upfield region between δ 1.18 and 3.46. The aromatic protons peaks were observed in the downfield region at δ 7.47–8.26. The chemical shift of the C12 proton DMSO-d6 is in the range of δ 7.75; however, in CDCl3, it is at about δ 7.59. The C13 proton in DMSO-d6 as a multiplet appeared in the range of δ 7.89–7.95 but in CDCl3 at δ 7.78. The C18 aliphatic proton showed in CDCl3 at δ 1.30 but in DMSO-d6 at δ 1.18 due to the solvent polarity. The 13C NMR spectrum shows the presence of 18 carbons. The characteristic signals at δ 203.29 and δ 202.51 were due to −C16=O using CDCl3 (Figure S10) and DMSO-d6 (Figure S11) solvents, respectively. The characteristic signals in the aliphatic region at δ 8.14 (C18), 30.91 (C17) and δ 8.42 (C18), 30.72 (C17) appeared using CDCl3 (Figure S10) and DMSO-d6 (Figure S11) solvents, respectively. All other aromatic carbons appeared in the region of δ 118.09–152.64. From DEPT-135 spectra, 30.91 (C17) and 30.72 (C17) were shown in CDCl3 (Figure S15) and DMSO-d6 (Figure S16) solvents, respectively, which is identified in the downfield region due to the even number of protons.
Table 2. 1H-NMR Chemical Shift δ (ppm) (Figures S5–S9).
| atoms | CDCl3 | DMSO-d6 | B3LYP/6-311G(d,p) |
|---|---|---|---|
| C2(H) | 7.47–7.56 | 7.56–7.64 | 7.56 |
| C3(H) | 7.51 | ||
| C4(H) | 7.52 | ||
| C5(H) | 7.64 | ||
| C6(H) | 7.66 | ||
| C8(H) | 8.08 | 7.89–7.95 | 8.3 |
| C11(H) | 8.26 | 8.26 | 8.38 |
| C12(H) | 7.59 | 7.75 | 7.79 |
| C13(H) | 7.78 | 7.89–7.95 | 7.6 |
| C14(H) | 7.97 | 7.89–7.95 | 8.14 |
| C17(H2) | 3.46 | 3.41 | 3.41 |
| C18(H3) | 1.30 | 1.18 | 1.19 |
Table 3. 13C-NMR Chemical Shift δ (ppm) (Figures S10–S14).
| atoms | CDCl3 | DMSO-d6 | B3LYP/6-311G(d,p) |
|---|---|---|---|
| C1 | 137.83 | 137.52 | 160.92 |
| C2 | 129.58 | 129.34 | 150.82 |
| C3 | 128.44 | 129.65 | 147.11 |
| C4 | 128.58 | 129.65 | 148.21 |
| C5 | 128.58 | 129.65 | 149.43 |
| C6 | 129.58 | 129.34 | 149.69 |
| C7 | 149.39 | 149.40 | 171.32 |
| C8 | 118.36 | 118.09 | 137.32 |
| C9 | 152.61 | 152.64 | 171.25 |
| C10 | 147.83 | 147.59 | 167.82 |
| C11 | 130.93 | 130.92 | 152.63 |
| C12 | 129.73 | 130.98 | 148.56 |
| C13 | 128.63 | 129.84 | 147.72 |
| C14 | 128.09 | 127.73 | 145.88 |
| C15 | 125.85 | 125.93 | 147.44 |
| C16 | 203.29 | 202.51 | 220.35 |
| C17 | 30.91 | 30.72 | 50.15 |
| C18 | 8.14 | 8.42 | 24.14 |
The 1H and 13C NMR spectra of molecule 3 were calculated by using B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels of density functional calculations, and experimental chemical shift values are represented as a distribution graph in Figure 3. The theoretical 1H and 13C chemical shift values compared with experimental 1H and 13C chemical shift values showed in Tables 2 and 3. The theoretical 1H and 13C chemical shift results for (3) are mostly closer to the experimental 1H and 13C shift data. The optimized electronic structure (Figure 1), FT-IR (Figures S2–S4), and NMR (Figures S7–S9 and S12–S14) spectroscopy of molecule 3 were performed using at B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels of theory. The absence of imaginary frequencies for the optimized geometry of molecule 3 suggests a suitable conversion of the model calculations.
Figure 3.
Distribution graphs between calculated and experimental 1H and 13C NMR chemical shifts for molecule 3.
4.2.4. Single-Crystal XRD Studies
4.2.4.1. Crystal and Molecular Structure
The molecular structure of 1-(4-phenylquinolin-2-yl)propan-1-one (3) crystallizes in a orthorhombic system, in the space group P212121 (Z = 4), with normal bond angles and distances62 and similar to related compounds.63,64 The fragments linked to the quinoline ring are practically planar, except for phenyl ring attached to quinoline ring, were their mean planes have a torsion angle of 49.5(1)°, in agreement with 56.75(8) and 61.35(6) found in other similar compounds63,64 (see Figure 4 for more details). A summary of the details about crystal data, collection parameters, and refinement is included in Table 4, and additional crystallographic details are included in the CIF files. ORTEP (Figure 4) views were drawn using OLEX2 software.45
Figure 4.

ORTEP plot for molecule 3. Thermal ellipsoids were drawn with 50% of probability.
Table 4. Crystal Data Parameters for Molecule 3.
| empirical formula | C18H15NO |
| CCDC number | 2077085 |
| formula mass, g mol–1 | 261.31 |
| collection T, K | 296.86 |
| crystal system | Orthorhombic |
| space group | P212121 |
| a (Å) | 7.2696(6) |
| b (Å) | 10.6810(8) |
| c (Å) | 17.8044(14) |
| V (Å3) | 1382.45(19) |
| Z | 4 |
| ρcalcd (g cm–3) | 1.255 |
| crystal size (mm) | 0.25 × 0.23 × 0.19 |
| F(000) | 552.0 |
| abs coeff (mm–1) | 0.078 |
| 2θ range (deg) | 5.958 to 56.534 |
| range h, k, l | –9/9, −14/14, −23/23 |
| no. total refl. | 26,163 |
| no. unique refl. | 3433 [Rint = 0.0836, Rsigma = 0.0468] |
| comp. θmax (%) | 99.9 |
| max/min transmission | 0.983/0.980 |
| data/restraints/parameters | 3433/0/183 |
| final R [I > 2σ(I)] | R1 = 0.0526, wR2 = 0.0980 |
| R indices (all data) | R1 = 0.0976, wR2 = 0.1162 |
| goodness of fit/F2 | 1.022 |
| largest diff. peak/hole (eÅ–3) | 0.23/–0.15 |
| Flack parameter | –0.7(10) |
The calculated geometrical parameters (bond length, bond angle, and dihedral angle) of molecule 3 and single-crystal X-ray diffraction (XRD) data are listed in Table 5, following the atom numbering scheme given in Figure 1.
Table 5. Optimized Geometrical Parameters (Bond Lengths, Bond Angles, and Dihedral Angels) of Molecule 3 Using B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) Levels of Theory in Comparison with Experimental XRD Studies.
| parameters | bond length (A) |
parameters | bond angles (deg) |
parameters | dihedral (deg) |
|||
|---|---|---|---|---|---|---|---|---|
| XRD | B3LYP/6-311G(d,p) | XRD | B3LYP/6-311G(d,p) | XRD | B3LYP/6-311G(d,p) | |||
| C1–C2 | 1.39 | 1.39 | C1–C2–C3 | 120.8 | 120.61 | C2–C1–C7–C8 | –47.23 | –55.86 |
| C2–C3 | 1.39 | 1.39 | C2–C3–C4 | 120.29 | 120.15 | C6–C1–C7–C15 | 132.91 | 121.9 |
| C3–C4 | 1.37 | 1.39 | C3–C4–C5 | 119.73 | 119.69 | C7–C15–C14–C13 | –178.86 | –179.78 |
| C4–C5 | 1.37 | 1.39 | C4–C5–C6 | 120.59 | 120.2 | C7–C8–C9–N1 | –0.97 | 0.1 |
| C5–C6 | 1.38 | 1.39 | C5–C6–C1 | 120.08 | 120.54 | C9–N1–C10–C11 | –177.84 | –178.85 |
| C1–C7 | 1.49 | 1.49 | C6–C1–C7 | 122.14 | 121.08 | C9–N1–C10–C15 | 1.29 | 0.587 |
| C7–C8 | 1.37 | 1.37 | C1–C7–C8 | 118.58 | 118.82 | C9–C16–C17–C18 | 179.8 | 179.85 |
| C7–C15 | 1.42 | 1.43 | C1–C7–C15 | 123.56 | 122.34 | N1–C9–C16–C17 | 4.27 | 0.369 |
| C8–C9 | 1.41 | 1.41 | C7–C8–C9 | 120.52 | 119.9 | N1–C10–C11–C12 | 178.71 | 178.52 |
| C9–N1 | 1.32 | 1.31 | C7–C15–C14 | 124.25 | 123.88 | |||
| N1–C10 | 1.37 | 1.36 | C8–C9–N1 | 123.52 | 123.76 | |||
| C10–C11 | 1.41 | 1.42 | C8–C9–C16 | 118.7 | 118.55 | |||
| C10–C15 | 1.43 | 1.42 | C9–N1–C10 | 117.4 | 118.01 | |||
| C11–C12 | 1.36 | 1.36 | N1–C10–C11 | 117.73 | 117.74 | |||
| C12–C13 | 1.4 | 1.41 | N1–C10–C15 | 123.03 | 122.73 | |||
| C13–C14 | 1.36 | 1.37 | C10–C11–C12 | 120.52 | 120.58 | |||
| C14–C15 | 1.42 | 1.42 | C10–C15–C14 | 118.12 | 118.37 | |||
| C9–C16 | 1.51 | 1.51 | C11–C12–C13 | 120.48 | 120.08 | |||
| C16–O1 | 1.21 | 1.21 | C12–C13–C14 | 120.61 | 120.66 | |||
| C16–C17 | 1.49 | 1.51 | C13–C14–C15 | 121.03 | 120.756 | |||
| C17–C18 | 1.51 | 1.52 | C9–C16–C17 | 118.37 | 117.73 | |||
| C9–C16–O1 | 119.4 | 119.67 | ||||||
| C16–C17–C18 | 113.8 | 112.87 | ||||||
The distribution graphs show the agreement between calculated and experimental structural parameters. Correlation coefficients (R2) are 0.9827, 0.9794, and 0.9973 for bond length, bond angles, and dihedral, respectively, in Figure 5. According to Table 5 and Figure 5, the calculated and experimental values of geometrical parameters were satisfactory.
Figure 5.
Correlation of experimental and calculated structural parameters for molecule 3. (A) Bond length, (B) bond angles, and (C) dihedral angles.
4.2.5. Noncovalent Interactions
Additionally, the crystal structure does not show classic hydrogen bonds between neighbor molecules. However, one C–H···O intermolecular bifurcated hydrogen bond interaction is observed with graph-set notation C11(8), C11(9), and R21(5).65 Large C–H···π interactions are also observed in the crystal structure of the title compound. Both interactions observed in the crystal structure lie in each screw axis present in the space group. Moreover, the distances between involved atoms is larger than the sum of the VdW radii60,61 (see Figure 6 and Table 6 for more details).
Figure 6.
Crystal packing of molecule 3.
Table 6. Hydrogen Bond Interactions for Molecule 3.
| atoms | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| C13–H13···O1b | 0.93 | 2.732 | 3.314(4) | 121.5 |
| C14–H14···O1b | 0.93 | 2.829 | 3.366(4) | 117.8 |
| C2–H2···CNT1c,a | 0.93 | 2.739 | 3.611(3) | 159.4 |
| C17–H17B···CNT2d,a | 0.97 | 2.841 | 3.639(4) | 146.7 |
| C18–H18C···CNT3d,a | 0.93 | 2.739 | 3.611(3) | 159.4 |
CNT = centroid; 1: C1/C2/C3/C4/C5/C6; 2: C7/C8/C9/N1/C10/C15; 3: C10/C11/C12/C13/C14/C15.
3/2 – x, 1 – y, −1/2 – z.
–1/2 + x, 1/2 – y, 1 – z.
–1/2 + x, 3/2 – y, 1 – z.
4.2.6. Hirshfeld Surface Analyses
Hirshfeld surface analysis was utilized as a complementary analysis for the crystal packing contacts. The intermolecular interactions are mainly constituted by C–H···O, which are shown as red (dnorm < VdW radii), white (dnorm = VdW radii), and blue (dnorm > VdW radius) spots in the dnorm surfaces for all compounds. Furthermore, there is evidence of some other interesting weak contacts with less impact on the crystal structure, such as C–H···π contacts. The reciprocal contacts and their corresponding contributions are displayed in Figure 7 and all fingerprint plots with dnorm (where dnorm = di + de) surfaces for their intermolecular contacts were calculated at the BLYP/6-31G(d,p) level of theory in the cluster of radius 3.8 Å around the molecule.57
Figure 7.
dnorm surface for molecule 3 (left) and its overall 2D fingerprint plot (right), indicating the most important contacts in the crystal packing. Red and blue colors indicate strong and weak interactions, respectively. Isovalues range from −0.0051 (red) to +1.3934 (blue).
The reciprocal contacts for C–H···O appear as a wide symmetrical wing with de + di ∼ 2.7, corresponding to classical hydrogen bond interactions. However, this is larger than the sum of the VdW radii, as mentioned above. In the case of C–H···N interactions, the distance is slightly higher than the VdW radii for N and H atoms (de + di > 2.75 Å),61 where the contribution is negligible. Remarkably, The interatomic contacts of C–H···H interactions display a di + de ∼ 2.4 Å = 2.4 Å, with a contribution of around 50.1%, creating a nonsignificant effect over molecular packing in the crystal structure stabilization, in other words, these contacts are slightly larger than the sum of the VdW radii for these atoms.60,61
In the Hirshfeld surface analysis, there is another type of weak interactions. For example, the contribution of H···π type interaction is around 34.6%, when de + di of ∼2.9 Å. This was analyzed with a shape index, allowing us to determine the presence of these weak interactions. The yellow-orange spots exhibit surface subsidence due to the proximity of the neighboring moieties and the blue-green spots demonstrate the reciprocal contacts of the moieties that generate the subsidence.
In this context, their corresponding counterparts, where the interaction between hydrogens and delocalized π-clouds bonds are demonstrated when constructing the crystal structure, are shown in Figure 8. However, the sum of VdW radii for C and H atoms interacting between them is slightly larger than their theoretical value (2.95 vs 2.9 Å).60,61 Thus, these types of interactions can be reflected weakly as for the dnorm surface.
Figure 8.
dnorm surface for molecule 3 (left) and its shape index plot (right) indicate the C–H···π contacts in the crystal packing. Isovalues range from −0.0051 (red/orange) to +1.3934 (blue).
4.2.7. Frontier Molecular Orbitals, MEP Maps, and MEP Contours
The DFT calculations of MOs and their energy diagrams were executed at the B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels of theory in the gas phase. Frontier molecular orbitals (FMOs), such as the HOMO and the LUMO, were exploited to determine the chemical reactivity. The distributions of the energy diagrams from HOMO – 2 to LUMO + 2 for molecule 3 are given in Figure 9 at the B3LYP/6-311G(d,p) level. The results at other calculation levels are listed in Figure S19 (Supporting Information). It is noticed that electrons in HOMO are mostly localized on the quinoline ring of molecule 3. The presence of electrons in LUMO is also located in the same region of the quinoline ring, indicating the presence of increased chemical activity. The values of hardness, softness, chemical potential, and electronegativity for molecule 3 in the gas phase with B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels of theory basis sets. In this report, the character of the MOs is calculated using the same levels of theory, and the density of the states (DOS) is shown in Figure 10. DOS plot exhibits the MO compositions at the different energy levels of molecule 3. Moreover, the DOS is also notable for numerous calculations, such as estimating the occupancy of states and virtual orbitals of molecule 3 using red and green lines in the spectrum.66,67 The mentioned quantum chemical parameters are calculated using eqs 1–5 and are given in Table 7.
Figure 9.

Molecular orbital (MO) energy diagrams (eV) from HOMO – 2 to LUMO + 2 at B3LYP/6-311G(d,p) molecule 3 in the gas phase.
Figure 10.

Simulated density of states spectrum of molecule 3 with (A) B3LYP/6-311G(d,p), (B) CAM-B3LYP/6-311G(d,p), and (C) M06-2X/6-311G(d,p) levels of theory.
Table 7. FMO Energies, Chemical Potential (μ), Hardness (η) Softness (S), and Electronegativity (χ) Parameters for the Molecule 3 Calculated with B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) Levels of Theory.
| property | B3LYP/6-311G(d,p) | CAM-B3LYP/6-311G(d,p) | M06-2X/6-311G(d,p) |
|---|---|---|---|
| EHOMO | –0.24103 | –0.29054 | –0.28706 |
| ELUMO | –0.08241 | –0.03665 | –0.04792 |
| EGAP | 0.15862 | 0.25389 | 0.23914 |
| chemical potential (μ) | –0.16172 | –0.16359 | –0.16749 |
| global hardness (η) | 0.07931 | 0.12695 | 0.11957 |
| global softness (S) | 6.30437 | 3.93856 | 4.18165 |
| global electronegativity (χ) | 0.16172 | 0.16359 | 0.16749 |
According to Table 7, selected quantum chemical calculations are close to each other in the CAM-B3LYP/6-311G(d,p) and M06-2X/6-311G(d,p) levels. The results in B3LYP/6-311G(d,p) are different from other. However, these results do not represent any errors. When the result of this study is comparable to previous reports,68 it is observed that the spherical softness of the studied molecule is quite large at each computational level, and it is lower in the energy gap. In this case, it states that the studied molecule can easily interact.
MEP maps are correlated to electron density on molecular surfaces. These maps can be depicted in nucleophilic or electrophilic active zones. MEP maps and contours of molecule 3 at B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels in the gas phase are represented in Figure 11. In the MEP contour map, the negative regions (designated as red) of MEP are related to electrophilic attacks, and positive regions (designated as blue) are related to nucleophilic reactivity. According to MEP maps in Figure 11, the environment of the oxygen atom in the carbonyl group with red color acts at the electrophilic reactive site. The surfaces around the nitrogen atom are blue color, which can act as a nucleophilic reactive site.
Figure 11.
MEP maps and contours of molecule 3 at B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) levels in the gas phase.
4.2.8. Thermodynamics Parameters
The thermodynamic parameters of molecule 3 have been calculated to get reliable relations among energetic, structural, and reactivity characteristics of the molecules. Table S3 (Supporting Information) shows the values of thermodynamic parameters such as, Gibbs free energy, entropy allows us to estimate the stability of the compound, and zero-point energy gives information about the nuclear motion of the structure.69
4.2.9. NBO Charges
Our interest is to compare the different methods to describe the distribution of electrons in the compound under study and evaluating the sensitivity of the calculated charges to changes in the quantum mechanical method used. The N(1) and the O(1) atoms have the highest negative charges. These charges, being relatively close in space, make this fragment of the molecule a potential coordination site with metal ions. Natural bond orbital (NBO) charges of atoms in studied compound are calculated at each calculation level and given in Table 8.
Table 8. NBO Atomic Charges (e) of the Molecule 3.
| atom | B3LYP | CAM-B3LYP | M06-2X |
|---|---|---|---|
| C1 | –0.058 | –0.060 | –0.064 |
| C2 | –0.186 | –0.190 | –0.192 |
| C3 | –0.192 | –0.194 | –0.196 |
| C4 | –0.194 | –0.200 | –0.201 |
| C5 | –0.194 | –0.197 | –0.199 |
| C6 | –0.194 | –0.199 | –0.201 |
| C7 | 0.018 | 0.023 | 0.018 |
| C8 | –0.200 | –0.210 | –0.212 |
| C9 | 0.126 | 0.130 | 0.131 |
| C10 | 0.164 | 0.162 | 0.163 |
| C11 | –0.171 | –0.176 | –0.177 |
| C12 | –0.192 | –0.197 | –0.199 |
| C13 | –0.184 | –0.188 | –0.190 |
| C14 | –0.187 | –0.191 | –0.194 |
| C15 | –0.067 | –0.069 | –0.073 |
| C16 | 0.538 | 0.550 | 0.550 |
| C17 | –0.464 | –0.475 | –0.481 |
| C18 | –0.569 | –0.582 | –0.583 |
| N1 | –0.439 | –0.440 | –0.433 |
| O1 | –0.548 | –0.552 | –0.549 |
| H2 | 0.212 | 0.216 | 0.219 |
| H3 | 0.207 | 0.211 | 0.213 |
| H4 | 0.205 | 0.209 | 0.212 |
| H5 | 0.206 | 0.210 | 0.213 |
| H6 | 0.212 | 0.216 | 0.219 |
| H8 | 0.240 | 0.246 | 0.248 |
| H11 | 0.220 | 0.224 | 0.227 |
| H12 | 0.208 | 0.212 | 0.214 |
| H13 | 0.207 | 0.211 | 0.213 |
| H14 | 0.219 | 0.223 | 0.226 |
| H17a | 0.229 | 0.233 | 0.236 |
| H17b | 0.229 | 0.234 | 0.236 |
| H18a | 0.202 | 0.206 | 0.207 |
| H18b | 0.195 | 0.199 | 0.201 |
| H18c | 0.201 | 0.206 | 0.207 |
It is interesting to notice that the dipole moment is higher for B3LYP calculation compared with CAM-B3LYP and M06-2X methods. Therefore, B3LYP describes a more polarized structure than the other DFT methods.
4.2.10. Determination of NLO Properties
We carried out the study to calculate the dipolar moment (u) linear polarizability (α) and the first order hyperpolarizability (β) using the B3LYP, CAM-B3LYP, and M06-2X functional with the 6-311G(d,p) basis set in the gas phase. These parameters are important to predict the NLO properties of molecules for their potential application in optoelectronic technologies in the field of telecommunications, signal processing, data storage, microscopy to higher harmonic, etc.70 For its part, urea is a reference substance for NLO material for comparative purposes.71,72Table 9 shows the dipole moments and static electronic polarizabilities of molecule 3 obtained from the calculated parameters.
Table 9. Dipole Moments and Static Electronic Polarizabilities of the Molecule 3 Calculated with B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) Levels of Theory.
| property | urea |
molecule 3 |
||||
|---|---|---|---|---|---|---|
| B3LYP | CAM-B3LYP | M06-2X | B3LYP | CAM-B3LYP | M06-2X | |
| μx | 0.0012 | 0.0014 | 0.0000 | –2.5628 | –2.5084 | –2.4506 |
| μy | –3.6204 | –3.6204 | –3.6049 | 2.2797 | 2.2452 | 2.2356 |
| μz | 0.0004 | 0.0001 | 0.0000 | –0.0981 | –0.0957 | –0.0983 |
| μa | 3.6204 | 3.6964 | 3.6049 | 3.4314 | 3.3678 | 3.3186 |
| αxx | –17.4265 | –17.3455 | –17.4046 | –106.5586 | –106.1839 | –104.7016 |
| αyy | –24.9745 | –24.978 | –25.0079 | –114.7163 | –114.8909 | –113.9694 |
| αzz | –24.9976 | –25.0503 | –25.0713 | –115.7369 | –116.0649 | –116.3475 |
| ⟨α⟩b | –22.2697 | –22.2333 | –22.2696 | –112.3373 | –112.3799 | –111.6728 |
| (Δα)b | 8.9713 | 8.9686 | 9.1274 | 15.9477 | 16.40179 | 17.2993 |
| βtotalc | 11.9617 | 12.3879 | 11.9143 | 57.6441 | 57.3569 | 55.5248 |
In Debye.
In (×10–23) esu.
In (×10–30) esu.
Interestingly, even though both molecule 3 and urea have relatively similar dipole moments, the linear polarizability and hyperpolarizability of molecule 3 are approximately 5 times higher compared to urea. Thus, the large magnitude of ⟨α⟩ and βtotal indicate that the molecule 3 is highly polarizable; therefore, this molecule 3 could potentially serve as NLO material.
5. Conclusions
In the present work, PPA-assisted synthesis of a novel 1-(4-phenylquinolin-2-yl)propan-1-one from 2-aminobenzophenone and pentan-2,3-dione under solvent-free Friedländer quinoline synthesis is shown. The synthesized 1-(4-phenylquinolin-2-yl)propan-1-one (3) is confirmed by molecular and structural analysis like FT-IR, 1H and 13C NMR chemical shifts, and single-crystal XRD techniques. DFT calculations for molecule 3 were measured and calculated using B3LYP/6-311G(d,p), CAM-B3LYP/6-311G(d,p), and M06-2X/6-311G(d,p) basis sets in the gas phase. As a result, it has been found that there is a good correlation between calculated and experimental data. The optimized geometry of the quinoline molecule was compared with the experimental XRD values. DFT calculations of the noncovalent interactions, Hirshfeld surface analysis, NLO properties, thermodynamic properties, and FMO acknowledged chemically active sites of the quinoline compound accountable for its chemical reactivity. The MEP results showed that the most reactive site for any nucleophilic attack is the nitrogen atom, while the most reactive site for any electrophilic attack is the oxygen atom. These findings may provide information about potential reactive regions on the whole molecule. It is interesting to observe that the dipole moment is higher for B3LYP calculation than for CAM-B3LYP and M06-2X methods. Therefore, B3LYP describes a more polarized structure than the other DFT methods.
Acknowledgments
S.R. is thankful to the financial support of FONDECYT for a Postdoctoral (Project no. 3190292) fellowship and Dr. Cristian Salas, Faculty of Chemistry and Pharmacy, Pontificia Universidad Católica de Chile, Chile, which are gratefully acknowledged. The authors also acknowledge Dr. Iván Brito, Facultad de Ciencias Básicas, Universidad de Antofagasta, Antofagasta, Chile and Dr. Werner Kaminsky, Department of Chemistry, University of Washington, Seattle, WA 98195, USA, for single-crystal XRD analysis.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.3c04360.
General procedure for synthesis, spectral data, and theoretical investigation of molecule 3 (PDF)
The Cambridge Crystallographic Data Centre has deposited CIF files for compound 3 with the CCDC number 2077085. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge, CB2 1EZ, UK. Fax: +44 (0) 1223 336033 or Email: deposit@ccdc.cam.ac.uk (CIF)
Author Contributions
Rajendran Satheeshkumar: conceptualization, methodology, and writing—original draft preparation; Rodrigo Montecinos: data curation and original draft preparation; Jonathan Cisterna, Kolandaivel Prabha, Nitin Bharat Charbe, and Sushesh Palakurthi: reviewing and editing; Roberto Acevedo and Karnam Jayarampillai Rajendra Prasad: reviewing and editing; and Koray Sayin and Murtuza Tambuwala: data curation and reviewing and editing.
Author Contributions
All the data out from software have been included in the paper.
Consent for publication: All authors gave consent for publication.
The authors declare no competing financial interest.
Supplementary Material
References
- Nainwal L. M.; Tasneem S.; Akhtar W.; Verma G.; Khan M. F.; Parvez S.; Shaquiquzzaman M.; Akhter M.; Alam M. M. Green Recipes to Quinoline: A Review. Eur. J. Med. Chem. 2019, 164, 121–170. 10.1016/j.ejmech.2018.11.026. [DOI] [PubMed] [Google Scholar]
- Morimoto Y.; Matsuda F.; Shirahama H. Total Synthesis of (±)-Virantmycin and Determination of Its Stereochemistry. Synlett 1991, 1991 (03), 202–203. 10.1055/s-1991-20680. [DOI] [Google Scholar]
- Michael J. P. Quinoline, Quinazoline and Acridone Alkaloids. Nat. Prod. Rep. 1997, 14 (6), 605–618. 10.1039/np9971400605. [DOI] [PubMed] [Google Scholar]
- Michael J. P. Quinoline, Quinazoline and Acridone Alkaloids. Nat. Prod. Rep. 2002, 19 (6), 742–760. 10.1039/b104971m. [DOI] [PubMed] [Google Scholar]
- Markees D. G.; Dewey V. C.; Kidder G. W. Antiprotozoal 4-Aryloxy-2-Aminoquinolines and Related Compounds. J. Med. Chem. 1970, 13 (2), 324–326. 10.1021/jm00296a048. [DOI] [PubMed] [Google Scholar]
- Campbell S. F.; Hardstone J. D.; Palmer M. J. 2,4-Diamino-6,7-dimethoxyquinoline Derivatives as .alpha.1-Adrenoceptor Antagonists and Antihypertensive Agents. J. Med. Chem. 1988, 31 (5), 1031–1035. 10.1021/jm00400a025. [DOI] [PubMed] [Google Scholar]
- Yadav P.; Shah K. Quinolines, a Perpetual, Multipurpose Scaffold in Medicinal Chemistry. Bioorg. Chem. 2021, 109, 104639. 10.1016/j.bioorg.2021.104639. [DOI] [PubMed] [Google Scholar]
- Kaur R.; Kumar K. Synthetic and Medicinal Perspective of Quinolines as Antiviral Agents. Eur. J. Med. Chem. 2021, 215, 113220. 10.1016/j.ejmech.2021.113220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skraup Z. H. Eine Synthese Des Chinolins. Monatsh. Chem. 1880, 1 (1), 316–318. 10.1007/BF01517073. [DOI] [Google Scholar]
- Li J. J.Skraup Quinoline Synthesis. In Name Reactions; Springer, 2003; pp 378–379. [Google Scholar]
- Doebner O.; von Miller W. Ueber Eine Dem Chinolin Homologe Base. Ber. Dtsch. Chem. Ges. 1881, 14 (2), 2812–2817. 10.1002/cber.188101402258. [DOI] [Google Scholar]
- Pfitzinger W. Chinolinderivate Aus Isatinsäure. J. Prakt. Chem. 1885, 33 (1), 100. 10.1002/prac.18850330110. [DOI] [Google Scholar]
- Conrad M.; Limpach L. Synthesen von Chinolinderivaten Mittelst Acetessigester. Ber. Dtsch. Chem. Ges. 1887, 20 (1), 944–948. 10.1002/cber.188702001215. [DOI] [Google Scholar]
- Surrey A. R.Combes Quinoline Synthesis, 2nd ed.; Academic Press Inc., 1961. 10.1016/b978-1-4832-3227-0.50027-4. [DOI] [Google Scholar]
- Povarov L. S. αβ-Unsaturated Ethers and their Analogues in Reactions of Diene Synthesis. Russ. Chem. Rev. 1967, 36 (9), 656–670. 10.1070/RC1967v036n09ABEH001680. [DOI] [Google Scholar]
- Povarov L. S. Reactions of Acetals With Unsaturated Ethers. Russ. Chem. Rev. 1965, 34 (9), 639–656. 10.1070/RC1965v034n09ABEH001546. [DOI] [Google Scholar]
- Cheng C.-C.; Yan S.-J. The Friedländer Synthesis of Quinolines. Org. React. 1982, 17, 37–201. 10.1002/0471264180.or028.02. [DOI] [Google Scholar]
- Friedländer P.; Gohring C. F. Ueber Eine Darstellungsmethode Im Pyridinkern Substituirter Chinolinderivate. Ber. Dtsch. Chem. Ges. 1883, 16 (2), 1833–1839. 10.1002/cber.18830160265. [DOI] [Google Scholar]
- Rajendran S.; Sivalingam K.; Karnam Jayarampillai R. P.; Wang W.-L. L.; Salas C. O. Friedländer’s Synthesis of Quinolines as a Pivotal Step in the Development of Bioactive Heterocyclic Derivatives in the Current Era of Medicinal Chemistry. Chem. Biol. Drug Des. 2022, 100 (6), 1042–1085. 10.1111/CBDD.14044. [DOI] [PubMed] [Google Scholar]
- Marco-Contelles J.; Pérez-Mayoral E.; Samadi A.; Carreiras M. D. C.; Soriano E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009, 109 (6), 2652–2671. 10.1021/cr800482c. [DOI] [PubMed] [Google Scholar]
- Shiri M.; Zolfigol M. A.; Kruger H. G.; Tanbakouchian Z.. Friedländer Annulation in the Synthesis of Azaheterocyclic Compounds; Academic Press, 2011; Vol. 102. 10.1016/B978-0-12-385464-3.00002-9. [DOI] [Google Scholar]
- Li J. J.Friedländer Quinoline Synthesis. In Name Reactions; Springer, 2021; pp 206–208. 10.1007/978-3-030-50865-4_54. [DOI] [Google Scholar]
- Ghobadi N.; Nazari N.; Gholamzadeh P. The Friedländer Reaction: A Powerful Strategy for the Synthesis of Heterocycles. Adv. Heterocycl. Chem. 2020, 132, 85–134. 10.1016/bs.aihch.2020.01.001. [DOI] [Google Scholar]
- Satheeshkumar R.; Rajendra Prasad K. J. Solvent-Free Synthesis of Dibenzo[b,j] [1,10]Phenanthroline Derivatives Using Eaton’s Reagent as Catalyst. Synth. Commun. 2017, 47 (10), 990–998. 10.1080/00397911.2017.1301480. [DOI] [Google Scholar]
- Satheeshkumar R.; Shankar R.; Kaminsky W.; Rajendra Prasad K. J. Novel Synthetic and Mechanistic Approach of TFA Catalysed Friedländer Synthesis of 2-Acylquinolines from Symmetrical and Unsymmetrical 1,2-Diketones with o-Aminoarylketones. ChemistrySelect 2016, 1 (21), 6823–6829. 10.1002/slct.201601624. [DOI] [Google Scholar]
- Satheeshkumar R.; Sayin K.; Kaminsky W.; Rajendra Prasad K. J. Indium Triflate and Ionic Liquid-Mediated Friedländer Synthesis of 2-Acylquinolines. Synth. Commun. 2017, 47 (21), 1940–1954. 10.1080/00397911.2017.1357185. [DOI] [Google Scholar]
- Satheeshkumar R.; Kaminsky W.; Sparkes H. A.; Rajendra Prasad K. J. Efficient Protocol for Synthesis of Pyrazolo[3,4-a]Acridines. Synth. Commun. 2015, 45 (19), 2203–2215. 10.1080/00397911.2015.1070433. [DOI] [Google Scholar]
- Satheeshkumar R.; Kaminsky W.; Rajendra Prasad K. J. Efficient Novel Synthesis of Pyrano[3,2-a]- and Pyrazolo[4,3-a]-Acridines. Synth. Commun. 2017, 47 (3), 245–255. 10.1080/00397911.2016.1262038. [DOI] [Google Scholar]
- Satheeshkumar R.; Shanmugaraj K.; Delgado T.; Bertrand J.; Brito I.; Salas C. O. Friedländer Synthesis of Novel Polycyclic Quinolines Using Solid SiO2/H2SO4 Catalyst. Org. Prep. Proced. Int. 2021, 53 (2), 138–144. 10.1080/00304948.2020.1865069. [DOI] [Google Scholar]
- Hasaninejad A.; Zare A.; Shekouhy M.; Ameri-Rad J. Sulfuric Acid-Modified PEG-6000 (PEG-OSO3H): An Efficient, Bio-Degradable and Reusable Polymeric Catalyst for the Solvent-Free Synthesis of Poly-Substituted Quinolines under Microwave Irradiation. Green Chem. 2011, 13 (4), 958–964. 10.1039/c0gc00953a. [DOI] [Google Scholar]
- McAteer C. H.; Murugan R.; Subba Rao Y. V.. Heterogeneously Catalyzed Synthesis of Heterocyclic Compounds; Academic Press, 2017; Vol. 121. 10.1016/BS.AIHCH.2016.03.003. [DOI] [Google Scholar]
- Satheeshkumar R.; Shankar R.; Kaminsky W.; Kalaiselvi S.; Padma V. V.; Rajendra Prasad K. J. Theoretical and Experimental Investigations on Molecular Structure of 7-Chloro-9-Phenyl-2,3-Dihydroacridin-4(1H)-One with Cytotoxic Studies. J. Mol. Struct. 2016, 1109, 247–257. 10.1016/j.molstruc.2016.01.002. [DOI] [Google Scholar]
- Satheeshkumar R.; Sayin K.; Kaminsky W.; Rajendra Prasad K. J. Synthesis, Spectral Analysis and Quantum Chemical Studies on Molecular Geometry, Chemical Reactivity of 7-Chloro-9-(2′-Chlorophenyl)-2,3-Dihydroacridin-4(1H)-One and 7-Chloro-9-(2′-Fluorophenyl)-2,3-Dihydroacridin-4(1H)-One. J. Mol. Struct. 2017, 1128, 279–289. 10.1016/j.molstruc.2016.08.080. [DOI] [Google Scholar]
- Satheeshkumar R.; Rajamanikandan R.; Ilanchelian M.; Sayin K.; Prasad K. J. R. Synthesis of Novel 1,10-Phenanthroline Derivatives and It Used as Probes for Sensitive Detection of Zn2+ and Cd2+ Metal Ions—Spectroscopic and Theoretical Approach. Spectrochim. Acta, Part A 2019, 221, 117196. 10.1016/j.saa.2019.117196. [DOI] [PubMed] [Google Scholar]
- Satheeshkumar R.; Sayin K.; Kaminsky W.; Rajendra Prasad K. J. Synthesis, Spectroscopic, In Vitro Cytotoxicity and Crystal Structures of Novel Fluorinated Dispiroheterocycles: DFT Approach. Monatsh. Chem. 2018, 149 (1), 141–147. 10.1007/s00706-017-2050-5. [DOI] [Google Scholar]
- Jayarajan R.; Satheeshkumar R.; Kottha T.; Subbaramanian S.; Sayin K.; Vasuki G. Water Mediated Synthesis of 6-Amino-5-cyano-2-oxo-N-(pyridin-2-yl)-4-(p-tolyl)-2H-[1,2′-bipyridine]-3-carboxamide and 6-amino-5-cyano-4-(4-fluorophenyl)-2-oxo-N-(pyridin-2-yl)-2H-[1,2′-bipyridine]-3-carboxamide—An Experimental and Computational Studies with Non-Linear Optical (NLO) and Molecular Docking Analyses. Spectrochim. Acta, Part A 2020, 229, 117861. 10.1016/j.saa.2019.117861. [DOI] [PubMed] [Google Scholar]
- Satheeshkumar R.; Prabha K.; Vennila K. N.; Sayin K.; Güney E.; Kaminsky W.; Acevedo R. Spectroscopic (FT-IR, NMR, Single Crystal XRD) and DFT Studies Including FMO, Mulliken Charges, and Hirshfeld Surface Analysis, Molecular Docking and ADME Analyses of 2-Amino-4′-Fluorobenzophenone (FAB). J. Mol. Struct. 2022, 1267, 133552. 10.1016/j.molstruc.2022.133552. [DOI] [Google Scholar]
- Popp F. D.; Mcewen W. E. Polyphosphoric Acids As A Reagent In Organic Chemistry. Chem. Rev. 1958, 58 (2), 321–401. 10.1021/cr50020a004. [DOI] [Google Scholar]
- So Y. H.; Heeschen J. P. Mechanism of Polyphosphoric Acid and Phosphorus Pentoxide-Methanesulfonic Acid as Synthetic Reagents for Benzoxazole Formation. J. Org. Chem. 1997, 62 (11), 3552–3561. 10.1021/jo960441u. [DOI] [Google Scholar]
- Prabha K.; Satheeshkumar R.; Rajendra Prasad K. J. Synthesis and Cytotoxicity of Novel Indoloquinolines and Benzonaphthyridines from 4-Chloro-2,8-Dimethylquinoline and Variety of Hetero Amines. ChemistrySelect 2021, 6 (28), 7136–7142. 10.1002/SLCT.202101269. [DOI] [Google Scholar]
- Kolandaivel P.; Rajendran S.; Karnam Jayarampillai R. P. Synthesis of Novel Benzo Naphtho Naphthyridines from 2,4-Dicloroquinolines. J. Heterocycl. Chem. 2021, 58 (9), 1809–1824. 10.1002/JHET.4312. [DOI] [Google Scholar]
- Bruker AXS Inc. APEX3 Package, APEX3, SAINT and SADABS; Bruker AXS Inc.: Madison, Wisconsin, USA, 2016.
- Sheldrick G. M.SADABS, Software for Empirical Absorption Correction; University of Göttingen: Germany, 2000.
- Sheldrick G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71 (1), 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2 : A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42 (2), 339–341. 10.1107/S0021889808042726. [DOI] [Google Scholar]
- Becke A. D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98 (7), 5648–5652. 10.1063/1.464913. [DOI] [Google Scholar]
- Yanai T.; Tew D. P.; Handy N. C. A New Hybrid Exchange-Correlation Functional Using the Coulomb-Attenuating Method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393 (1–3), 51–57. 10.1016/j.cplett.2004.06.011. [DOI] [Google Scholar]
- Zhao Y.; Truhlar D. G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120 (1–3), 215–241. 10.1007/s00214-007-0310-x. [DOI] [Google Scholar]
- Krishnan R.; Binkley J. S.; Seeger R.; Pople J. A. Self-consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72 (1), 650–654. 10.1063/1.438955. [DOI] [Google Scholar]
- Dennington R.; Todd Keith J. M.. GaussView, Version 5, 2009.
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenb D. J.. Gaussian 09, Revision A.02, 2009.
- PerkinElmer . ChemBioDraw Ultra, Version (13.0.0.3015); CambridgeSoftWaltham: MA, USA, 2012.
- Kohn W.; Sham L. J. Quantum Density Oscillations in an Inhomogeneous Electron Gas. Phys. Rev. 1965, 137 (6A), A1697–A1705. 10.1103/PhysRev.137.A1697. [DOI] [Google Scholar]
- Jesudason E. P.; Sridhar S. K.; Malar E. P.; Shanmugapandiyan P.; Inayathullah M.; Arul V.; Selvaraj D.; Jayakumar R. Synthesis, Pharmacological Screening, Quantum Chemical and In Vitro Permeability Studies of N-Mannich Bases of Benzimidazoles through Bovine Cornea. Eur. J. Med. Chem. 2009, 44 (5), 2307–2312. 10.1016/j.ejmech.2008.03.043. [DOI] [PubMed] [Google Scholar]
- Gökce H.; Bahçeli S. A Study on Quantum Chemical Calculations of 3-4-Nitrobenzaldehyde Oximes. Spectrochim. Acta, Part A 2011, 79 (5), 1783–1793. 10.1016/J.SAA.2011.05.057. [DOI] [PubMed] [Google Scholar]
- McKinnon J. J.; Jayatilaka D.; Spackman M. A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, (37), 3814–3816. 10.1039/b704980c. [DOI] [PubMed] [Google Scholar]
- Wolff S. K.; Grimwood D. J.; McKinnon J. J.; Turner M. J.; Jayatilaka D.; Spackman M. A.. Crystal Explorer, (Version 17.5); University of Western Australia, 2012.
- Spackman M. A.; McKinnon J. J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4 (66), 378–392. 10.1039/B203191B. [DOI] [Google Scholar]
- Spackman M. A.; McKinnon J. J.; Jayatilaka D. Electrostatic Potentials Mapped on Hirshfeld Surfaces Provide Direct Insight into Intermolecular Interactions in Crystals. CrystEngComm 2008, 10, 377–388. 10.1039/b715227b. [DOI] [Google Scholar]
- Bondi A. Van Der Waals Volumes and Radii. J. Phys. Chem. 1964, 68 (3), 441–451. 10.1021/j100785a001. [DOI] [Google Scholar]
- Batsanov S. S. Van Der Waals Radii of Elements. Inorg. Mater. 2001, 37 (9), 871–885. 10.1023/A:1011625728803. [DOI] [Google Scholar]
- Allen F. H.; Kennard O.; Watson D. G.; Brammer L.; Orpen A. G.; Taylor R. Tables of Bond Lengths Determined by X-Ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc., Perkin Trans. 2 1987, (12), 1–19. 10.1039/P298700000S1. [DOI] [Google Scholar]
- Murugavel S.; Jacob Prasanna Stephen C. S.; Subashini R.; AnanthaKrishnan D. Synthesis, Structural Elucidation, Antioxidant, CT-DNA Binding and Molecular Docking Studies of Novel Chloroquinoline Derivatives: Promising Antioxidant and Anti-Diabetic Agents. J. Photochem. Photobiol., B 2017, 173, 216–230. 10.1016/j.jphotobiol.2017.05.043. [DOI] [PubMed] [Google Scholar]
- Wang C. C.; Chan C. K.; Lai C. Y. Environmentally Friendly Nafion-Mediated Friedländer Quinoline Synthesis under Microwave Irradiation: Application to One-Pot Synthesis of Substituted Quinolinyl Chalcones. Synthesis (Stuttg). 2020, 52 (12), 1779–1794. 10.1055/s-0039-1690088. [DOI] [Google Scholar]
- Bernstein J.; Davis R. E.; Shimoni L.; Chang N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chem., Int. Ed. Engl. 1995, 34 (15), 1555–1573. 10.1002/anie.199515551. [DOI] [Google Scholar]
- Saral A.; Sudha P.; Muthu S.; Irfan A. Computational, Spectroscopic and Molecular Docking Investigation on a Bioactive Anti-Cancer Drug: 2-Methyl-8-Nitro Quinoline. J. Mol. Struct. 2022, 1247, 131414. 10.1016/j.molstruc.2021.131414. [DOI] [Google Scholar]
- Uzun S.; Esen Z.; Koç E.; Usta N. C.; Ceylan M. Experimental and Density Functional Theory (MEP, FMO, NLO, Fukui Functions) and Antibacterial Activity Studies on 2-Amino-4- (4-Nitrophenyl) −5,6-Dihydrobenzo [h] Quinoline-3-Carbonitrile. J. Mol. Struct. 2019, 1178, 450–457. 10.1016/j.molstruc.2018.10.001. [DOI] [Google Scholar]
- Sayin K.; Karakaş D.; Kariper S. E.; Sayin T. A. Computational Study of Some Fluoroquinolones: Structural, Spectral and Docking Investigations. J. Mol. Struct. 2018, 1156, 172–181. 10.1016/j.molstruc.2017.11.091. [DOI] [Google Scholar]
- Kesharwani M. K.; Brauer B.; Martin J. M. L. Frequency and Zero-Point Vibrational Energy Scale Factors for Double-Hybrid Density Functionals (and Other Selected Methods): Can Anharmonic Force Fields Be Avoided?. J. Phys. Chem. A 2015, 119 (9), 1701–1714. 10.1021/jp508422u. [DOI] [PubMed] [Google Scholar]
- Garmire E.; Durfee C. G.; Storz T.; Garlick J.; Hill S.; Squier J. A.; Kirchner M.; Taft G.; Shea K.; Kapteyn H.; Murnane M.; Backus S.; Wang Q.; Liu Z.; Chen X.; Fan S.; Zhang X.; Zhang H.; Tao X.; Li S.; Rong H.; Jones R.; Liu A.; Cohen O.; Hak D.; Fang A.; Paniccia M.; Trebino R.; DeLong K. W.; Fittinghoff D. N.; Sweetser J. N.; Krumbugel M. A.; Richman B. A.; Kane D. J.; Hollemeyer K.; Altmeyer W.; Heinzle E.; Pitra C.; Wang G.; König K.; Halbhuber K.; Blanche A.; Bablumian A.; Voorakaranam R.; Christenson C.; Lin W.; Gu T.; Flores D.; Wang P.; Hsieh W.; Kathaperumal M.; Rachwal B.; Siddiqui O.; Thomas J.; Norwood R. A.; Yamamoto M. Nonlinear Optics in Daily Life. Opt. Express 2013, 21 (25), 30532–30544. 10.1364/OE.21.030532. [DOI] [PubMed] [Google Scholar]
- Satheeshkumar R.; Montecinos R.; Vera A.; Prasad K. J. R.; Kaminsky W.; Salas C. O. Experimental and Theoretical Physicochemical Study of a New Dispirocompound: 4′-(4-Fluorophenyl)-2′,7-Dimethyl-1,4-Dihydro-3H-Dispiro[Cyclopent[b]Indol-2,5′-[1,2]Oxazinan-6′,3′-Indolin]-2′,3-dione. J. Mol. Struct. 2021, 1227, 129431. 10.1016/j.molstruc.2020.129431. [DOI] [Google Scholar]
- Kerru N.; Gummidi L.; Bhaskaruni S. V. H. S.; Narayana Maddila S.; Singh P.; Jonnalagadda S. B. A Comparison between Observed and Dft Calculations on Structure of 5-(4-Chlorophenyl)-2-Amino-1,3,4-Thiadiazole. Sci. Rep. 2019, 9, 19280. 10.1038/s41598-019-55793-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.